Abstract
Neoadjuvant chemotherapy (NC) improves overall survival in patients with resectable muscle-invasive urothelial cancer of the bladder (MIBC). However uptake of NC in Canada is dis-appointingly low. Following a detailed literature review and in consultation with urologic oncology, the Canadian Association of Genitourinary Medical Oncologists (CAGMO) has developed a consensus statement for the use of NC in MIBC. Our primary goal is to increase the uptake of NC for MIBC in Canada and improve patient outcomes.
Introduction
MIBC is the sixth most common malignancy diagnosed in Canada with 7800 new cases and 2100 cancer-related deaths annually.1 At diagnosis, 30% of patients have muscle-invasive disease, which is defined pathologically as organ confined (pT2), or extravesical disease (pT3 or pT4).2 In these patients, despite radical cystectomy (RC) and lymph node dissection only about 50% of patients are cured and most patients subsequently die of metastatic disease within 3 years of diagnosis. For MIBC patients treated with local therapy alone, the overall survival (OS) rates are 52% to 77% for pT2 disease, 40% to 64% for pT3 disease, and only 26% to 44% for pT4 or node-positive disease.3 Attempts to improve these outcomes have focused not only on improved surgical techniques and use of extended lymph node dissection, but also on the use of perioperative chemotherapy.
All patients with suspected MIBC first require a transure-thral resection of the bladder tumour (TURBT) with adequate muscle sampling to confirm the presence of muscle-invasion. Once confirmed, patients with MIBC should be considered for neoadjuvant chemotherapy (NC) which should begin as soon as possible after diagnosis. This recommendation is based on a large meta-analysis of 11 randomized trials of NC, which showed a 5% OS benefit with cisplatin-based combination regimens.4 Close follow-up (clinical and radiographic) during NC is crucial to monitor for toxicity and/or disease progression that may necessitate early discontinuation of NC and definitive local management. After NC is complete, and once blood counts are adequate, patients should undergo RC and lymph node dissection. For patients who are not surgical candidates, bladder sparing approaches may also be an option after NC; however, a comprehensive discussion of bladder preservation is beyond the scope of this article.
Where NC is not an option, or if patients have already had definitive surgery adjuvant chemotherapy (AC) administered in a timely manner post surgery can be considered. The 2005 Advanced Bladder Cancer (ABC) meta-analysis systemically reviewed 6 adjuvant trials and though limited by small patient numbers and imbalances between patient groups, did show a 25% relative risk reduction in death.5 There was however, insufficient evidence to recommend AC over NC which remains the preferred option.
Despite Level 1 evidence for NC, several studies including a Canadian survey of medical oncologists have shown that referrals for and uptake of NC remains low.6 To improve the uptake of NC, coordination of NC and definitive surgical management is essential and requires a streamlined referral process and close multidisciplinary collaboration.
The aims of this CAGMO initiative were therefore to:
Conduct a literature review on NC in MIBC and understand barriers to its use.
Develop a consensus statement on the use of NC in MIBC, informed by input from medical and urologic oncology.
Publish a consensus statement advocating for the use of NC in Canada.
Assess the impact of this consensus statement, on the uptake of NC for MIBC in Canada, 12 months post-publication.
Methods
Following the 2012 CAGMO Annual Meeting, where challenges relating to bladder cancer care were identified, a consensus statement on the use of NC in MIBC was drafted and circulated to a core group of medical oncologists (SS, LW, SN, NB, and DR). The document was then reviewed with two urologic oncologists (AZ and PB) and is presented here. Twelve months post-publication of this consensus statement a survey to assess uptake of NC in Canada will be administered.
Discussion
Neoadjuvant chemotherapy for bladder MIUC
The practice of NC is well-established in treating many malignancies, resulting in tumour downsizing and improved outcomes In MIBC, RC, with curative intent, is associated with a high failure rate, and provides the impetus to use perioperative systemic chemotherapy to improve outcomes.3 Level 1 evidence supports the use of NC in MIBC, with an OS benefit. There have been several randomized clinical trials evaluating the use of neoadjuvant platinum-based regimens in MIBC (Table 1); 3 key trials are highlighted below.
Table 1.
