Abstract
Livers from cull ewes and market lambs raised in Ontario were obtained to determine the status of specific minerals and vitamin E. Values for copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) obtained by atomic absorption and inductively coupled plasma — atomic emission spectroscopy (ICP-AES) were found to be statistically different but sufficiently biologically similar to allow the use of ICP-AES for screening groups of samples for deficient or toxic levels of those minerals. Toxic levels of cadmium were not found. Toxic levels of aluminum were found in 1 cull ewe and 1 market lamb. A significant proportion of both market lamb samples (40.0%) and cull ewe samples (50.0%) had high to toxic levels of Cu. In market lambs, Fe, Mn, molybdenum (Mo), selenium (Se), and Zn were not found to be important determinants of Cu level. In cull ewes, Fe, Mn, and Zn play a moderate role in the variability of liver Cu levels. Selenium was found to be present at marginal levels in 3.3% of cull ewe samples and in 42.6% of market lamb samples. Vitamin E was found to be low to deficient in 10.0% of cull ewe samples and in 90.0% of market lamb samples. In market lambs, only Mo was associated with Se levels, and no minerals were associated with vitamin E levels. In cull ewes, there was a strong association between Se and vitamin E. This survey demonstrates that marked nutritional imbalances of Cu, Se, and vitamin E exist in cull ewes and market lambs in Ontario.
Introduction
Sheep are more susceptible to copper (Cu) toxicosis than are other domestic species. Copper levels in the diet of between 5 to 10 mg/kg dry matter are recommended with normal dietary levels of sulphur and molybdenum (Mo). This is approximately one quarter of the daily requirements of cattle and one tenth that of pigs (1). From 1990 to 1995 in Ontario, 151 of 887 diagnoses of metal toxicoses in domestic animals were Cu toxicosis of sheep (2). The clinical signs of chronic Cu toxicosis in sheep are an acute presentation of anorexia, respiratory distress, severe hemolytic anemia, methemoglobinuria, and jaundice (1). Common sources of excess dietary Cu in sheep are rations or mineral mixes formulated for cattle or swine; accidental contamination of sheep rations with copper-containing feeds; copper accumulating forages; and, less frequently, from water with a high Cu content (3,4,5). Insufficient dietary Mo (less than 1.0 mg/kg dry weight [DW]) may also cause excessive accumulation of Cu in the liver (1,6). Copper deficiency has been diagnosed in Ontario, but it is not common.
Insufficient dietary selenium (Se), sometimes with concurrent deficiency of vitamin E, causes various syndromes of which nutritional muscular dystrophy, or white muscle disease, in ruminants is best known (7). Selenium deficiency may be associated with an increased prevalence of infectious disease, reproductive failure, reduced growth, and productivity (8). Free ions of nutritionally required metals, such as Cu, Se, iron (Fe), and zinc (Zn), produce oxidative stress when present in excess; these elements are required for the body's antioxidant system. Soils and feeds in Ontario are known to be deficient in Se, and nutritional muscular dystrophy is still commonly diagnosed in lambs (9). Vitamin E levels in feed in Ontario vary depending on the grain and forage type, growth, and storage conditions. Supplementation with Vitamin E and Se is done most commonly with injectable products at birth and less commonly with the mineral supplements fed to ewes and market lambs.
The analysis of tissue for metals in diagnostic laboratories has traditionally been done by atomic absorption (AA) spectroscopy, which is highly sensitive and very specific, but the analysis is done 1 metal at a time, which is both time consuming and expensive. The use of inductively coupled argon plasma atomic emission spectroscopy (ICP-AES) would permit multiple metal determination during a single analysis. Unfortunately, the sensitivity of ICP-AES for some metals of interest in animal production (Mo and Se) is not equal to that of AA analysis. The ICP-AES analysis can detect toxic levels of these metals, but it does not determine nutritionally significant concentrations. With improved technology, ICP-AES is slowly proving itself as a useful diagnostic tool.
Despite a broad awareness among producers of the risk of Cu toxicosis and vitamin E and Se deficiency, diseases caused by these nutritional imbalances continue to plague the sheep industry in Ontario. In addition, little is known about the levels of Zn, Fe, and manganese (Mn), or the levels of specific heavy metals (cadmium [Cd] and aluminium [Al]), in sheep in Ontario. This survey was undertaken to determine the prevalence of specific mineral and vitamin E imbalances in cull ewes and market lambs in Ontario.
Materials and methods
Sample collection
During July and August 1998, abattoirs in Ontario that process Ontario-grown lamb and adult sheep were identified; those within a 3-hour driving distance of the University of Guelph were contacted for permission to collect samples. In total, 7 abattoirs that were currently killing sheep, lambs, or both, participated in the survey. Samples from cull rams (> 1 y of age) were excluded, because the number of culled rams processed is low compared with that of culled ewes and because of known differences in management and feeding practices between ewes and rams. Sex was not recorded for market lambs, as both genders are raised similarly (a lamb is a sheep < 12 mo of age, as determined by incisor eruption).
