Abstract
Single nucleotide polymorphisms in the promoter region of interleukin-18 (IL-18), an inflammatory cytokine, have been linked to susceptibility to many diseases, including cancer and immune dysfunction. Here, we explored the potential association between the IL-18 -607C/A (rs1946518) promoter region polymorphism and susceptibility to ischemic stroke (IS). This locus was amplified from peripheral blood samples of 386 IS patients (cases) and 364 healthy individuals (controls) by the polymerase chain reaction with sequence-specific primers. Significant differences were observed by the χ2 test in the -607C/A (rs1946518) genotype and allele frequencies between cases and controls (P < 0.05). Furthermore, after excluding for age, gender, smoking status, and hypertension, logistic regression indicated that IS susceptibility of -607C carriers increased 1.6 times (OR = 1.601, 95%CI = 1.148-2.233, P = 0.006) compared to -607A carriers. Additionally, similar increases in IS risk were noted for male patients or patients less than 65 years old. In conclusion, IL-18 -607C/A (rs1946518) promoter polymorphism is associated with IS susceptibility, and the C allele may confer increased IS risk.
Keywords: IL-18, Polymorphism, Ischemic stroke, Correlation
Introduction
Ischemic stroke (IS) is characterized by local brain tissue disintegration or destruction resulting from sudden reduced arterial perfusion of the local blood supply, completely interrupted blood flow, or inadequate oxygen or sugar supply (1). Risk factors for IS include diabetes, high cholesterol, and high blood pressure (1). Indeed, IS is a major cause of morbidity and mortality worldwide. While the etiology of stroke has not been completely defined, pathological studies have demonstrated the roles of atherosclerosis, particularly the inflammatory cytokines and the responses involved in arterial injury, in the pathogenesis of ischemic events (2,3).
Interestingly, susceptibility to IS has been associated with polymorphisms, particularly single nucleotide polymorphisms (SNPs), in the genes producing cytokines like IL-6, TGF-β1, and TNF-α (4-6). IL-18, a member of the interleukin-1 family, is a pleiotropic pro-inflammatory cytokine (7 that functions in the inflammatory response (8). IL-18 stimulates cell-mediated immunity and induces natural killer and T cells to release interferons (7). It can also induce severe inflammatory reactions, leading to disease processes. In patients experiencing IS, peripheral blood levels of IL-18 have been found to be significantly higher compared to healthy individuals (9,10). Specifically, an SNP in the IL-18 promoter (designated -607C/A) has been associated with the development of cardiovascular disease (11,12), which can include vascular endothelial damage and formation of atherosclerosis, processes that can induce stroke. This polymorphism affects the expression and activity of IL-18 (13-15), thereby offering a potential mechanistic basis leading to increased susceptibility to cardiovascular disease. To determine whether alteration in IL-18 may also affect susceptibility to IS, we investigated whether the IL-18 promoter -607C/A (rs1946518) SNP is associated with increased incidence of IS.
Material and Methods
Subjects
This prospective clinical study identified 386 IS patients who received treatment in the Neurology Department, the First Hospital of Yancheng City (Yancheng, China). IS was confirmed in all patients by clinical diagnosis, radiological examination, cardiac function test, and supersonic examination and met WHO Diagnostic Criteria for Stroke (16). Patients with the following diseases were excluded: diabetes, liver or kidney dysfunction, myocardial infarction, asthma, cancer, peripheral vascular disease, or a history of cardiovascular surgery. An additional 364 healthy individuals who received physical examination in our hospital during the same period of time were selected as the control group. These individuals were ≥40 years old and exhibited no history of diabetes, liver or kidney disease, asthma, or cerebrovascular disease. Smoking history was obtained and was defined as at least 1 cigarette daily for more than 6 months. The study protocols were approved by the Ethics Committee of Yancheng Health Vocational and Technical College and all participants gave written informed consent to participate.
DNA extraction
Venous blood (3'mL) was collected in the early morning from each fasting patient and placed in a 2.5% EDTA anticoagulant tube. DNA was extracted using a genomic DNA extraction kit (Takara, Japan) according to manufacturer instructions. Extracted DNA samples were preserved at -70°C.
