Abstract
Graft-versus-host disease (GVHD) is a significant cause of morbidity and mortality following allogenic haematopoietic stem-cell transplantation and thus the focus of much ongoing research. Despite considerable advances in our understanding of the pathophysiology, diagnosis and predisposing factors for both acute and chronic forms of the disease, a standardised therapeutic strategy is still lacking. There is good evidence for initial treatment of both acute and chronic forms of the disease with corticosteroid therapy. However, the most effective approach to steroid-refractory disease remains controversial, with current practice based mainly on smaller studies and varying considerably between local institutions. Timely diagnosis, multidisciplinary working and good supportive care, including infection prophylaxis, are clearly important in optimizing response and survival in such patients. It is hoped that in the future systematic research strategies and the identification of novel therapeutic targets may improve outcome further. The following review aims to outline some of the existing options for the treatment and management of acute and chronic GVHD.
Keywords: graft-versus-host disease, GVHD, therapy
Introduction
Despite decades of research and improvements in post-transplant immunosuppressive therapies, graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality in allogenic haematopoietic stem-cell transplant (HSCT) recipients. Classically defined by Billingham in 1966 as a syndrome in which immunocompetent donor cells recognize and attack host tissues in an immunocompromised recipient, GVHD demonstrates a heterogeneous clinical presentation primarily involving the skin, mucosa, gastrointestinal tract, liver and lungs [Billingham, 1966]. Historically, features occurring within 100 days of HSCT were classified as acute GVHD (aGVHD) and those occurring beyond 100 days as chronic GVHD (cGVHD). However, it is now recognized that clinical features of cGVHD can occur within 100 days of transplant and that features of aGVHD and cGVHD may coexist, leading to new definitions in which diagnosis focuses on the constellation of symptoms rather than their time of onset [Filipovich et al. 2005].
Depending on a number of patient- and transplant-related variables, the incidence of aGVHD ranges from 10% to 80% with symptoms usually developing 2–3 weeks post transplant. Risk factors for aGVHD include degree of human leukocyte antigen (HLA) mismatch, older age, previous donor alloimmunization and the nature of GVHD prophylaxis. It is estimated that cGVHD affects 30–70% of allogenic HSCT recipients surviving beyond 100 days, with a median onset of 4–6 months following HSCT. Although associated with reduced relapse rate in patients transplanted for leukaemia, cGVHD remains the leading cause of late death in HSCT survivors. With increasing use of HSCT in older recipients, mismatched and unrelated donors and mobilized peripheral blood stem-cell grafts, the clinical and economic impact of GVHD looks set to further increase in future years [Lee et al. 2002; Flowers et al. 2011].
Management of GVHD is challenging. Immuno-suppression with corticosteroids forms the basis of first-line therapy in both acute and chronic GVHD, producing sustained responses in less than 50% of patients with aGVHD and 40–50% of patients with cGVHD depending on initial disease severity. Despite ongoing research, there are few randomized controlled trials focusing on management of steroid-refractory GVHD. Evaluation of therapeutic options is complicated by the heterogeneous nature of the patient group (variable organ involvement, age, conditioning regimens, GVHD prophylaxis and type of HSCT), lack of a clear definition of corticosteroid-refractory disease and inconsistent treatment end points. As a consequence, there remains no clear consensus as to the best second- and third-line options in GVHD management and local treatment regimens often depend largely on financial considerations, availability of therapies, and the preferences and experience of treating physicians.
Pathophysiology
As yet, the pathophysiology underlying aGVHD and cGVHD remains incompletely understood. One commonly quoted model suggests three distinct stages in the development of aGVHD: a conditioning regimen which damages host tissues, including intestinal mucosa and liver; activation of donor T cells against host antigens and subsequent clonal T-cell expansion; and release of inflammatory cytokines such as interleukin 1 (IL-1) and tumour necrosis factor α (TNFα), leading to further host tissue damage [Ferrara et al. 1999]. Several mechanisms have been implicated in cGVHD pathogenesis, including persistence of donor-derived alloreactive T cells, autoreactive T cells, B cells producing antibodies against the host, and mechanisms of chronic inflammation leading to end organ fibrosis. The existence of such complex parallel networks remains subject to much ongoing research, not least because they form the basis for many new and existing therapeutic targets (reviewed by Ferrara and colleagues) [Ferrara et al. 2009].
Management of acute graft-versus-host disease
Overview
Acute GVHD classically affects the skin, liver and gastrointestinal tract. Using criteria first published by Glucksberg and colleagues in 1974, it is graded based on degree of organ involvement (surface area of skin rash, serum bilirubin and volume of diarrhoea) and assessment of clinical status [Glucksberg et al. 1974]. While comparison of published data is limited to some extent by variation in diagnosis and grading between physicians, it is recognized that the overall grade of aGVHD has a major impact on survival post HSCT, with transplant-related mortality (TRM) ranging from 28% in grade 0 aGVHD to 92% in grade IV aGVHD in one large-scale study [Gratwohl et al. 1995].
Although beyond the scope of this review, much emphasis in current transplant management is based on preventing development of aGVHD. Current prophylactic regimens aim to either suppress donor T-cell function with immunomodulatory agents [e.g. ciclosporin, methotrexate, tacrolimus, mycophenolate mofetil (MMF)] or deplete donor T cells before or after stem-cell infusion using monoclonal (e.g. alemtuzumab, anti-CD52) or polyclonal antibodies [antithymocyte globulin (ATG)].
First-line management of acute graft-versus-host disease
Grade I aGVHD may respond to topical steroid therapy alone. Calcineurin inhibitor levels should be optimized to ensure therapeutic dosing. In cases which do not resolve with topical management systemic treatment may be required.
Systemic corticosteroids remain the mainstay of first-line treatment in grade II–IV aGVHD. A number of studies have looked at response of patients with aGVHD to steroid therapy, although variation in dose, regimen and length of therapy means that there remains no clear consensus as to optimal use. In 1990, Martin and colleagues retrospectively analyzed the results of treatment in 740 patients with grade II–IV aGVHD. Of these, primary treatment was with corticosteroids in 531 patients. Overall complete or partial responses were reported in 44%, with improvement rates in skin disease, evaluable liver disease and evaluable gut disease of 43%, 35% and 50% respectively [Martin et al. 1990]. Broadly comparable results were obtained by Weisdorf and colleagues and MacMillan and colleagues in retrospective studies of 197 patients and 443 patients [Weisdorf et al. 1990; MacMillan et al. 2002b]. Significantly, response to primary therapy has been shown to correlate well with post-transplant survival [Saliba et al. 2012].