In order to detect elevated liver Cu levels (greater than 5509 μmol/L [350 μgm/g] dry weight [DW]) at a prevalence of 5% or more with 99% confidence, 60 samples from each group (cull ewes and market lambs) were required. Sheep originating from outside Ontario were excluded from sampling. A random number table was used to select a set number of animals for sampling and the samples were obtained as the preselected animals came through the kill-line. No more than 30% of the animals killed on a particular day were selected for sampling and, if the origin of the animals was known, no more than 4 sheep or lambs from the same farm were selected. A total of 60 samples from market lambs and 54 samples from adult cull ewes were obtained. These samples were taken from 7 different abattoirs on 8 different days during July and August 1998, for a total of 114 samples.
The caudate lobe of the liver was removed immediately after slaughter by using a clean scalpel blade, placed in a whirl pack bag, and transported on ice to the Animal Health Laboratory at the University of Guelph. Samples were assigned random numbers and labeled adult or market lamb and with the source of the animal. At the laboratory, the livers were divided into 4 aliquots by using a clean scalpel blade; each aliquot weighed approximately 50 to 100 g and was stored at −20°C.
Metal analysis
Tissue digestion — All liver samples were freeze-dried and microwave digested with concentrated nitric acid for metal analysis. Digested samples for Se and Mo analysis were reduced by using hydrochloric acid and heating at 90°C for 30 min. All samples were taken up to a constant volume for analysis.
Atomic absorption spectroscopy — Samples were analyzed for Cd, Cu, Fe, Mn, and Zn by using atomic absorption (AA) spectroscopy (5100 Atomic absorption flame spectrometer; Perkin-Elmer, Norwalk, Connecticut, USA) and a FIAS-100 hydride generator system using NaBH4 in HCl (Perkin-Elmer). A technique similar to that described by Diaz et al (10) was used for Se analysis. Molybdenum was analyzed by the graphite furnace procedure (Perkin-Elmer 5100ZL AA; Perkin-Elmer) (11). All analyses were performed in duplicate in peak height mode to determine absorbence values.
Inductively coupled argon plasma atomic emission spectroscopy — Metal analyses for Al, Cu, Zn, Fe, and Mn were performed using a spectrometer with gem tip cross-flow pneumatic nebulizer (Ash Atom Scan 16ICP-AES; Thermo Jarrell Ash, Franklin, Massachusetts, USA). The procedure was performed at emission lines 396.15, 324.8, 213.9, 259.9, and 260.5 nm for Al, Cu, Zn, and Fe, respectively (12), and the ICP was operated under conditions recommended by the manufacturer. Background-corrected intensities were measured at normal resolution by the area- processing mode. All mineral concentrations are reported in SI units of liver DW.
Vitamin E analysis
Vitamin E (α-tocopherol) levels were determined by using a high-performance liquid chromatography (HPLC) procedure (13). In brief, liver was freeze-dried and then digested with ascorbic acid and potassium hydroxide. Vitamin E was extracted with isooctane and then analyzed by HPLC with a 5-μm spherical silica column 3.9 × 150 nm (Waters Canada, Mississauga, Ontario) An ultraviolet detector with an excitation wavelength of 296 nm and an emission wavelength of 325 nm was used to visualize vitamin E separation. Vitamin E concentrations are reported in SI units of liver DW.
Reported reference values, if given in wet weight (WW), were converted to DW for comparison purposes by multiplying reported WW values by 3.5, which is the mid-point of the standard conversion rates used (range 3.0 to 4.0) (15). Reference ranges reported in ppm or μgm/g were converted to SI units (μmol/kg) by using the conversion factors provided by Puls (15).
Statistical analyses
The data were entered into a spreadsheet (Excel; Microsoft Canada, Mississauga, Ontario) and all statistical analyses were performed using a statistical analysis system (SAS System, version 6.12; SAS Institute, Cary, North Carolina, USA ). For both market lambs and cull ewes, proc univariate was used to determine the univariate features of each substance, as well as the ratio of WW to DW for each liver sample. For all analyses that examined differences between groups, non-normal variables were log transformed and tested for normality by using the Shapiro Wilk test for normality (14). Proc means (SAS System) was used to determine if significant differences exist between values as determined by AA and ICP-AES for Cu, Fe, Mn, and Zn. This was done by testing if the difference between the 2 means was different than zero. Proc t-test (SAS System) was used to test if the substance in question was different between cull ewes and market lambs. To allow for comparison, Cd values that were reported as < 0.05 (below detectable limits) were changed to a value of 0. For both market lambs and cull ewes, to determine possible relationships between Cu and various metals known to interact with Cu (specifically Fe, Mn, Mo, Se, and Zn), all variables and interaction terms were examined by using proc corr (SAS System). All variables with a significant correlation to Cu (P> < 0.10) were offered to a regression model for both market lambs and cull ewes and models were ranked by best C(p). These models were then offered to proc reg (SAS System) and evaluated by significance of the variables (P> < 0.05), value of R-square, and normality of the residuals. Variables that were a component of a significant interaction term were included, regardless of significance. Model residuals were tested for normality by using the Shapiro Wilk test for normality. Only models with normally distributed residuals were accepted. These steps were repeated when determining the relationship between Se and interacting minerals (Cu, Fe, Mn, Mo, and Zn), as well as vitamin E. Vitamin E was also examined for possible association with Se, Cu, Fe, Mn, Mo, and Zn by using the same procedure.
Results
The ratio of WW to DW for market lambs ranged from 2.40 to 2.799, with a mean value of 2.562 and a standard deviation of 0.082. The ratio of WW to DW for cull ewes ranged from 2.279 to 2.906, with a mean value of 2.609 and a standard deviation of 0.137.