List of neoadjuvant chemotherapy trials included in the 2005 ABC meta-analysis
| Author/year | No. patients | Stage | NC regimen | Definitive treatment | OS benefit |
|---|---|---|---|---|---|
| Wallace/199129 | 159 | T2–4NXM0 | Cisplatin 100 mg/m2 | 45–50 Gy in 22F 65 Gy in 22F + |
No |
| Raghavan/199130 | 96 | T2–4NXM0 | Cisplatin 70 mg/m2 | RC + pelvic lymphadenectomy | No |
| Martinez-Pineiro/199531 | 122 | T2–4ANX-2M0 | Cisplatin 100 mg/m2 | RC + pelvic lymphadenectomy | No |
| Malmstrom/199632 | 325 | T1 (grade3) T2–4ANXM0 |
Cisplatin 70 mg/m2 Doxorubicin 30 mg/m2 |
20 Gy in 5F + RC + pelvic lymphadenectomy |
Yes for T3–T4 (p=0.03) |
| Abol-Enein/199733 | 196 | T2–4ANXM0 | Carboplatin 300 mg/m2 Methotrexate 50 mg/m2 Vinblastine 4 mg/m2 |
RC + pelvic lymphadenectomy | Not reported |
| Bassi/199934 | 206 | T2–4N0M0 | Cisplatin 70 mg/m2 Methotrexate 30 mg/m2 Vinblastine 3 mg/m2 |
RC + pelvic lymphadenectomy | Not reported |
| International Collaboration/1999,7 updated 20118 | 976 | T2 (grade 3) T3–T4ANO,NXM0 |
Cisplatin 100 mg/m2 Vinblastine 4 mg/m2 Methotrexate 30 mg/m2 |
60 Gy in 30F (or) 20 Gy in 5F + RC (or) RC and pelvic lymphadenectomy | Yes on 2011 update (p=0.037) |
| Sherif/200235 | 317 | T2–4ANXM0 | Cisplatin 100 mg/m2 Methotrexate 250 mg/m2 |
RC + pelvic lymphadenectomy | No |
| Sengelov/200236 | 153 | T2–T4bN0NXM0 | Cisplatin 100 mg/m2 Methotrexate 250 mg/m2 |
60 Gy in 30F (or) RC | No (p=0.76) |
| Cortesi/unpublished | 171 | T2–4N0M0 | Cisplatin 70 mg/m2 Methotrexate 30 mg/m2 Vinblastine 3 mg/m2 Epirubicin 40 mg/m2 Methotrexate 30 mg/m2 |
RC | Not reported |
| Grossman/20039 | 317 | T2–T4ANXM0 | Vinblastine 3 mg/m2 Doxorubicin 30 mg/m2 Cisplatin 70 mg/m2 |
RC | No (p=0.06) |
ABC: advanced bladder cancer; NC: neoadjuvant chemotherapy; OS: overall survival; RC: radical cystectomy; F: fractions.
In the EORTC/MRC Phase III international multi-institutional trial, 976 patients with T2–T4a N0 or NX M0 disease (of which 58% were T3) were randomized to 3 cycles of cisplatin, methotrexate and vinblastine (CMV) followed by definitive local therapy (RC and/or radiotherapy) or definitive therapy alone.7 Although initially reported as a negative trial, with longer follow-up NC showed a statistically significant 16% reduction in risk of death (hazard ratio [HR] 0.84, 95% confidence interval [CI], 0.72–0.99, p = 0.037); improvement in 3-year OS from 50–56%; 10-year OS from 30% to 36%; and median survival from 37 to 44 mos.7,8
These results are similar to the Southwest Oncology Group (SWOG) trial reported by Grossman and colleagues, in which 317 patients with clinical stage T2–T4a, N0, M0 (of which 60% were T3 or T4a), were randomized to 3 cycles of methotrexate, vinblastine, adriamycin and cisplatin (MVAC) followed by RC or RC alone. NC showed a statistically significant 25% reduction in risk of death (HR 0.75, 95% CI, 0.57–1.0, p = 0.06); and improved median survival from 46 to 77 months. Importantly, 38% of patients receiving NC had no residual invasive disease (pT0) at the time of cystectomy compared to only 15% in the group who did not have NC (p < 0.001). Furthermore, 85% of patients who were pT0 at cystectomy were alive at 5 years. There were no toxic deaths or increase in postoperative complications in patients who received NC.9
Although some of the NC trials were small, did not use cisplatin-based combination regimens, closed early, or used different local therapies (RC and/or radiation) a large meta-analysis by the ABC Collaboration has confirmed an OS benefit of NC (Table 1). The 2003 meta-analysis reviewed data from 2688 individual patients and 10 randomized clinical trials of platinum-based NC for biopsy-proven cT2–cT4A MIBC. They showed an absolute OS benefit of 5% at 5 years, with OS increasing from 45% to 50%, regardless of the type of definitive local therapy, which included RC, radiotherapy, and combined RC and radiotherapy. This analysis did not suggest improved OS with single agent cisplatin, and it was not possible to assess the effect of carboplatin-based versus cisplatin-based regimens.10 An updated ABC Meta-Analysis in 2005 including the SWOG trial discussed above, confirmed the 5% absolute survival improvement (p = 0.003) and 9% improvement in disease-free survival at 5 years (p < 0.0001).4 Unfortunately, toxicity and quality of life were not assessed in these meta-analyses (Table 1).