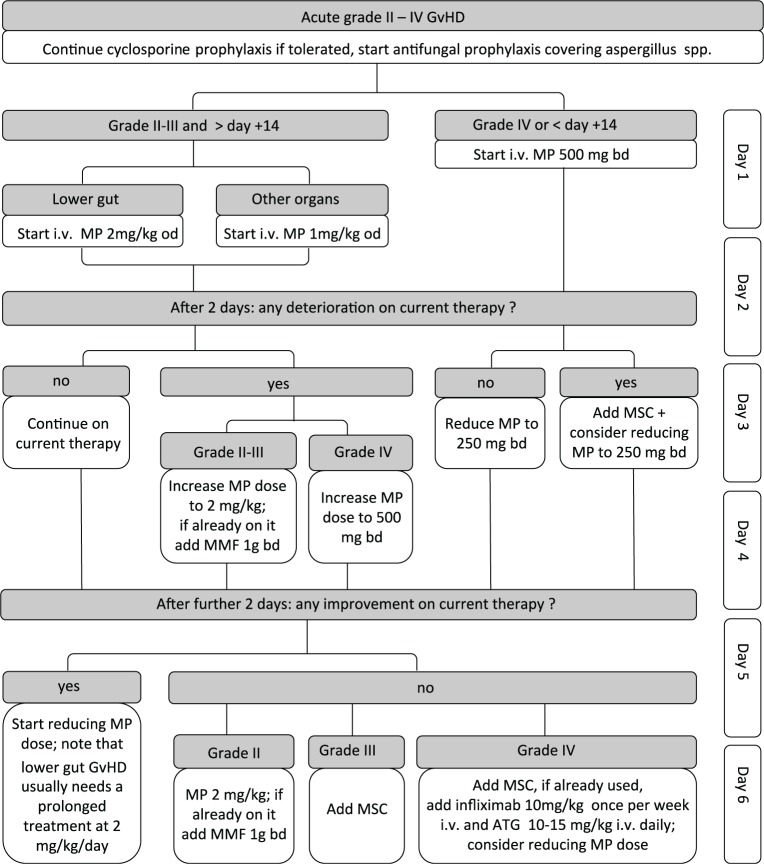
In most centres, the starting dose of prednisolone or methylprednisolone is 1–2 mg/kg/day depending on GVHD severity. Figure 1 outlines the current initial treatment strategy at our centre. No significant difference in outcome was identified in patients with grade I/II aGVHD treated with 1 or 2 mg/kg/day of corticosteroid in one retrospective study. The small number of patients with grade III/IV aGVHD at diagnosis limited conclusions in this group [Mielcarek et al. 2009]. Equally, a prospective randomized trial comparing 2 mg/kg/day of methylprednisolone with 10 mg/kg/day over a 5-day period with subsequent tapering doses found no significant difference in response of aGVHD, evolution to more severe disease, 3-year TRM or 3-year survival. Logically, patients who had responded to 2 mg/kg/day of steroid therapy by day 5 showed a lower TRM than nonresponders [Van Lint et al. 1998, 2006].
Figure 1.
First six days of systemic therapy for acute graft-versus-host disease grade I–IV as used at the Hammersmith Hospital, London. Please note this represents our local protocol and is not a generalized guideline.
ATG, antithymocyte globulin; bd, twice daily; GVHD, graft-versus-host disease; i.v., intravenous; MMF, mycophenolate mofetil; MP, methylprednisolone; MSC, mesenchymal stem cell; od, once daily.
Steroids have many well documented side effects, including immunosuppression, hyperglycaemia and osteopenia, meaning that a balance must be drawn between benefit and risk of prolonged courses. There remains no consensus as to the best way of tapering steroids in responding patients. One prospective trial randomized 30 patients with aGVHD who had responded to initial steroid treatment to short (86-day) versus long (147-day) taper of steroid dose. Patients in the short taper group achieved resolution of aGVHD after a median of 42 days compared with 30 days in the longer taper group. No difference was reported in incidence of steroid-related side effects, cGVHD or 6-month survival [Hings et al. 1993]. Practically, when steroid taper leads to worsening of symptoms, simply increasing the steroid dose and reducing again gradually may prove an effective solution. As fungal and viral infections are frequent complications of prolonged treatment, anti-infective prophylaxis is recommended.
In an effort to improve disease response, additional first-line therapies have been studied. In a four-arm phase II trial, 180 patients with aGVHD were randomized to receive 2 mg/kg/day of methylprednisolone plus either etanercept, MMF, denileukin or pentostatin as initial therapy. Complete response (CR) rates at day 28 were 26%, 60%, 53% and 38% respectively with 9-month overall survival of 47%, 64%, 49% and 47% [Alousi et al. 2009]. Based on these findings, a phase III trial comparing steroid therapy plus MMF with steroid therapy alone was initiated (BMT CTN Study 0802). Preliminary results suggest no benefit of the addition of MMF to standard first-line therapy [Bolanos-Meade et al. 2013].
Second-line management of acute graft-versus-host disease
Although the definition of steroid-refractory aGVHD remains subject to ongoing debate, it is generally acknowledged that if aGVHD worsens in any organ over 3 days of treatment or there is no response to steroid therapy in 5–14 days, secondary therapy should be considered [Dignan et al. 2012a]. Numerous therapeutic agents have been studied in this context (Table 1). However, as yet, none have demonstrated convincing long-term benefit. Therefore the outcome of refractory aGVHD is poor with a high rate of morbidity and mortality figures approaching 80% [Van Lint et al. 2006; MacMillan et al. 2002a].
Table 1.