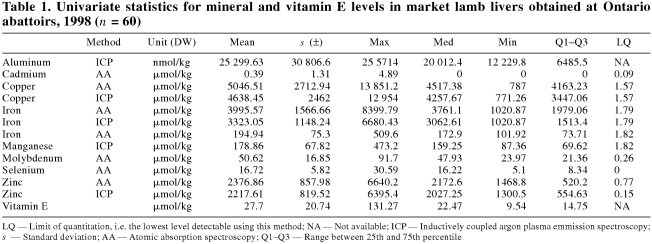
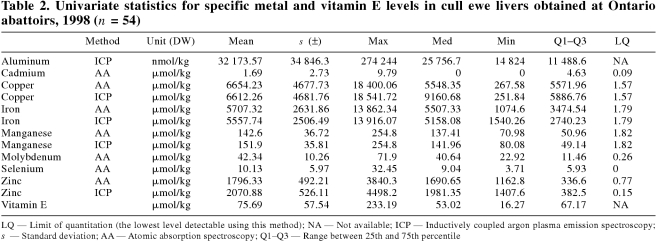
The univariate statistics for all minerals and for vitamin E are presented in Table 1 for market lambs and in Table 2 for cull ewes. The log mean values for Cu, Fe, Mn, and Zn, as derived by AA and ICP-AES, were compared and significant differences existed for all of them, with the exception of Fe in cull ewes. For market lambs, ICP-AES consistently underestimated the values as determined by AA (P> < 0.0001). For cull ewes, Cu as determined by ICP-AES was underestimated, and Mn and Zn were overestimated (P> < 0.0001). For purposes of all further analyses, the values for Cu, Fe, Mn, and Zn, as determined by AA, were used.
Table 1.
Table 2.
Aluminum values in 1 sample from the market lamb group (255 714 nmol/kg DW) and 1 sample from the cull ewe group (274 244 nmol/kg DW) were in the toxic range (> 81 717 nmol/kg DW) (15). The lamb and ewe were both purchased by the same abattoir from the same stockyards but were not killed on the same day (2 d apart). All values of Cd were within the normal range (< 43.6 μmol/kg) (15).
By comparing mean values between lambs and cull ewes, it was found that all minerals, as well as vitamin E were statistically different, with the exception of Cu. Iron (P> < 0.0005), Al (P> < 0.005), Cd (P> < 0.05), and vitamin E (P> < 0.0001) all had higher mean values in cull ewes than in market lambs. Mean values of Mn (P> < 0.0001), Zn (P> < 0.0001), Mo (P> < 0.01), and Se (P> < 0.0001) were all lower in cull ewes than in lambs.
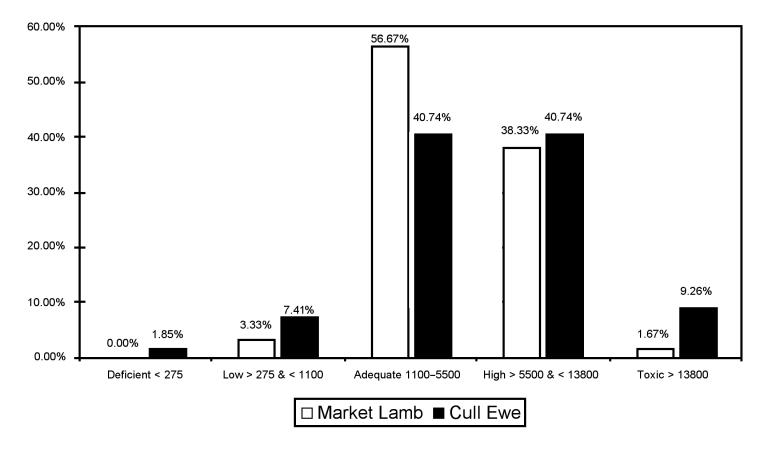
In Figure 1, Cu levels for lambs and ewes are shown with regards to distribution within toxic, high, low, and deficient ranges, as reported by Puls (15). Despite having similar mean values, there is a difference in the distribution of Cu levels between market lambs and cull ewes. Forty percent of market lamb livers and 50% of cull ewe livers had values in the high to toxic range. Only 1 sheep sample could be considered deficient. That particular animal also had a Mo level of 36.47 μmol/kg DW, well below the reported toxic level of 312.6 μmol/kg DW associated with molybdenosis (15).
Figure 1. Distribution of liver copper levels in market lambs and cull ewe livers obtained from abattoirs in Ontario as determined by atomic absorption spectroscopy analysis (μmol/kg DW) (15).
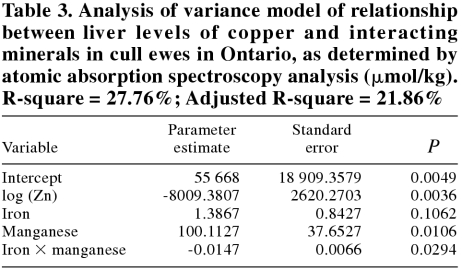
No significant model could be developed for predicting Cu values in market lambs. For cull ewes, the model with the best C(p) and R-square value with normally distributed residuals is presented in Table 3.
Table 3.