Advantages of NC
There are a number of potential advantages of NC, including:
Improved overall survival (Level 1 evidence).
Down-staging of the primary tumour which may facilitate surgery.
In vivo assessment of chemo-sensitivity.
Treatment of micro-metastatic disease (postulated to be the reason for the survival benefit).
Improved tolerability of chemotherapy prior to RC. Postoperatively, 64% of patients may experience complications within 90 days of RC, and as a result up to 30% may be unable to receive chemotherapy postoperatively due to these complications.11 NC may, therefore, be more feasible than AC and result in more patients receiving the benefit of systemic treatment.
The fact that there is no evidence of a detrimental effect in delaying RC for chemotherapy administration,12 and RC ideally within 4 to 6 weeks of completing NC but within 10 weeks of NC, is feasible without compromising survival.13
Disadvantages of NC may include:
Potential for disease progression in patients with chemo-resistant disease; however, with close clinical monitoring and restaging scans performed after 2 cycles of NC, definitive RC can be performed in a timely manner in patients not responding to NC.
NC-related complications, such as infections, which may potentially delay RC. Increased risk of post-RC complications after exposure to NC; however, these concerns have not been borne out by reports of surgical morbidity.14–17
Chemotherapy regimens for NC
The optimum NC regimen is unknown, although standard MVAC is the regimen with the most robust evidence. Dose-dense MVAC (ddMVAC) given every 2 weeks, with growth-factor support is also a reasonable option. GC (gemcitabine and cisplatin) is the most commonly used regimen in Canada although it lacks prospective randomized Phase III data in support of its use. The efficacy and use of GC in the neoadjuvant setting is extrapolated from the metastatic setting, where Phase III data showed similar efficacy, but less toxicity compared with MVAC.18 In the neoadjuvant setting, as with other cancers, pathological down-staging appears to be an important surrogate endpoint, where patients who have no residual invasive disease at the time of RC have improved survival.19 Neoadjuvant MVAC has shown a pathological down-staging (to pT0) rate of 38%, which is the highest reported to date. A recent pooled analysis of 7 studies published from 2007 to 2012 evaluated clinical outcomes with neoadjuvant GC (n = 164 patients) and revealed pathological down-staging to pT0 and to less than pT2 rates of 26% and 47% of patients, respectively.20 Despite the challenges of cross trial comparisons and acknowledging that these results appear inferior to the results from neoadjuvant MVAC, 57% of patients receiving MVAC experienced grade 3/4 granulocytopenia, as compared to 38% who experienced grade 3/4 haematological toxicities with neoadjuvant GC.9,20 Therefore, the better toxicity profile of GC makes it a reasonable option despite the lack of strong evidence.
In metastatic disease, substituting carboplatin for cisplatin in cisplatin-unfit patients (those with multiple comorbidities, poor functional status or renal impairment) is a common practice. However, the ABC meta-analyses only included 1 trial with a carboplatin-containing regimen versus 10 trials with cisplatin-based protocols.4 There is therefore no evidence to support the use of carboplatin in the neoadjuvant setting, and thus carboplatin cannot be recommended. Cisplatin-unfit patients should forego NC and proceed immediately to definitive local therapy.