Therapy commonly used for steroid-refractory acute graft-versus-host disease and usual dosing regimens.
| Therapy | Usual dosing | Comments |
|---|---|---|
| Antithymocyte globulin | 10–15 mg /kg daily for 3–10 days intravenously | Steroid sparing. Durable effects infrequent. Considerable acute toxicity during administration, infections |
| Alemtuzumab | 10 mg daily for 5 days intravenously | Better tolerated than antithymocyte globulin. Significant infections (including CMV reactivation). Rarely durable responses |
| Dacluzimab | 5 mg/kg once a week intravenously | Limited availability. Infections |
| Infliximab | 10 mg/kg once a week intravenously | Preferable in gut disease. Considerable risk of infections |
| Extracorporeal photophoresis | Optimal treatment schedule and duration not yet established | Spares steroids. Excellent safety profile. Requires venous access and minimal platelet count of 30 × 109/liter |
| Mycophenolate mofetil | 1g twice per day orally or intravenously | Spares steroids. Gut toxicity may mimic gut GVHD. Can cause nausea and diarrhoea but usually well tolerated |
| Sirolimus | 1–4 mg/day orally, adjusted to trough serum levels of 4–12 ng/ml | Requires close monitoring. Increased risk of thrombotic microangiopathy when used with calcineurin inhibitors or with higher levels. Can cause hyperlipidaemia, myelosuppression, seizures and renal dysfunction |
| Pentostatin | 1.5 mg/m2/day intravenously for 3 days | Can cause infection and cytopenias |
| Mesenchymal stem cells | 1–6 million cells/kg intravenously, dose can be escalated in responders | Spares steroids. Preferable in gut disease. Generally low side-effect profile |
CMV, cytomegalovirus; GVHD, graft-versus-host disease.
Antithymocyte globulin
A number of retrospective studies have suggested the benefit of ATG in steroid-refractory aGVHD, particularly when used early in the disease and in the context of skin involvement [MacMillan et al. 2002a]. However, evidence that ATG improves survival in this context is lacking. In one prospective study, patients (n = 61) not responding to 5 days of 2 mg/kg/day methylprednisolone were randomized to receive 10 days of 5 mg/kg/day methylprednisolone, either alone or in combination with rabbit ATG. No significant difference was reported between the groups in terms of survival, TRM or response rate [Van Lint et al. 2006].
Alemtuzumab (Campath)
Similarly, small studies using the humanized monoclonal anti-CD52 antibody alemtuzumab in steroid-refractory disease have failed to show convincing long-term benefit. In one prospective study, 18 patients with steroid-refractory aGVHD received subcutaneous alemtuzumab for 5 days. At day 28, 15 patients had responded to treatment. Ten of these responders were still alive at 11 months. Fourteen patients developed infections, including cytomegalovirus reactivation in 11 patients [Gomez-Almaguer et al. 2008]. In a subsequent phase II trial of 10 adult patients with grade III/IV aGVHD, five responded to treatment but all 10 died within a median period of 40 days [Martinez et al. 2009].
Interleukin-2 receptor antagonists
Several monoclonal IL-2 receptor antibodies, thought to act by targeting activated T cells, have been trialled in the treatment of steroid-refractory aGVHD. Although of limited availability in Europe, a number of studies have suggested a potential role for daclizumab, with a favourable toxicity profile [Przepiorka et al. 2000]. However, a multicentre randomized control trial comparing use of methlyprednisolone 2 mg/kg/day with or without daclizumab for the initial treatment of aGVHD demonstrated a significantly worse 100-day survival in the daclizumab group (77% versus 94%, p = 0.02) at interim analysis which was sufficient to halt the trial. This was secondary to both relapse and GVHD-related mortality [Lee et al. 2004]. Several smaller, largely retrospective studies have demonstrated clinical response in patients with steroid-refractory aGVHD treated with other IL-2 receptor antagonists: inolimomab [Pinana et al. 2006], denileukin [Ho et al. 2004], basiliximab [Wang et al. 2011]. However, a recent retrospective study of 92 patients with corticosteroid-refractory aGVHD treated with inolimomab reported an overall response rate of 42% on day +30, but an overall survival of just 33% at 2 years in the day 30 responders, suggesting that the initial benefit of treatment may not correlate well with long-term survival [Garcia-Cadenas et al. 2013]. Of interest is a potential role for IL-2 in the management of cGVHD (see below).
Antitumour necrosis factor antibodies
The inflammatory cytokine TNFα has been implicated in the pathophysiology of GVHD, hypothesized to activate antigen-presenting cells (APCs), recruit effector cells and induce cellular apoptosis. Trials involving the anti-TNFα monoclonal antibody infliximab have produced mixed results in patients with steroid-refractory aGVHD. Retrospective studies have suggested some benefit of infliximab, particularly in patients with primarily gut involvement, albeit associated with an increased risk of infection [Couriel et al. 2004]. More recent reports are less positive. When Couriel and colleagues randomized 63 patients with refractory aGVHD to receive either steroid therapy alone or steroid plus infliximab, GVHD-related mortality, non-relapse mortality and overall survival were not significantly different between the groups [Couriel et al. 2009]. Etanercept, a recombinant human soluble TNF-α receptor fusion protein which competes for TNFα binding and renders it inactive, has also shown some promise. In 13 patients with steroid-refractory aGVHD, six responded to etanercept therapy, with the best response seen in those with gastrointestinal disease [Busca et al. 2007].
Combination therapies may also be efficacious. Of 22 children with steroid-resistant aGVHD treated with a combination of daclizumab and infliximab, 19 responded and 15 were still alive at a median of 31 months. Only two of the deaths were attributed to infection [Rao et al. 2009].
Extracorporeal photophoresis
Experience with extracorporeal photophoresis (ECP) in aGVHD is less than with chronic forms of the disease. The treatment consists of a combination of leucapheresis and photodynamic therapy, in which patient blood is exposed to 8-methoxypsoralen followed by ultraviolet A irradiation, before being reinfused. This is thought to induce leukocyte apoptosis, phagocytosis of apoptotic cells by APCs and a switch in APC activity in favour of immunomodulation. ECP has a good safety profile, with no significant increase in infection, secondary malignancy or disease relapse reported [Couriel et al. 2006]. Retrospective studies have suggested ECP therapy is of benefit in aGVHD [Perfetti et al. 2008]. A prospective phase II study included 59 patients with steroid-dependent or steroid-refractory GVHD treated with weekly ECP until maximum disease response. CR was achieved in 82% of patients with cutaneous, 61% with liver and 61% with gut involvement. TRM at 4 years was 14% in patients who achieved CR and 73% in those who did not [Greinix et al. 2006].