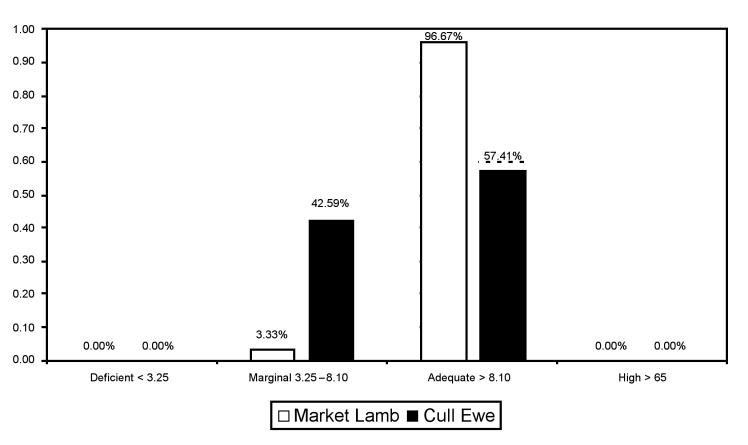
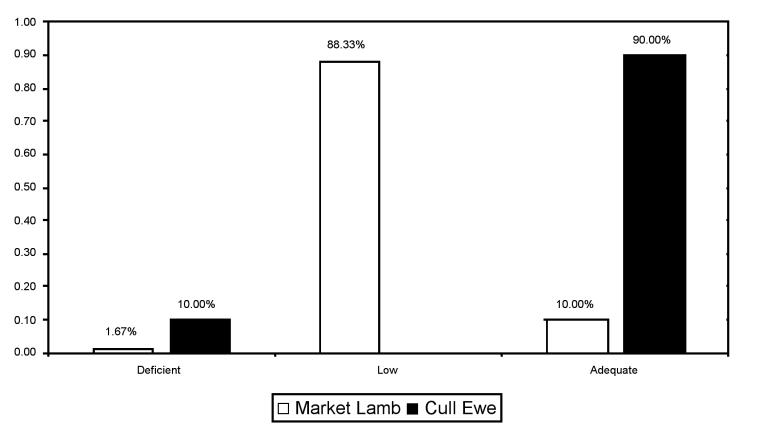
The distribution of Se and vitamin E levels in market lambs and cull ewes is shown in Figures 2 and 3. Of note, a high percentage of cull ewes had Se levels in the marginal range (3.25 to 8.10 μmol/kg DW) and the majority of market lambs had vitamin E levels in either the deficient (< 11.6 μmol/kg DW) or the marginal (11.6 to 23.2 μmol/kg DW) ranges (15,16). As well, a significant proportion of cull ewes had deficient (< 23.2 μmol/kg DW) vitamin E levels (16).
Figure 2. Distribution of selenium levels in market lambs and cull ewe livers from abattoirs in Ontario as determined by atomic absorption spectroscopy analysis (μmol/kg) (15).
Figure 3. Distribution of liver vitamin E levels in market lambs and cull ewe livers obtained from abattoirs in Ontario (μmol/kg DW).
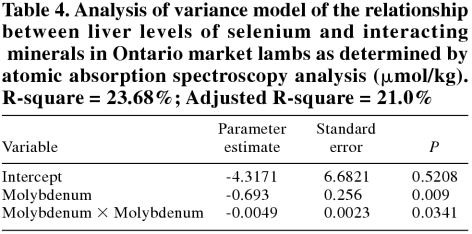
In market lambs, Se had significant correlations with Mn and Mo. However, the best model for predicting liver Se levels is presented in Table 4. No variables were significantly associated with vitamin E levels in the livers of market lambs.
Table 4.
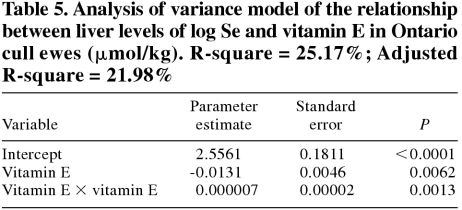
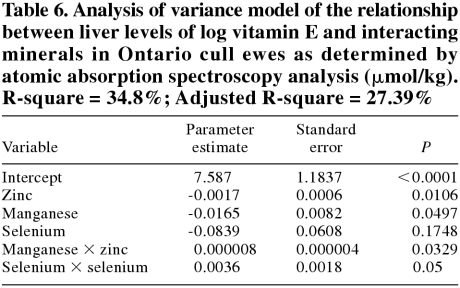
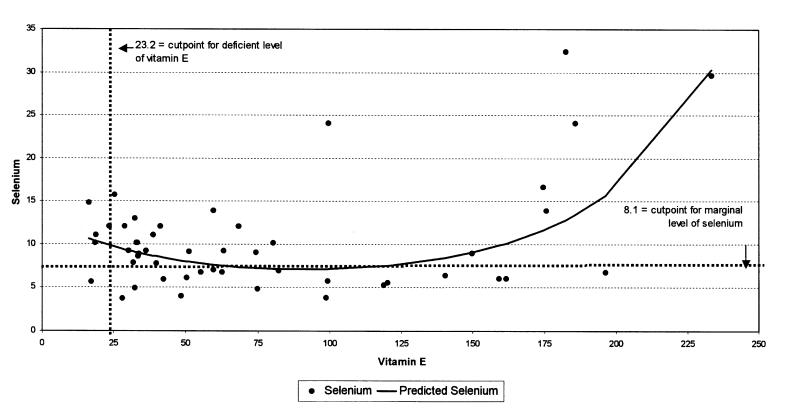
In cull ewes, the best model for predicting liver Se levels is presented in Table 5. The relationship between Se and this model is shown in Figure 3. The best model for predicting vitamin E levels in cull ewes is presented in Table 6.