Detection of IL-18 promoter polymorphism
PCR primers used to amplify the promoter region of the IL-18 gene were designed with the Primer-5 software according to a previous study (17) and were synthesized by Sangon Biological Engineering (China). Specific upstream primers were used to detect the C allele (5"-GTTGCAGAAAGTGTAAAAATTATTAC-3") or the A allele (5"-GTTGCAGAAAGTGTAAAAATTATTAA-3"). Other primers included a forward primer for internal reference (5"-CTTTGCTATCATTCCAGGAA-3") and a downstream primer (5"-TAACCTCATTCAGGACTTCC-3"). The reaction mix (total volume, 50'µL) contained 50'ng template DNA, 5'µL 10X PCR buffer, 2.5'U Taq enzyme, 4'µL dNTP mixture, 5'µL MgCl2, 0.3'μM each of the two specific primers, and 0.6'μM forward and downstream primers. PCR cycling conditions were 94°C for 4'min; 8 cycles of 94°C for 40's, 64°C for 40's, and extension at 72°C for 40's; 30 cycles of 94°C for 40's, 56°C for 40's, and 72°C for 40's; 72°C for 5'min. Amplified products were detected by separation on 2% agarose gel with ethidium bromide staining.
Statistical analysis
Hardy-Weinberg equilibrium analysis was used to detect group representativeness of IL-18 alleles in patient and control groups. The SPSS17.0 statistical software was applied for statistical analysis. The two-sample t-test was used to compare difference in age between subjects younger than 65 years old and older than 65 years; the χ2 test was used to compare age, gender, smoking status, high blood pressure, and genotype and allele frequencies between the two groups. The relationship of gene polymorphism and IS was analyzed by the odds ratio (OR) and its 95% confidence interval (95%CI) was obtained by logistic regression. All analyses were two-sided and P < 0.05 was considered to be statistically significant. The statistical power analysis was performed using the QUANTO software.
Results
Study population
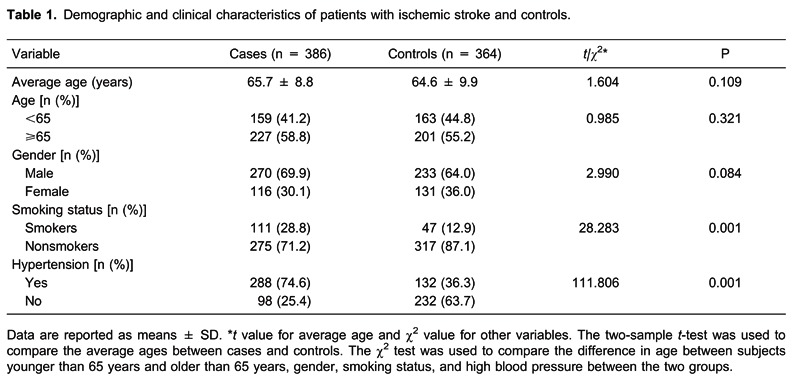
The clinical and demographic information about the study subjects, specifically age, gender, smoking status, and blood pressure, is shown in Table 1. No statistical difference was observed in age or gender distribution between the control and patient groups. However, significantly higher proportions of IS patients smoked or had hypertension compared to the control group (28.8 and 74.6% vs 12.9 and 36.3%, respectively; P < 0.05). Our data showed statistical power to detect association (86.33%) in this sample.
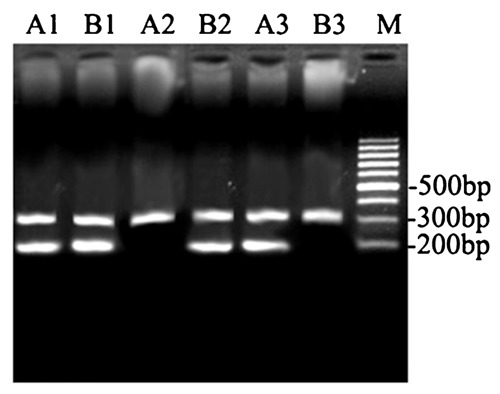
IL-18 gene promoter -607C/A (rs1946518) polymorphism
PCR amplification of the -607C/A (rs1946518) locus in the IL-18 gene promoter produced both a locus-specific product of 196'bp and an internal reference product of 301'bp. The two alleles, C and A, of this locus were amplified using primers specific for these alleles (Figure 1), allowing us to distinguish CC, CA, and AA genotypes.