Mycophenolate mofetil
MMF, which inhibits purine synthesis in lymphocytes and has the advantage of oral or intravenous administration, has been studied in a number of small retrospective and prospective trials, with a possible role as a steroid-sparing agent in refractory disease. In 2009, Furlong and colleagues demonstrated clinical response in 9 of 19 patients with steroid-resistant aGVHD, although they found only a 16% 1-year survival [Furlong et al. 2009].
Sirolimus
The mammalian target of rapamycin inhibitor sirolimus has also been used in the treatment of steroid-refractory aGVHD, although concerns have been raised over its side effects, which include hyperlipidaemia, myelosuppression, seizures and thrombotic microangiopathy (TAM). In one pilot study of 21 patients with steroid-refractory grade III/IV aGVHD treated with sirolimus, the reported response rate was 57% (CR 24%) but the drug was discontinued in 10 patients due to toxicity or disease progression [Benito et al. 2001]. Broadly similar results were observed in retrospective studies [Hoda et al. 2010].
Pentostatin
In a phase I dose escalation study involving 23 patients with steroid-refractory aGVHD treated with the nucleotide analogue pentostatin, Bolanos-Meade and colleagues demonstrated CR in 14 patients but a median survival of only 85 days. Notably, several of these patients had already received additional aGVHD therapies [Bolanos-Meade et al. 2005]. A small retrospective series of 12 patients reported an overall response to pentostatin of 50% [Pidala et al. 2010].
Mesenchymal stem cells
Since the first treatment of steroid-resistant aGVHD in a 9-year-old boy with haploidentical third-party derived mesenchymal stem cells (MSCs) [Le Blanc et al. 2004], a number of phase I–II studies have suggested that MSC infusion is safe and may be effective in aGVHD treatment. One nonrandomized multicentre phase II study of 55 patients with refractory aGVHD treated with MSCs from either HLA-identical stem-cell donors, haploidentical family donors or unrelated HLA-mismatched donors demonstrated CR in 30 patients and improvement of GVHD in nine patients [Le Blanc et al. 2008]. Research in this area is ongoing, and growing evidence that MSCs may play a key role in modulating inflammation [Dazzi et al. 2012] perhaps suggests a more central role in the future management of GVHD.
Management of chronic graft-versus-host disease
Overview
Chronic GVHD commonly affects the skin, eyes, mouth, liver, gastrointestinal tract, lungs and genitalia and is classified as mild, moderate or severe according to National Institutes of Health consensus criteria, reflecting the number of organs involved, severity of GVHD and whether or not there is pulmonary involvement [Filipovich et al. 2005]. Several risk factors for cGVHD have been identified, including degree of HLA mismatch, age of recipient and prior aGVHD. Considerations regarding the impact of GVHD on survival following HSCT must be balanced with the fact that GVHD is associated with lower rates of relapse in leukaemia (the graft versus leukaemia effect). Data are conflicting as to how well this correlates with GVHD severity [Horowitz et al. 1990; Lee et al. 2002; Gratwohl et al. 2002].
Given the paucity of robust data, particularly regarding management of steroid-refractory disease, it is recommended that patients are enrolled in clinical trials whenever possible.
First-line management of chronic graft-versus-host disease
While patients with mild cGVHD may respond well to topical therapies, systemic treatment is usually indicated in moderate to severe cGVHD [Filipovich et al. 2005]. Corticosteroids remain the mainstay of first-line treatment, either alone or in combination with other agents. The standard starting dose is 1 mg/kg/day and there are very few data supporting use of higher or lower doses in clinical practice. Duration of immunosuppressive therapy is determined largely by clinical response, but is often prolonged, with median duration estimated at 2–3 years [Stewart et al. 2004]. Rate of steroid taper varies considerably with no randomized trials comparing protocols.
Early studies suggested benefit of the calcineurin inhibitor ciclosporin in first-line treatment of cGVHD and more recently Koc and colleagues randomized 287 patients to receive either cyclosporine plus 1 mg/kg prednisolone or prednisolone alone as primary treatment for cGVHD. No significant difference in transplant-related mortality (17% versus 13%), progression to secondary therapy (11% versus 17%) or duration of immunosuppression was noted between the two arms of the trial. The incidence of avascular necrosis was higher in the prednisolone-only arm (22% versus 13%), however, suggesting that while combination therapy may not result in a survival advantage, it could help ameliorate steroid-related side effects [Koc et al. 2002]. In 2009, Martin and colleagues initiated a double-blind randomized multicentre trial investigating the effect of adding MMF to first-line management of cGVHD on disease outcome. The study was closed early, however, following interim analysis revealing no benefit from adding MMF and a marginally higher death rate in the MMF arm [Martin et al. 2009].
Second-line management of chronic graft-versus-host disease
One of the many difficulties of cGVHD management and research is reproducibly defining response to treatment. Martin and colleagues suggest that failure of primary therapy is indicated by progression of cGVHD despite prednisolone at doses of 1 mg/kg/day for 2 weeks, a lack of improvement after 4–8 weeks of sustained treatment or an inability to taper steroids [Martin et al. 2006]. Many of these end points are subjective and some manifestations of cGVHD are irreversible despite appropriate therapy. As yet, there remains no standard treatment for steroid-refractory cGVHD. Commonly used treatments are discussed below and summarized in Table 2.
Table 2.