Table 5.
Table 6.
Discussion
The market lamb samples in this survey represented late winter and spring born lambs raised for market under Ontario conditions; as such, they provide a representative sample of the status of market lambs raised in Ontario. The population from which the adult samples were obtained was healthy cull ewes and may represent the mineral and vitamin E status of adults on the farm of origin.
The conversion factors for liver WW to DW are significantly lower than the 3 to 4 generally accepted (15). A possible explanation is that, in this study, the lambs and ewes were exsanguinated prior to sampling, which would lower the volume of blood remaining in the liver. This differs from reports in the literature, which include mostly cases that died naturally. This variation from the accepted levels points out the risks of converting DW values to WW for purposes of reporting, without knowing the true conversion ratio.
Traditionally toxic and nutritionally required metals have been analyzed individually by using AA spectroscopy; a time consuming method that provides the extreme specificity and sensitivity needed for tissue analysis. The ICP-AES method permits the analysis of multiple metals in a single sample at the same time, thus reducing the cost and time of analysis. Limitations of the ICP-AES method include low sensitivity for some metals and interferences produced by metals with similar ion line emissions. However, technical advances are rapidly improving the ICP-AES performance. The lack of sensitivity to detect physiological levels of Se and Mo by using ICP-AES necessitated analysis by AA. Although statistical differences in Cu, Fe, Mn, and Zn levels were found between ICP-AES and AA analysis, these differences were not physiologically large. So this study demonstrated the possible applicability of ICP-AES for analysis of Al, Cu, Zn, Fe, and Mn in ovine liver tissues in cases of suspected toxicity or deficiency.
Although not nutritionally necessary, Al and Cd are known environmental toxins. Cadmium accumulation does not appear to be a problem in sheep in Ontario. However, toxic Al values were found, indicating that there could be a sporadic problem with Al toxicity in sheep and lambs raised in Ontario. Aluminum, as fly ash or as a contaminant of sewage spread onto pastures, has been implicated as a dietary source of toxic levels in domestic animals. It was not possible to trace the origin of the ewe and lamb, as sheep from across the province are routinely sold at this stockyard. In order to more fully understand the implications of this finding, a survey designed specifically to determine the geographic source of sheep containing high levels of Al would need to be conducted.
It can be speculated that the cause of the difference in levels of minerals and vitamin E between lambs and cull ewes was due to a difference in feeding practices between these 2 groups. However, no information could be gathered on diet, in this study. It is much more informative to examine the distribution of samples into categories of deficiency or toxicity. This information can then be used to determine the approximate risk of the sheep population in Ontario for deficiency or toxicity and then take appropriate educational steps to prevent disease.
Liver Cu levels greater than 13 800 μmol/kg DW are taken as evidence that the animal was at risk of an episode of clinical Cu toxicity; however, lower levels can also be associated with mortality due to an acute ingestion of high levels of Cu (17). A retrospective survey of submissions from clinical cases of Cu toxicosis to a veterinary diagnostic toxicology laboratory in Ontario reported a range of 13 773 to 55 520 μmol/kg DW (875 to 3521 μg/g) (2). Texel lambs dying of copper toxicosis had liver levels at necropsy of 25 971 μmol/g (1650 mg/kg) DW (18). Other cases report levels of 480 mg/kg WW (26 443 μmol/kg DW) (19), 350 ppm WW (19 282 μmol/kg DW) (20), 1473 mg/kg DW (23 185 μmol/kg) (5), and 3368.4 μmol/kg WW (11 790 μmol/kg DW) (21). This survey supports the observation that copper deficiency in sheep is uncommon in Ontario. However, the high percentage of samples from both the market lambs and cull ewes that had high to toxic levels of Cu means that risk of death due to copper toxicosis is significant in sheep raised in Ontario.
Several papers have described the breed susceptibility with respect to Cu accumulation in the liver (18,22,23). In these studies, it was demonstrated that Texel cross sheep are the most susceptible and that Romanov and Finnish Landrace sheep are the most resistant to toxic Cu accumulation in the liver. Intermediate risk breeds include East Friesian, Dorset, Montadale, and Suffolk. One study found that a Cu level of 10 ppm DW in a diet was insufficiently low to prevent Cu accumulation in the liver of Texel and Texel cross sheep (22). In this study, the sheep accumulated a mean value of 600 mg/kg DW (9444 μmol/kg DW), with only 1 mortality due to Cu toxicosis. According to the authors, susceptible breeds are more efficient in utilizing Cu from the ration as a protection against copper deficiency. In the present study, breed was not recorded. The most common breeds of sheep in Ontario are Suffolk, Dorset, Rideau Arcott, Romanov, Finnish Landrace, and their crosses. Thus our study likely included breeds of moderate or low susceptibility to copper toxicity. The Texel breed is not common, but its use as a terminal sire is becoming increasingly popular. It may be that the trend towards using more susceptible breeds may increase the risk of copper toxicosis in the sheep population in Ontario.