Patient selection for NC
Selection of patients for NC requires careful assessment of both functional status and comorbidities (in particular presence of renal impairment) that may preclude safe administration of cisplatin-based combination chemotherapy. In addition it may be important to address the anxieties related to deferred surgery.
The published meta-analyses show an OS benefit in all subgroups with T2–T4 disease. However, trials within the meta-analyses did not include clinically node-positive bladder cancers or upper tract UC.4 We believe extrapolation of data to patients with upper tract UC is reasonable (Level 3 evidence, expert opinion), and an informed discussion with these patients on an individualized basis about the benefits and risks of NC is appropriate. Unfortunately, there is no data from prospective, randomized controlled trials of upper tract UC (including that of ureteral disease) to inform such a discussion. Given the obligatory loss of renal function following radical nephroureterectomy, if systemic perioperative chemotherapy is to be considered, it would seem most feasible to be administered prior to surgery.21 Pure non-urothelial cancers were also not represented in the trials, and there is no data to support perioperative chemotherapy for non-UCs of the urothelial tract, unless a component of urothelial histology is present. This highlights the importance of accurate uro-pathological reporting about histological variants, as this not only influences whether NC should be administered or not, but it also has been shown to be a strong independent predictor of upstaging at time of RC.22
Barriers to NC and reasons for poor uptake
Despite Level 1 evidence for the use of NC for MIBC, the incorporation of NC as part of standard practice has proven to be quite challenging across North America. In a large retrospective study, Feifer and colleagues analyzed all T2–4 N0M0 MIBC patients (4541 patients from 14 academic institutions) undergoing RC from 2003 to 2008. They found 66% of potentially eligible patients undergoing RC did not receive perioperative chemotherapy. Only 12% of patients received NC, and 35% of those patients received non-cisplatin based regimens.23 Low uptake of NC was also found in two retrospective Canadian studies. In a study by Yafi and colleagues, of 2287 patients treated with RC between 1998 and 2008, only 3.1% of patients received NC while 19.4% received AC.24 A study by Booth and colleagues, of 2738 MIBC patients treated with RC between 1994 and 2008 showed NC rates to be 3% to 6% and AC rates to be 16% to 23%.25 Although in some cases there are reasons to avoid NC (such as preoperative renal dysfunction, poor performance status, and symptomatic disease), these studies do suggest a significant number of eligible patients are not being offered NC.
There are likely several reasons to explain the low uptake of NC. In a study reported by Raj and colleagues, among 145 patients who underwent RC for preoperative clinical stage ≥T2 disease, where only 17% received cisplatin-based NC, the main reasons cited for lack of use were age, comorbidities, concerns over toxicity and the modest nature of benefit.26 This latter point may particularly be an issue in patients with clinically staged, cT2 disease where the relative benefit from NC appears smaller compared to that of T3 or T4a disease, but nevertheless there is still a 5% OS benefit at 5 years. Another reason to offer NC to patients with cT2 disease is that a significant number of patients are upstaged at the time of RC. Contemporary series show that up to 73% of patients with cT2 disease are actually upstaged at RC,27 and as such may derive greater relative benefit from NC than initially expected preoperatively. Encouraging data from a recent Canadian survey of medical oncologists and urologists suggests that 96% and 88%, respectively would offer NC, however the referral rate and use of NC is still relatively low.6,28 This may be due to a lack of a multidisciplinary approach up front, and could possibly be addressed by the implementation of a streamlined referral process which ensures referrals to medical oncology, timely completion of NC and subsequent RC or where appropriate, bladder sparing therapies.
As Canadian medical oncologists treating urothelial cancer, CAGMO feels it is imperative that all patients with potentially resectable MIBC without contraindications for cisplatin-based combination chemotherapy should be considered for NC.
Conclusion
Despite Level 1 evidence of improved patient outcomes associated with NC for MIBC, the uptake of NC in Canada and internationally remains disappointingly low. NC is feasible, safe, and when delivered in a timely manner does not negatively affect surgical outcomes. Patients do require close monitoring and follow-up medically and surgically while on treatment to address toxicities and potential disease progression; this ensures the best outcomes for all patients.