Therapy commonly used for steroid-refractory chronic graft-versus-host disease and usual dosing regimens.
| Therapy | Usual dosing | Comments |
|---|---|---|
| Extracorporeal photophoresis | Fortnightly paired treatments for an assessment period of 3 months | Spares steroids. Excellent safety profile. Particularly efficacious in cutaneous chronic GVHD. Requires venous access and minimal platelet count of 30 × 109/liter |
| Mycophenolate mofetil | 1 g twice per day orally or intravenously | Spares steroids. Gut toxicity may mimic gut GVHD. Can cause nausea and diarrhoea but usually well tolerated |
| Methotrexate | 5 mg/m2 or 7.5 mg/m2 per week orally | Spares steroids. Most benefit in skin and mouth GVHD. Can cause cytopenias |
| Rituximab | 375 mg/m2 weekly intravenously. Assess at 4 weeks | Benefit in skin and musculoskeletal GVHD. Risk of infection |
| Sirolimus | 1–4 mg/day orally, adjusted to trough serum levels of 4–12 ng/ml | Requires close monitoring. Increased risk of thrombotic microangiopathy when used with calcineurin inhibitors. Can cause hyperlipidaemia, myelosuppression, seizures and renal dysfunction |
| Pentostatin | 4 mg/m2 every 2 weeks intravenously | Can cause infection and cytopenias |
| Thalidomide | 100–800 mg/day orally in divided doses | Doses >200 mg are poorly tolerated. Side effects include neurotoxicity, constipation, sedation and thrombosis |
| Imatinib | 100–200 mg once daily orally increased to 400 mg daily if tolerated | Best response in refractory sclerotic chronic GVHD. Some benefit in mild–moderate lung GVHD. May cause fluid retention, shortness of breath and myelosuppression |
GVHD, graft-versus-host disease.
Extracorporeal photophoresis
ECP has been extensively evaluated in cGVHD management, with a number of studies demonstrating benefit in steroid-refractory disease, especially involving the skin, mouth or liver [Couriel et al. 2006; Scarisbrick et al. 2008]. In 2008, Flowers and colleagues performed a prospective multicentre trial of 95 steroid-refractory or steroid-dependent patients with cutaneous cGVHD comparing ECP plus standard treatment to standard treatment alone [Flowers et al. 2008]. The primary end point was defined as a change in total skin score (TSS) measured by a blinded physician at 12 weeks. As with many trials in cGVHD management, conclusions were limited by the large variation in immunosuppressive regimens used. Although statistically significant results were not obtained for the primary end point, the proportion of patients reaching at least a 50% reduction in steroid dose and 25% decrease from baseline in TSS was greater in the ECP arm, suggesting that ECP may have a role as a steroid-sparing agent. The response to ECP of gut and lung cGVHD has been reported but is less consistent. Although associated with relatively few significant side effects, ECP does require effective venous access, which can be challenging.
Mycophenolate mofetil
Although not infrequently used in the management of steroid-refractory cGVHD, published data surrounding the efficacy of MMF in this context is limited. Lopez and colleagues reported the use of MMF as second-line therapy in 24 patients, with five demonstrating CR and 13 partial response [Lopez et al. 2005]. An earlier retrospective study reported an overall response rate of 72% in 18 patients treated with MMF for refractory cGVHD [Busca et al. 2003]. Common side effects include infections, cytopenias and gastrointestinal toxicity, with the latter potentially mimicking the effects of gut GVHD both clinically and histologically, and thus complicating diagnosis and assessment of response.
Methotrexate
Low-dose methotrexate is well established as an anti-inflammatory agent in the management of autoimmune disease. It has been shown to be well tolerated and potentially efficacious in a number of smaller studies of patients with steroid-refractory cGVHD [Huang et al. 2005; Inagaki et al. 2008]. More recently, Wang and colleagues evaluated the role of methotrexate as a first-line therapy in 86 patients with cGVHD in combination with other immunosuppressive agents. The overall response rate was 86%, with most marked benefit in cutaneous disease [Wang et al. 2009].
Rituximab
Based on the hypothesis that B cells play a role in cGVHD pathogenesis, the anti-CD20 monoclonal antibody rituximab has been trialled in cGVHD management and shown some benefit. Cutler and colleagues reported a 70% response rate in 21 patients with steroid-refractory cGVHD treated with 38 cycles of rituximab [Cutler et al. 2006]. Notably, responses were limited to patients with skin or musculoskeletal manifestations and many responses were partial. Similarly, in a meta-analysis of seven studies involving 111 patients, the pooled response rate to rituximab therapy was 66%, although the varying criteria for reporting organ response and dosage reduction of steroids limited interpretation [Kharfan-Dabaja et al. 2009].
Sirolimus
Sirolimus has been evaluated in the second-line treatment of cGVHD, mainly in combination with other immunosuppressive agents. Johnston and colleagues showed an initial clinical response to sirolimus therapy in 15 of 16 patients with cGVHD, although documented adverse events included renal impairment, TAM and disease relapse, leading to discontinuation of treatment in six patients [Johnston et al. 2005]. Similarly, Couriel and colleagues reported an overall response rate of 63% in 35 patients with steroid-resistant cGVHD treated with sirolimus, tacrolimus and methylprednisolone [Couriel et al. 2005]. Notably, four patients developed TAM requiring cessation of therapy and there was a high rate of infectious complications. It is suggested that patients treated with sirolimus should be carefully monitored for renal complications and hyperlipidaemia. Interactions with other medications, especially calcineurin inhibitors and azoles, should be avoided if possible.
Pentostatin
Following its successful use in steroid-refractory aGVHD, pentostatin has also been trialled in the second-line treatment of cGVHD. One phase II study of 58 patients with refractory cGVHD who were given pentostatin every second week for a median of 12 doses reported an overall response rate of 55%, although it should be noted that most patients were heavily pretreated [Jacobsohn et al. 2007]. Similar results were seen in a number of retrospective series. Infections appear to be a frequent complication of pentostatin treatment in cGVHD.
Thalidomide
Thalidomide demonstrates diverse immunomodulatory activities, inhibiting cytokines such as IL-6 and IL-12, reducing TNFα expression, downregulating surface adhesion molecules and suppressing angiogenesis. In 1992, Vogelsang and colleagues reported complete or partial response in 14 of 44 and 12 of 44 patients with refractory cGVHD treated with thalidomide therapy and subsequent studies have produced broadly similar results [Vogelsang et al. 1992]. Thalidomide is, however, associated with numerous side effects, including neurotoxicity, sedation, constipation and thrombosis. Indeed, in a randomized, placebo-controlled trial of thalidomide as first-line therapy for cGVHD, treatment with the study drug was discontinued before resolution of cGVHD in 23 of the 25 patients taking thalidomide due to an unacceptable side-effect profile [Koc et al. 2000].