Figure 1. PCR amplification with specific primers for the identification of IL-18 promoter -607C/A (rs1946518) polymorphisms in ischemic stroke patients and healthy controls. Samples 1, 2, and 3 were amplified with common downstream primers and internal reference upstream primers; samples A1, A2, and A3 were amplified with specific upstream primers (for the C allele); samples B1, B2, and B3 were amplified with specific upstream primers (for the A allele). The genotypes of samples 1, 2, and 3 were CA, AA, and CC, respectively. Lane M = DNA molecular weight marker.
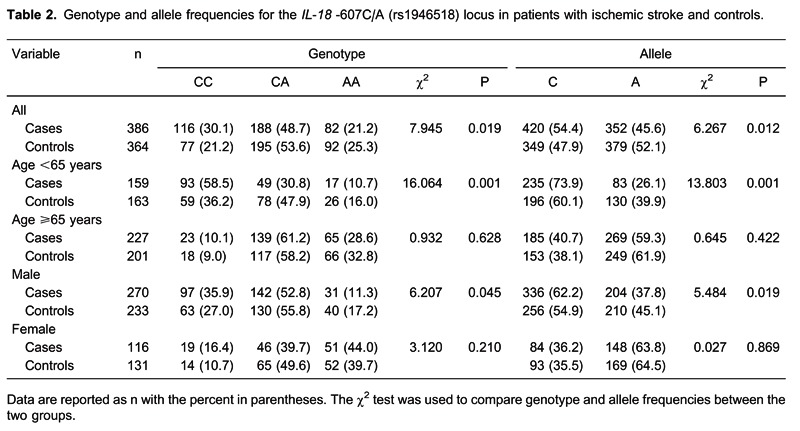
Hardy-Weinberg analysis indicated that genotype distributions for this locus were in equilibrium for both the control and patient groups (P > 0.05). Statistically significant differences in genotype and allele frequencies were detected for the IL-18 promoter -607C/A (rs1946518) locus between the control and patient groups (both P < 0.05, Table 2). Additionally, genotype and allele frequencies were significantly different between male patients and male controls as well as between patients and controls <65 years old (both P < 0.05). No differences were noted for female patients versus female controls or for patients versus controls ≥65 years old (both P > 0.05).
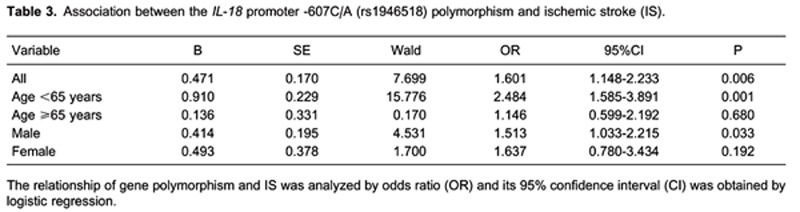
Relationship between genotype and IS
Further analysis of this SNP by logistic regression showed that, after excluding the impact of age, gender, smoking status, and high blood pressure, IS risk was increased 1.6 times in patients carrying the -607C allele compared to patients carrying the -607A allele (OR = 1.601, 95%CI = 1.148-2.233, P < 0.01; Table 3). Similarly, increased risk was noted for male patients and patients <65 years old (P < 0.05).
Discussion
SNPs are commonly found in humans, with an incidence of 1% in the whole population (18). These genetic variations are influenced by many factors, including race and environment. Identifying relationships between SNPs and disease pathology is critical for the development of novel treatment and preventive measures for a variety of human diseases.