There are many potential dietary sources of excess Cu reported in the literature. Sheep and lambs are commonly fed stored feeds, often including commercial supplements. Some ingredients used in the manufacture of supplements may naturally be high in Cu, such as beet pulp, brewer's grain, corn distiller's grain, molasses, and soyabean meal (24). Many sheep feeds are prepared at small feed mills that also prepare riskier feeds, such as dairy and beef premixes, as well as swine feeds. This increases the risk of quality assurance errors, such as mislabeling of feeds, or batch contamination. Because the sheep industry is small, some of the operators of small feed mills may not be fully acquainted with the risk of Cu to sheep. In response to this, the Ontario Grain and Feed Association (OGFA) has produced guidelines for Ontario feed mills to help to avoid these problems. Forages and water are another potential source of excess Cu. The OGFA performed a survey of Cu analyses of forage and water samples in 1997 and 1998 (25). Mean values for all forages were generally less than 10 μg/g DW, although some sample values were greater than 15 μg/g DW (considered risky) and 1 sample was recorded at 58.4 μg/g DW. Water sample values were generally much lower than the upper acceptable limit for livestock of 0.5 ppm. The increasing number of swine and poultry farms that spread copper-laden manure on Ontario pastures will only increase the risk of high copper content of forages. It is recommended that all forages be analyzed for Cu, as well as for Mo, prior to feeding to sheep.
The lack of any significant correlations between Cu and interacting minerals in market lambs supports the hypothesis that high liver Cu levels are more likely due to too much dietary Cu rather than to Cu interactions with other minerals. On the other hand, some interacting minerals appear to play a role in Cu liver levels in cull ewes, although this role is not large. Despite evidence that adequate Se protects against Cu accumulation in the liver of sheep (1), there was no evidence of this association in this survey. The lack of importance of this association is supported by Sivertsen et al (26), who examined the livers of normal and copper poisoned lambs and found no difference in Se levels, and Bires et al (27), who found that additional supplementation with Se did not protect sheep from experimentally produced Cu toxicity.
Higher dietary Zn levels have been shown to lower Cu accumulation, and Zn has been used in flock outbreaks of Cu toxicosis as part of a treatment protocol to reduce Cu liver levels (1,7,18). Despite this, Saylor and Leach (28) did not find that Zn supplementation lowered liver Cu levels when supplemented up to 543 μg/g DW in the ration as inorganic Zn for 60 d. This finding was repeated by Van Ryssen (29), who fed up to 300 mg Zn (as Zn2Na EDTA) per kg DW feed over 88 d to mutton Merino lambs, and found no decrease in liver Cu levels over controls. However, van der Schee et al (18) found that Zn supplementation of values up to 479 ppm did lower Cu levels in the Texel and Texel X Friesian milksheep but not in pure Friesian milksheep, leading to the supposition that there may be genetic factors involved.
Molybdenum is an important determinant of Cu storage, particularly in the presence of sulphur (6,20). Low levels of Mo in the ration (< 1 ppm) are associated with Cu accumulation into the toxic range, even in the face of diets with acceptable levels of Cu (7–11 ppm DM) (6). Inorganic forms of Mo, such as tetrathiomolybdate, are useful as chelating agents to increase bile excretion of Cu from sheep suffering from Cu toxicosis (31,32). In 1 trial, Mo at 30 mg/head/d, was effective in reducing liver Cu from a mean value of 898 μg/g DW (14 134 μmol/kg DW) to a mean value of 479 μg/g DW (7539 μmol/kg DW) (29). From these findings, it can be assumed that dietary Mo is very important in preventing or treating Cu toxicosis. In this survey, however, liver Mo levels were not significant in determining liver Cu status in either cull ewes or market lambs.
Despite a high level of awareness of the risk of disease due to vitamin E and Se deficiency in Ontario, a sizable proportion of cull ewe samples were found to have insufficient Se. All Ontario soils are considered to be low to deficient in Se; that is, approximately 50% to 80% of all forage and grain contains less than 0.10 ppm Se (6). Selenium deficiency diseases in lambs and ewes are well documented. The most commonly known disease is nutritional muscular dystrophy, or white muscle disease, but other diseases, such as Se-responsive unthriftiness in lambs, infertility in ewes, early embryonic death, periodontal disease, decreased wool productivity, and lowered immune response may also be important sources of loss due to this deficiency (1,17). In Canada, Se cannot be included in sheep feeds at a rate exceeding 0.3 mg/kg DW for complete feeds, 0.7 mg/kg DW for limit fed feeds and 90 mg/kg DW in free choice trace mineralized salts (33). However, veterinarians can prescribe Se supplementation in excess of these amounts, if it is deemed to be therapeutically necessary. It is clear from this survey, however, that despite knowledge of white muscle preventative procedures in newborn lambs, cull ewes and to a lesser extent market lambs are not receiving adequate Se supplementation. Some Ontario producers are supplementing ewe rations in excess of National Research Council (NRC) requirements by feeding mineral mix free choice with Se added at 60 ppm. Given an estimated intake of 15 gm of mineral mix/ewe/d, this diet would deliver 0.9 mg of Se/ewe/d, compared with NRC guidelines of 0.1 to 0.2 mg/kg diet dry matter (6). Dry matter intakes for nursing ewes are estimated at between 2 and 3 kg, thus suggesting an upper limit intake of 0.6 mg of Se/ewe/d (6). This increasingly popular practice has raised concerns about the possible risk of Se toxicosis, as well as of excessive accumulation of Se, which can be a health risk to humans. No evidence of high levels of Se was found in this survey. The reason for this may be that an insufficient number of producers are supplementing ewes with high levels of Se for high levels to be detected by this survey, or that the high levels are not sufficiently high to cause toxic accumulation. A project investigating this particular issue is currently underway.