Imatinib
In recent years, an adjuvant role for the tyrosine kinase inhibitor (TKI) imatinib in the treatment of refractory cGVHD has emerged, thought to act by inhibiting the TGFβ and platelet-derived growth factor pathways and thus reducing fibrosis. In 2009, Magro and colleagues evaluated the use of imatinib for the treatment of refractory sclerotic cGVHD in 14 patients, demonstrating a 50% response rate and reduction in corticosteroid requirement [Magro et al. 2009]. Interestingly, in nine patients with lung GVHD, some benefit was suggested from imatinib therapy in mild–moderate disease, but only minor improvement was noted in more severe conditions, raising the question of whether established fibrosis can be reversed [Stadler et al. 2009]. Side effects of imatinib include myelosuppression, shortness of breath and fluid retention.
Supportive care
Despite improvements in diagnosis and supportive care, cGVHD remains the major cause of late nonrelapse morbidity and mortality following HSCT, with deaths primarily resulting from infections related to the immunosuppressive effect of cGVHD or its treatments [Socie et al. 1999]. Appropriate antimicrobial prophylaxis is of great importance, with adequate protection against Pneumocystis jiroveci, Streptococcus pneumoniae, viral and fungal infections according to local protocol (see Dignan and colleagues for a review) [Dignan et al. 2012b]. Patients should be educated as to the risk of sepsis and advised to seek medical attention at an early stage.
The heterogenous nature of disease manifestations in cGVHD means that a multidisciplinary approach to patient management is highly beneficial, with appropriate subspecialty opinion, early dietary review and a consideration of the impact of therapy on growth and development in younger patients. Long-term systemic steroid use is associated with a wide range of adverse effects, including infection, hyperglycaemia, hypertension, osteoporosis and dyspepsia. When steroid therapy cannot be avoided, patients should be monitored closely, with prescription of gastric protection, consideration of bone protection, and regular monitoring of blood glucose and blood pressure. It is also recognized that patient quality of life after HSCT may be compromised by cGVHD [Pidala et al. 2011] and this should be acknowledged and formally assessed.
Novel therapies and further directions
Clearly there remains an urgent clinical need to optimize current therapies and to develop novel treatments for GVHD. Each of the therapeutic agents currently in use is associated with a significant risk of failure. Rather than developing universal treatment algorithms, it may be that we need to consider individualized therapy based on better characterization of GVHD subtypes, stages and the pathophysiological mechanisms involved.
Promising new directions include the inhibition of histone acetylation by vorinostat leading to a reduction in proinflammatory cytokine production, modulation of APC function and enhancement of regulatory T-cell number and activity [Choi et al. 2012]. Similarly, administration of daily low-dose IL-2 to patients with cGVHD was associated with sustained expansion of these cells and amelioration of GVHD [Koreth et al. 2011]. In a clinical study of haploidentical transplantation, investigators in Perugia demonstrated that adoptive transfer of regulatory T cells prevented GVHD in the absence of any post-transplantation immunosuppression [Di Ianni et al. 2011]. Equally, blockage of the chemokine receptor CCR5 by maraviroc inhibited lymphocyte chemotaxis and prevented development of GVHD in an early clinical study [Reshef et al. 2012]. Bortezomib has been shown to induce apoptosis in activated T cells via caspase activation and cleavage of the antiapoptotic bcl-2 protein and has recently entered early clinical trials [Koreth et al. 2012]. Targeting inflamasome and danger signalling pathways may have a potential role in influencing the early stages of GVHD development (reviewed by Wolf and colleagues) [Wolf et al. 2012].
Conclusion
The treatment and management of GVHD is continually changing as new therapies are proposed, trialled and validated. Although there is good evidence for the first-line management of both aGVHD and cGVHD with steroids and steroid-sparing agents, the most appropriate second-line treatment in both conditions remains unclear. Clearly there is a need for large systematic randomized studies to assess the efficacy and side effects of second-line therapies in GVHD management. By standardizing assessment tools for disease severity, outcome and quality of life, it is hoped that some of the variability seen in previous studies may be overcome. These trials should be conducted in conjunction with detailed laboratory analysis in an attempt to identify potential biomarkers predicting therapeutic responses with individual drugs as both aGVHD and cGVHD are biologically and clinically heterogeneous. Exciting prospects are on the horizon, in particular a role for MSCs in aGVHD management, and TKIs for the treatment of cGVHD. However, these remain to be validated in clinical practice. As our understanding of the pathophysiology underlying GVHD increases it seems likely that further therapeutic targets will continue to be identified.
Footnotes
Funding: The authors are grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme.
Conflict of interest statement: The authors have no potential conflicts of interest relevant to this article.
Contributor Information
Catherine Garnett, Imperial College London at Hammersmith Hospital, London, UK.
Jane F. Apperley, Imperial College London at Hammersmith Hospital, London, UK
Jiří Pavlů, Department of Haematology, Imperial College Healthcare NHS Trust, Room 2.20 Catherine Lewis Centre, Hammersmith Hospital, Du Cane Road, London W12 0HS, UK.