The contribution of SNPs in IL-18 to variations in expression and activity of this inflammatory cytokine has become important for the understanding of diseases related to immune function. Many studies have cloned and analyzed the promoter region of IL-18 to characterize gene expression and regulation (13). By means of various analyses, several SNPs have been identified in the promoter region of IL-18 exon 2, at -137, -607, and -656 loci (19). The -607C/A (rs1946518) variation is located in the binding region of nuclear factor cAMP-response element binding protein and histone H4 transcription factor (8). The SNP affects biological functions of IL-18, and, thus, this locus is currently the most widely studied IL-18 polymorphism (8). Recent studies of the IL-18 polymorphism have focused on allergic diseases, viral infections, autoimmune diseases, and cancers. Results indicate that the -607C/A (rs1946518) locus is correlated with allergic asthma, allergic rhinitis, nasopharyngeal cancer, chronic hepatitis B virus, and human immunodeficiency virus infection, among other diseases (20-24). Previously, the association of two functional polymorphisms in the IL-18 promoter, -607C/A (rs1946518) and -137G/C (rs187238), with the risk of IS was investigated in a Han Chinese population, and the results revealed that the -607C allele was associated with an increased risk of IS and the presence of the -137G allele was correlated with an increased risk of IS in the subtype of patients with large artery atherosclerosis (25). However, the study did not present results indicating an effect of age or gender.
Here, we further investigated the correlation between variants of IL-18 and risk of IS. Both the C and A alleles of the IL-18 promoter region -607 locus were detected in IS patients and healthy controls, and statistically significant differences in genotype and allele frequency were detected between these populations. Additionally, genotype and allele frequencies differed between patients and controls for males and for those <65 years of age. IS risk was increased 1.6 times in patients carrying -607C compared to patients carrying -607A after excluding for age, gender, smoking status, and hypertension. These results indicate an association between the -607C/A (rs1946518) polymorphism and the occurrence of cerebral infarction; individuals carrying the A allele have a higher risk of IS. The C allele may confer increased risk by increasing the expression of IL-18, and highly expressed IL-18 can increase the risk of IS. Whether a difference in protein expression is present in stroke patients with the C allele versus those with the A allele requires further investigation.
In summary, these results show that the IL-18 promoter polymorphism is correlated with susceptibility to IS and that the C allele at the -607 locus confers increased risk of IS. These findings add to the body of evidence demonstrating a role for IL-18 in disease susceptibility and pathology. Additional research will provide a detailed understanding of the pathological mechanisms linking IL-18 to IS.
Footnotes
First published online May 24, 2013.
References
- 1.Flossmann E, Schulz UG, Rothwell PM. Potential confounding by intermediate phenotypes in studies of the genetics of ischaemic stroke. Cerebrovasc Dis. 2005;19:1–10. doi: 10.1159/000081905. [DOI] [PubMed] [Google Scholar]
- 2.Yip HK, Sun CK, Chang LT, Wu CJ, Chua S, Fu M. Strong suppression of high-sensitivity C-reactive protein level and its mediated pro-atherosclerotic effects with simvastatin: in vivo and in vitro studies. Int J Cardiol. 2007;121:253–260. doi: 10.1016/j.ijcard.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 3.Nakase T, Yamazaki T, Ogura N, Suzuki A, Nagata K. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J Neurol Sci. 2008;271:104–109. doi: 10.1016/j.jns.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Balding J, Livingstone WJ, Pittock SJ, Mynett-Johnson L, Ahern T, Hodgson A, et al. The IL-6 G-174C polymorphism may be associated with ischaemic stroke in patients without a history of hypertension. Ir J Med Sci. 2004;173:200–203. doi: 10.1007/BF02914551. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Lee C. The gene encoding transforming growth factor beta 1 confers risk of ischemic stroke and vascular dementia. Stroke. 2006;37:2843–2845. doi: 10.1161/01.STR.0000244782.76917.87. [DOI] [PubMed] [Google Scholar]