Referral processes and lack of coordinated care in a multidisciplinary setting are barriers that can be overcome. CAGMO acknowledges that as we dig deeper for reasons why NC uptake in this setting is poor, the answers are likely more complex than it appears at first sight. CAGMO strongly recommends the establishment of a streamlined referral processes and excellent interdisciplinary communication in a team environment, as well as the consideration of NC for all patients with MIBC to optimize patient outcomes. It is our hope that this 2013 CAGMO Consensus Statement will facilitate these developments in Canada.
Fig. 1.
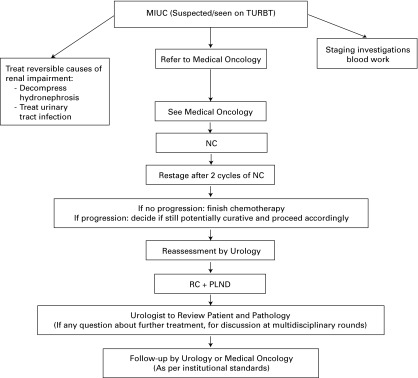
Patient flow from initial transurethral resection of bladder tumour to follow-up with a target timelines. TURBT: transurethral resection of the bladder tumour; NC: neoadjuvant chemotherapy; RC: radical cystectomy; PLND: pelvic lymph node dissection.
Target timelines:
I. TURBT to Pathology Review and Urology Review: 2 weeks.
II. Urology to Medical Oncology: 2 weeks.
III. Medical Oncology to commencement of NC: As soon as possible, maximum of 2 weeks.
IV. Completion of NC to definitive surgical management: within 4–6 weeks.
Appendix 1.
CAGMO Position Statement
General introduction
|
Eligibility for neoadjuvant chemotherapy
|
Caveats
|
Exclusion criteria
|
Caveats
|
Staging
|
Chemotherapy options
|
Caveats
|
Monitoring during neoadjuvant chemotherapy
|
References
- 1. Canadian Cancer Society. Bladder Cancer Statistics; 2012. http://www.cancer.ca/en/cancer-information/cancer-type/bladder/statistics/?region=on. Accessed September 11, 2013.
- 2.Rosenberg JE, Carroll PR, Small EJ. Update on chemotherapy for advanced bladder cancer. J Urol. 2005;174:14–20. doi: 10.1097/01.ju.0000162039.38023.5f. [DOI] [PubMed] [Google Scholar]
- 3.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 205–6. [DOI] [PubMed] [Google Scholar]
- 5.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189–99. doi: 10.1016/j.eururo.2005.04.005. discussion 199–201. [DOI] [PubMed] [Google Scholar]
- 6.Hsu T, North S, Eigl BJ, Chi KN, Canil CM, Wood L, Lau A, Panzarella T, Sridhar SS. The neoadjuvant management of bladder cancer in Canada: A survey of genitourinary medical oncologists. J Clin Oncol. 2011;29(suppl 7):285. doi: 10.5489/cuaj.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Collaboration of trialists, MRC/EORTC et al. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet. 1999;354:533–40. doi: 10.1016/S0140-6736(99)02292-8. [DOI] [PubMed] [Google Scholar]
- 8.International Collaboration of Trialists, MRC/EORTC et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011 Jun 1;29(16):2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 10.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003 Jun 7;361(9373):1927–34. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 11.Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009 Jan;55(1):177–85. doi: 10.1016/j.eururo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Izawa JI, Chin JL, Winquist E. Timing cystectomy and perioperative chemotherapy in the treatment of muscle invasive bladder cancer. Can J Urol. 2006 Jun;13(Suppl 3):48–53. [PubMed] [Google Scholar]
- 13.Alva AS, Tallman CT, He C, et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: a multidisciplinary approach. Cancer. 2012 Jan 1;118(1):44–53. doi: 10.1002/cncr.26240. Epub 2011 May 19. [DOI] [PubMed] [Google Scholar]
- 14.Hall MC, Swanson DA, Dinney CP. Complications of radical cystectomy: impact of the timing of perioperative chemotherapy. Urology. 1996;47:826–30. doi: 10.1016/S0090-4295(96)00073-8. [DOI] [PubMed] [Google Scholar]