References
- Alousi A., Weisdorf D., Logan B., Bolanos-Meade J., Carter S., DiFronzo N., et al. (2009) Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 114: 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito A., Furlong T., Martin P., Anasetti C., Appelbaum F., Doney K., et al. (2001) Sirolimus (raptomycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation 72: 1924–1929 [DOI] [PubMed] [Google Scholar]
- Billingham R. (1966) The biology of graft-versus-host reactions. Harvey Lect 62: 21–78 [PubMed] [Google Scholar]
- Bolanos-Meade J., Jacobsohn D., Margolis J., Ogden A., Wientjes M., Byrd J., et al. (2005) Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol 23: 2661–2668 [DOI] [PubMed] [Google Scholar]
- Bolanos-Meade J., Logan B., Alousi A., Antin J., et al. (2013) A multi-center, randomised, double blind phase III clinical trial comparing steroids/placebo vs. Steroids/mycophenolate mofetil as initial therapy for acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 19: S137 [Google Scholar]
- Busca A., Locatelli F., Marmont F., Audisio E, Falda M. (2003) Response to mycophenolate mofetil therapy in refractory chronic graft-versus-host disease. Haematologica 88: 837–839 [PubMed] [Google Scholar]
- Busca A., Locatelli F., Marmont F., Ceretto C, Falda M. (2007) Recombinant human soluble tumour necrosis factor receptor fusion protein as treatment for steroid-refractory graft-versus-host disease following allogenic stem cell transplantation. Am J Hematol 82: 45–52 [DOI] [PubMed] [Google Scholar]
- Choi S., DiPersio J., Braun T., Hou G., Stockerl-Goldstein K., Tawara I., et al. (2012) Targeting histone deacetylases as a new strategy for graft versus host disease prevention. Blood 120: 740.a [Google Scholar]
- Couriel D., Hosing C., Saliba R., Shpall E., Anderlini P., Rhodes B., et al. (2006) Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 107: 3074–3080 [DOI] [PubMed] [Google Scholar]
- Couriel D., Saliba R., de Lima M., Giralt S., Andersson B., Khouri I., et al. (2009) A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 15: 1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couriel D., Saliba R., Escalon M., Hsu Y., Ghosh S., Ippoliti C., et al. (2005) Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol 130: 409–417 [DOI] [PubMed] [Google Scholar]
- Couriel D., Saliba R., Hicks K., Ippoliti C., de Lima M., Hosing C., et al. (2004) Tumour necrosis factor alpha blockade for the treatment of aGVHD. Blood 104: 649–654 [DOI] [PubMed] [Google Scholar]
- Cutler C., Miklos D., Kim H., Treister N., Woo S., Bienfang D., et al. (2006) Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 108: 756–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi F., Lopes L., Weng L. (2012) Mesenchymal stromal cells: a key player in ‘innate tolerance’? Immunology 137: 206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignan F., Amrolia P., Clark A., Cornish J., Jackson G, Mahendra P., et al. (2012a) Diagnosis and management of chronic graft-versus-host disease. BCSH guideline. Br J Haematol 158: 46–61 [DOI] [PubMed] [Google Scholar]
- Dignan F., Scarisbrick J., Cornish J., Clark A., Amrolia P., Jackson G., et al. (2012b) Organ-specific management and supportive care in chronic graft-versus-host disease. BCSH guideline. Br J Haematol 158: 62–78 [DOI] [PubMed] [Google Scholar]
- Di Ianni M., Falzetti F., Carotti A., Terenzi A., Catellino F., Bonifacio E., et al. (2011) Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117: 3921–3928 [DOI] [PubMed] [Google Scholar]
- Ferrara J., Levine J., Reddy P., Holler E. (2009) Graft-versus-host disease. Lancet 373: 1550–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J., Levy R., Chao N. (1999) Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood and Bone Marrow Transplant 5: 347–356 [DOI] [PubMed] [Google Scholar]
- Filipovich A., Weisdorf D., Pavletic S., Socie G., Wingard J., Lee S., et al. (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Bone Marrow Transplant 11: 945–956 [DOI] [PubMed] [Google Scholar]
- Flowers M., Inamoto Y., Carpenter P., Lee S., Kiem H., Petersdorf E., et al. (2011) Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117: 3214–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers M., Inamoto Y., Carpenter P., Lee S., Hans-Peter K., Petersdorf E., et al. (2011) Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117: 3214–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong T., Martin P., Flowers M., Carnevale-Schianca F., Yatscoff R., Chauncey T., et al. (2009) Therapy with mycophenolate mofetil for refractory acute and chronic GVHD. Biol Blood Bone Marrow Transplant 44: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cadenas I., Valcarcel D., Martino R., Pinana J., Novelli S., Esquirol A., et al. (2013) Updated experience with inolimomab as treatment for corticosteroid-refractory acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 19: 435–439 [DOI] [PubMed] [Google Scholar]
- Glucksberg H., Storb R., Fefer A., Buckner C., Neiman P., Clift R., et al. (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18: 295–304 [DOI] [PubMed] [Google Scholar]
- Gomez-Almaguer D., Ruiz-Arguelles G., del Carmen Tarin-Arzaga L., Gonzalez-Llano O., Gutierrez-Aguirre H., Caniu-Rodriguez O., et al. (2008) Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 14: 10–15 [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Brand R., Apperley J., Biezen A., Bandini G., Devergie A., et al. (2002) Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukaemia. Blood 100: 3877–3886 [DOI] [PubMed] [Google Scholar]
- Gratwohl A., Hermans J., Apperley J., Arcese W., Bacigalupo A., Bandini G., et al. (1995) Acute graft-versus host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Bone Marrow Transplantation. Blood 86: 813–818 [PubMed] [Google Scholar]
- Gomez-Almaguer D., Ruiz-Arguelles G., del Carmen Tarin-Arzaga L., Gonzalez-Llano O., Gutierrez-Aguirre H., Caniu-Rodriguez O., et al. (2008) Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 14: 10–15 [DOI] [PubMed] [Google Scholar]
- Greinix H., Knobler R., Worel N., Schneider B., Schneeberger A., Hoecker P., et al. (2006) The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica 91: 405–408 [PubMed] [Google Scholar]
- Hings I., Filipovich A., Miller W., Blazar B., McGlave P., Ramsay N., et al. (1993) Prednisolone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation 56: 577–580 [DOI] [PubMed] [Google Scholar]
- Ho V., Zahrieh D., Hochberg E., Micale E., Levin J., Reynolds C., et al. (2004) Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogenic stem cell transplantation. Blood 104: 1224–1226 [DOI] [PubMed] [Google Scholar]