- 6.Lee BC, Ahn SY, Doo HK, Yim SV, Lee HJ, Jin SY, et al. Susceptibility for ischemic stroke in Korean population is associated with polymorphisms of the interleukin-1 receptor antagonist and tumor necrosis factor-alpha genes, but not the interleukin-1beta gene. Neurosci Lett. 2004;357:33–36. doi: 10.1016/j.neulet.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/S0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 8.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 9.Yuen CM, Chiu CA, Chang LT, Liou CW, Lu CH, Youssef AA, et al. Level and value of interleukin-18 after acute ischemic stroke. Circ J. 2007;71:1691–1696. doi: 10.1253/circj.71.1691. [DOI] [PubMed] [Google Scholar]
- 10.Zaremba J, Losy J. Interleukin-18 in acute ischaemic stroke patients. Neurol Sci. 2003;24:117–124. doi: 10.1007/s10072-003-0096-0. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Tang Q, Jiang H, Ding X, Liu Y, Zhu R, et al. Promoter polymorphism of interleukin-18 in angiographically proven coronary artery disease. Angiology. 2009;60:180–185. doi: 10.1177/0003319708321103. [DOI] [PubMed] [Google Scholar]
- 12.Pei F, Han Y, Zhang X, Yan C, Huang M, Huang L, et al. Association of interleukin-18 gene promoter polymorphisms with risk of acute myocardial infarction in northern Chinese Han population. Clin Chem Lab Med. 2009;47:523–529. doi: 10.1515/CCLM.2009.130. [DOI] [PubMed] [Google Scholar]
- 13.Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–152. doi: 10.1016/S0165-5728(00)00407-0. [DOI] [PubMed] [Google Scholar]
- 14.Kalina U, Ballas K, Koyama N, Kauschat D, Miething C, Arnemann J, et al. Genomic organization and regulation of the human interleukin-18 gene. Scand J Immunol. 2000;52:525–530. doi: 10.1046/j.1365-3083.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 15.Khripko OP, Sennikova NS, Lopatnikova JA, Khripko JI, Filipenko ML, Khrapov EA, et al. Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediators Inflamm. 2008;2008:309721. doi: 10.1155/2008/309721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke - 1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.STR.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 17.Yu JT, Tan L, Song JH, Sun YP, Chen W, Miao D, et al. Interleukin-18 promoter polymorphisms and risk of late onset Alzheimer's disease. Brain Res. 2009;1253:169–175. doi: 10.1016/j.brainres.2008.11.083. [DOI] [PubMed] [Google Scholar]
- 18.Saito H. Translation of the human genome into clinical study. Allergol Int. 2003;52:65–70. doi: 10.1046/j.1440-1592.2003.00288.x. [DOI] [Google Scholar]
- 19.Mi YY, Yu QQ, Yu ML, Xu B, Zhang LF, Cheng W, et al. Review and pooled analysis of studies on -607(C/A) and -137(G/C) polymorphisms in IL-18 and cancer risk. Med Oncol. 2011;28:1107–1115. doi: 10.1007/s12032-010-9569-1. [DOI] [PubMed] [Google Scholar]
- 20.Imboden M, Nicod L, Nieters A, Glaus E, Matyas G, Bircher AJ, et al. The common G-allele of interleukin-18 single-nucleotide polymorphism is a genetic risk factor for atopic asthma. The SAPALDIA Cohort Study. Clin Exp Allergy. 2006;36:211–218. doi: 10.1111/j.1365-2222.2006.02424.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee HM, Park SA, Chung SW, Woo JS, Chae SW, Lee SH, et al. Interleukin-18/-607 gene polymorphism in allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2006;70:1085–1088. doi: 10.1016/j.ijporl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Pratesi C, Bortolin MT, Bidoli E, Tedeschi R, Vaccher E, Dolcetti R, et al. Interleukin-10 and interleukin-18 promoter polymorphisms in an Italian cohort of patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother. 2006;55:23–30. doi: 10.1007/s00262-005-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang PA, Wu JM, Li Y, Yang XS. Association of polymorphisms of interleukin-18 gene promoter region with chronic hepatitis B in Chinese Han population. World J Gastroenterol. 2005;11:1594–1598. doi: 10.3748/wjg.v11.i11.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segat L, Bevilacqua D, Boniotto M, Arraes LC, de Souza PR, de Lima Filho JL, et al. IL-18 gene promoter polymorphism is involved in HIV-1 infection in a Brazilian pediatric population. Immunogenetics. 2006;58:471–473. doi: 10.1007/s00251-006-0104-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang N, Yu JT, Yu NN, Lu RC, Ma T, Wang ND, et al. Interleukin-18 promoter polymorphisms and risk of ischemic stroke. Brain Res Bull. 2010;81:590–594. doi: 10.1016/j.brainresbull.2010.01.008. [DOI] [PubMed] [Google Scholar]