- 15.Lawrentschuk N, Colombo R, Hakenberg OW, et al. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. 2010;57:983–1001. doi: 10.1016/j.eururo.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Svatek RS, Fisher MB, Matin SF, et al. Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. J Urol. 2010;183:929–34. doi: 10.1016/j.juro.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Svatek RS, Fisher MB, Williams MB, et al. Age and body mass index are independent risk factors for the development of postoperative paralytic ileus after radical cystectomy. Urology. 2010;76:1419–24. doi: 10.1016/j.urology.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 18.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 19.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–38. doi: 10.1016/j.eururo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Yuh BE, Ruel N, Wilson TG, et al. Pooled analysis of clinical outcomes with neoadjuvant Cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. J Urol. 2013;189:1682–6. doi: 10.1016/j.juro.2012.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. 2013;189:1214–21. doi: 10.1016/j.juro.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 22.Turker P, Bostrom PJ, Wroclawski ML, et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int. 2012;110:804–11. doi: 10.1111/j.1464-410X.2012.10939.x. [DOI] [PubMed] [Google Scholar]
- 23.Feifer A, Taylor JM, Shouery M, et al. Muscle-invasive Bladder Cancer Quality of Care Consortium, Multi-institutional quality-of-care initiative for nonmetastatic, muscle-invasive, transitional cell carcinoma of the bladder: Phase I. J Clin Oncol. 2011;29(7Suppl):240. [Google Scholar]
- 24.Yafi FA, Aprikian AG, Chin JL, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU Int. 2010;108:539–45. doi: 10.1111/j.1464-410X.2010.09912.x. [DOI] [PubMed] [Google Scholar]
- 25.Booth CM, Siemens RD, Li G, et al. Neoadjuvant (NACT) and Adjuvant Chemotherapy (ACT) for Muscle-Invasive Bladder Cancer: A Population-Based Outcomes Study. Ann Oncol. 2012;23(suppl 9):ix258–ix293. doi: 10.1093/annonc/mds399. 7880. [DOI] [Google Scholar]
- 26.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011 Jan 15;117(2):276–82. doi: 10.1002/cncr.25429. Epub 2010 Sep 9. [DOI] [PubMed] [Google Scholar]
- 27.Canter D, Long C, Kutikov A, et al. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107:58–62. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- 28.Sridhar SS, Chi KN, North SA, et al. The neoadjuvant management of muscle-invasive bladder cancer (MIBC) in Canada: A national survey of urologists. J Clin Oncol. 2012;30(suppl 5):303. [Google Scholar]
- 29.Wallace DM, Raghavan D, Kelly KA, et al. Neo-adjuvant (pre-emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. Br J Urol. 1991;67:608–15. doi: 10.1111/j.1464-410x.1991.tb15225.x. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan D, Pearson B, Watt WH, et al. Cytotoxic chemotherapy for advanced bladder cancer: cisplatin-containing regimens. Semin Oncol. 1991;18(1 Suppl 3):56–63. [PubMed] [Google Scholar]
- 31.Martinez-Piñeiro JA, Gonzalez Martin M, Arocena F, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: a prospective randomized phase III study. J Urol. 1995;153(3 Pt 2):964–73. [PubMed] [Google Scholar]
- 32.Malmström PU, Rintala E, Wahlqvist R, et al. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. J Urol. 1996;155:1903–6. [PubMed] [Google Scholar]
- 33.Abol-Enein H, El-Mekrech M, El-Baz M, et al. Neoadjuvant chemotherapy in the treatment of invasive transitional bladder cancer. A controlled prospective randomized study. Br J Urol. 1997;80 abstract 191. [Google Scholar]
- 34.Bassi P, Ferrante GD, Piazza N, et al. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol. 1999;161:1494–7. [PubMed] [Google Scholar]
- 35.Sherif A, Rintala E, Mestad O, et al. Neoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder cancer--Nordic cystectomy trial 2. Scand J Urol Nephrol. 2002;36:419–25. doi: 10.1080/003655902762467567. [DOI] [PubMed] [Google Scholar]
- 36.Sengeløv L, von der Maase H, Lundbeck F, et al. Neoadjuvant chemotherapy with cisplatin and methotrexate in patients with muscle-invasive bladder tumours. Acta Oncol. 2002;41:447–56. doi: 10.1080/028418602320405041. [DOI] [PubMed] [Google Scholar]