Vitamin E deficiency is sometimes mistakenly considered to be a clinical syndrome only in combination with Se deficiency. However, clinical vitamin E deficiency in the face of normal Se values has been reported as presenting as myodegeneration, rupture of the liver capsule, or both (34,35). Vitamin E content of feeds is variable, with high levels present in growing pastures and newly harvested mixed grass forages. Vitamin E levels decrease quickly in stored forages, so that even by early winter, levels may be very low. Complete feeds for commercial lamb are often supplemented with vitamin E at a rate of, for example, 22 IU/kg DM. Market lambs in Ontario are raised in a variety of ways, but lambs born in late winter and early spring are most often fed grain or commercial complete feeds with some stored forage offered. Lambs on these rations often achieve very high growth rates of over 400 gm/d. At this growth rate, 22 IU of vitamin E is said to be adequate in the ration (36). However, based on the results of this survey, it is quite clear that market lambs are not ingesting sufficient vitamin E.
In market lambs, no relationship between vitamin E and Se was found in this survey. The positive relationship found between Mo and Se in the regression model cannot be explained biologically and may be an artifact of the study, since no direct interaction between Mo and Se has been demonstrated (15). However, with cull ewes there is a strong association between vitamin E and Se. A sizable proportion of samples with marginal Se values had adequate vitamin E, which may indicate that these sheep had access to feeds containing high levels of vitamin E, such as pasture or new forages, but received inadequate levels of supplement that contained Se. A much smaller proportion of samples had adequate Se but were deficient in vitamin E. These sheep may have been fed older stored forages but had access to a Se containing mineral. Samples with adequate Se levels generally also had adequate vitamin E levels.
It is unknown if the sampling of cull ewes accurately reflects the status of ewes on-farm. However, the traditional time for culling a ewe is either soon after weaning or when reproductive failure is detected (failure to lamb or failure to be pregnant at the time of ultrasound scanning for pregnancy). At both these times, the ewes selected for culling would have been managed as productive ewes until just prior to culling, so it is possible that this survey does adequately identify a problem of marginal Se status in the general ewe population.
This survey has highlighted that marked nutritional imbalances exist in market lambs and cull ewes raised in Ontario, specifically high to toxic levels of Cu and low to deficient levels of vitamin E and Se. Most of these imbalances can be corrected through education of producers by nutritional consultants regarding the proper nutritional management of sheep. Forage analysis for Cu; avoidance of feeds naturally high in Cu; avoidance of management practices that may increase Cu content of forages, such a spreading swine manure on pastures and hay fields; improved quality assurance practices at feed mills regarding the preparation of feeds intended for sheep (sequencing of feeds through the pelleter); and supplementation of rations with Mo, if Cu levels are high in the feed, are all management practices that will reduce the risk of Cu toxicosis. Producers should also ensure that NRC levels of Se are available to market lambs and ewes year round and that vitamin E be supplemented to ewes and lambs being fed stored forages.CVJ
Figure 4. Relationship between selenium and vitamin E in cull ewe livers obtained from Ontario abattoirs (mmol/kg DW). R-square = 25.2% and Adjusted R-square = 22.0%.
Footnotes
Funding for this project was provided by The Ontario Sheep Marketing Agency and The Gartshore Memorial Sheep Research Fund.
Address all correspondence and reprint requests to Dr. Menzies; e-mail: pmenzies@ovc.uoguelph.ca
References
- 1.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. In: Veterinary Medicine — a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 9th ed. New York: WB Saunders, 2000: 1599–1603.
- 2.Hoff B, Boermans HJ, Baird J. Retrospective study of toxic metal analyses requested at a veterinary diagnostic toxicology laboratory in Ontario (1990–1995). Can Vet J 1998;39:39–43. [PMC free article] [PubMed]
- 3.García-Fernández AJ, Motas-Guzmán, Navas I, María-Mojica P, Romero D. Sunflower meal as cause of chronic copper poisoning in lambs of southeastern Spain. Can Vet J 1999;40:799–801. [PMC free article] [PubMed]
- 4.Dubreuil P, Sauvageau R. Intoxication chronique au cuivre chez des agneaux lourds par l'eau d'abreuvement. Can Vet J 1993;34:428–430. [PMC free article] [PubMed]
- 5.Kerr LA, McGavin HD. Chronic copper poisoning in sheep grazing pastures fertilized with swine manure. J Am Vet Med Assoc 1991; 198:99–101. [PubMed]
- 6.Subcommittee on Sheep Nutrition. Nutrient Requirements of Sheep. 6th rev. ed. National Research Council. Washington DC: Nat Acad Pr, 1985:17–18.
- 7.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. In: Veterinary Medicine — a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 9th ed. New York: WB Saunders. 2000: 1515–1553.
- 8.Pherson BO. Diseases and diffuse disorders related to selenium deficiencies in ruminants. Nor J Ag Sci 1993;11:79–93.
- 9.Arthur JR. Selenium content of some feed ingredients available in Canada. Can J Anim Sci 1971;51:71–74.
- 10.Diaz JP, Navarro M, Lopez H, Lopez MC. Determination of selenium levels in dairy products and drinks by hydride generation atomic absorption spectrometry. Food Addit Contam 1997;14:109–114. [DOI] [PubMed]
- 11.Tahan JE, Sanchez JM, Granadillo VA, Cubillan HS, Romero RA. Concentration of total Al, Cr, Cu, Fe, Hg, Na, Pb, and Zn in commercial canned seafood determined by atomic spectrometric means after mineralization by microwave heating. J Agric Food Chem 1995;43:910–915.
- 12.Hamalova M, Hodslavska J, Janos P. Determination of phosphorus, potassium, and magnesium in fertilizers by inductively coupled plasma — atomic emission spectroscopy and comparison with other techniques. J AOAC Int 1997;80:1151–1155.
- 13.Liu Q, Scheller KK, Schaefer DM. Technical note: a simplified procedure for vitamin E determination in beef muscle. J Anim Sci 1996;74:2406–2410. [DOI] [PubMed]
- 14.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika, 1965;52:591–611.
- 15.Puls R. Mineral Levels in Animal Health. 5th edition. Clearbrook, British Columbia: Sherpa Int., 1994.
- 16.Stowe HD, Herdt TH. Clinical assessment of selenium status of livestock. J Anim Sci 1992;70:3928–3933. [DOI] [PubMed]
- 17.Sharman GAM, Angus KW. Inorganic and organic poisons. In: Martin WB, Aitken ID, eds. Diseases in Sheep, 2nd ed. 1991:320–321.
- 18.van der Shee, van den Assem, van der Berg. Breed differences in sheep with respect to the interaction between zinc and the accumulation of copper in the liver. Vet Q 1983;5:171–174. [DOI] [PubMed]
- 19.Bulgin MS. Copper toxicosis in sheep from natural food stuffs. Proc Am Assoc Small Ruminant Pract, 1993;3–17.
- 20.Ross CE. Chronic copper poisoning in a lamb. Compend Contin Educ Pract Vet 1983;5:559–561.
- 21.Lewis NJ, Fallah-Rad AH, Connor ML. Copper toxicity in confinement-housed ram lambs. Can Vet J 1997;38:496–498. [PMC free article] [PubMed]
- 22.van der Berg R, Levels FH, van der Schee W. Breed differences in sheep with respect to the accumulation of copper in the liver. Vet Q 1983;5:26–31. [DOI] [PubMed]
- 23.Littledike ET, Young ID. Effect of sire and dam breed on copper status of fat lambs. J Anim Sci 1993;71:774–778. [DOI] [PubMed]
- 24.Subcommittee on Sheep Nutrition. Nutrient Requirements of Sheep, 6th rev. ed. National Research Council. Washington DC: Nat Acad Pr, 1985:50.
- 25.Vagge AS, Piett R, Cowan D. Copper content of Ontario forages. A Report of the Ontario Grain and Feed Association ad-hoc Sheep Committee. 1999.
- 26.Sivertsen T, Karlsen JT, Norheim G, Frøslie A. Concentration of selenium in liver in relation to copper levels in normal and copper-poisoned sheep. Acta Vet Scand 1978;19:472–474. [DOI] [PMC free article] [PubMed]
- 27.Bires J, Kovac G, Vrzgula L. Interactions between copper and selenium in sheep in the course of experimentally-produced copper intoxication. Vet Hum Toxicol 1991;33:489–491. [PubMed]
- 28.Saylor WW, Leach RM. Intracellular distribution of copper and zinc in sheep: Effect of age and dietary levels of metals. J Nutr 1980;110:448–459. [DOI] [PubMed]
- 29.Van Ryssen JB. The effectiveness of using supplementary zinc and molybdenum to reduce the copper content in the liver of hypercuprotic sheep. J S Afr Vet Assoc 1994;65:59–63. [PubMed]
- 30.Van Ryssen JBJ, Stielau WJ. Effect of different levels of dietary molybdenum on Cu and Mo metabolism in sheep fed on high levels of Cu. Br J Nutr 1980;45:203–210. [DOI] [PubMed]
- 31.Allen JD, Gawthorne JM. Involvement of organic molybdenum compounds in the interaction between copper, molybdenum and sulfur. J Inorg Biochem 1986;27:95–112. [DOI] [PubMed]
- 32.Gooneratne SR, Christensen DA. Effect of chelating agents on the excretion of copper, zinc and iron in the bile and urine of sheep. Vet J 1997;153:171–178. [DOI] [PubMed]
- 33.Selenium supplementation of livestock feeds. Agriculture and Agrifood Canada Publication T-3-112, 1992.
- 34.Mass J, Bulgin MS, Anderson BC, Frye TM. Nutritional myodegeneration associated with vitamin E deficiency and normal selenium status in lambs. J Am Vet Med Assoc 1984;184:201–204. [PubMed]
- 35.Hovers KA. Fatal syndrome in young lambs associated with vitamin E deficiency. Proc Sheep Vet Soc 1994;18:183–185.
- 36.Subcommittee on Sheep Nutrition. Nutrient Requirements of Sheep, 6th rev ed. National Research Council, Washington DC: Nat Acad Pr, 1985:45–47.