- Hoda D., Pidala J., Salgado-Vila N., Kim J., Perkins J., Bookout R., et al. (2010) Sirolimus for treatment of steroid-refractory acute graft versus host disease. Bone Marrow Transplantation 45: 1347–1351 [DOI] [PubMed] [Google Scholar]
- Horowitz M., Gale R., Sondel P., Goldman J., Kersey J., Kolb H., et al. (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75: 555–562 [PubMed] [Google Scholar]
- Huang X., Jiang Q., Chen H., Xu L., Liu D., Chen Y., et al. (2005) Low dose methotrexate for the treatment of graft-versus-host disease after allogenic hematopoetic stem cell transplantation. Bone Marrow Transplant 36: 343–348 [DOI] [PubMed] [Google Scholar]
- Inagaki J., Nagatoshi Y., Hatano M, Isomura N., Sakiyama M., Okamura J. (2008) Low-dose MTX for the treatment of acute and chronic graft-versus host disease in children. Bone Marrow Transplant 41: 571–577 [DOI] [PubMed] [Google Scholar]
- Jacobsohn D., Chen A., Zahurak M., Piantadosi S., Anders V, Bolanos-Meade J., et al. (2007) Phase II study of pentostatin in patients with corticosteroid-refractory chronic graft-versus-host disease. J Clin Oncol 25: 4255–4261 [DOI] [PubMed] [Google Scholar]
- Johnston L., Brown J., Shizuru J., Stockerl-Goldstein K., Stuart M., Blume K., et al. (2005) Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Bone Marrow Transplant 11: 47–55 [DOI] [PubMed] [Google Scholar]
- Kharfan-Dabaja M., Mhaskar A., Djulbegovic B., Cutler C., Mohty M., Kumar A. (2009) Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Bone Marrow Transplant 15: 1005–1013 [DOI] [PubMed] [Google Scholar]
- Koc S., Leisenring W., Flowers M., Anasetti C., Deeg H., Nash R., et al. (2000) Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood 96: 3995–3996 [PubMed] [Google Scholar]
- Koc S., Leisenring W., Flowers M., Anasetti C., Deeg H., Nash R., et al. (2002) Therapy for chronic graft versus host disease: a randomised trial comparing cyclosporine plus prednisolone versus prednisolone alone. Blood 100: 48–51 [DOI] [PubMed] [Google Scholar]
- Koreth J., Matsuoka K., Kim H., McDonough S., Bindra B., Alyea E., 3rd, et al. (2011) Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365: 2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J., Stevenson K., Kim H., McDonough S., Bindra B., Armand P., et al. (2012) Bortezomib-based graft-versus-host prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol 30: 3202–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., et al. (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371: 1579–1586 [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., et al. (2004) Treatment of severe graft versus host disease with third party haploidentical mesenchymal stem cells. Lancet 363: 1439–1441 [DOI] [PubMed] [Google Scholar]
- Lee S., Klein J., Barrett A., Ringden O., Antin J., Cahn J., et al. (2002) Severity of chronic graft-versus-host disease: association with treatment related mortality and relapse. Blood 100: 406–414 [DOI] [PubMed] [Google Scholar]
- Lee S., Zahrieh D., Agura E., MacMillan M., Maziarz R., McCarthy P., et al. (2004) Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood 104: 1559–1564 [DOI] [PubMed] [Google Scholar]
- Lopez F., Parker P., Nademanee A., Rodriguez R., Al-Kadhimi Z., Bhatia R., et al. (2005) Efficacy of mycophenolate mofetil in the treatment of chronic graft-versus-host disease. Biol Blood Bone Marrow Transplant 11: 307–313 [DOI] [PubMed] [Google Scholar]
- MacMillan M., Weisdorf D., DeFor T., Burns L., Ransay N., Wagner J., et al. (2002a) Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 8: 40–46 [DOI] [PubMed] [Google Scholar]
- MacMillan M., Weisdorf D., Davies S., DeFor T., Burns L., Ramsay N., et al. (2002b) Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 8: 40–46 [DOI] [PubMed] [Google Scholar]
- Magro L., Mohty M., Catteau B., Coiteux V, Chevallier P, Terriou L., et al. (2009) Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood 114: 719–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Schoch G., Fisher L., Byers V., Anasetti C., Appelbaum F., et al. (1990) A retrospective analysis of therapy for acute graft-versus-host disease initial treatment. Blood 76: 1464–1472 [PubMed] [Google Scholar]
- Martin P., Storer B., Rowley S., Flowers M., Lee S., Carpenter P., et al. (2009) Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 113: 5074–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Weisdorf D., Przepiorka D, Hirschfield S., Farrell A., Rizzo J., et al. (2006) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group Report. Biol Blood Bone Marrow Transplant 12: 491–505 [DOI] [PubMed] [Google Scholar]
- Martinez C., Solano C., Ferra C., Sampol A, Valcarcel D, Perez-Simon J. (2009) Alemtuzumab as treatment of steroid-refractory graft versus host disease: results of a phase II study. Biol Blood Bone Marrow Transplant 15: 639–642 [DOI] [PubMed] [Google Scholar]
- Mielcarek M., Storer B., Boeckh M., Carpenter P., McDonald G., Deeg H., et al. (2009) Initial therapy of acute graft-versus-host disease with low-dose prednisolone does not compromise patient outcomes. Blood 113: 2888–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti P., Carlier P., Strada P., Gualandi F., Occhini D., Van Lint M., et al. (2008) Extracorporeal photophoresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant 42: 609–617 [DOI] [PubMed] [Google Scholar]
- Pidala J., Kim J., Roman-Diaz J., Shapiro J., Nishihori T., Bookout R., et al. (2010) Pentostatin as rescue therapy for glucocorticoid-refractory acute and chronic graft-versus-host disease. Ann Transplant 15: 21–29 [PubMed] [Google Scholar]
- Pidala J., Kurland B., Chai X., Majhail N., Weisdorf D., Pavletic S., et al. (2011) Patient-reported quality of life is associated with severity of chronic graft versus host disease as measured by NIH criteria: report of baseline data from the Chronic GvHD Consortium. Blood 117: 4651–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinana J., Valcarcel D., Martino R., Moreno M., Sureda A., Briones J., et al. (2006) Encouraging results with inolimomab (anti-IL-2 receptor) as treatment for refractory acute graft-versus-host disease. Biol Blood Bone Marrow Transplant 12: 1135–1141 [DOI] [PubMed] [Google Scholar]
- Przepiorka D., Kernan N., Ippoliti C., Papadopoulos E., Giralt S., Khouri I, et al. (2000) Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood 95: 83–89 [PubMed] [Google Scholar]
- Rao K., Rao A., Karlsson H., Jaqani M., Veys P., Amrolia P. (2009) Improved survival and preserved antiviral responses after combination therapy with daclizumab and infliximab in steroid-refractory graft-versus-host disease. J Pediatr Hematol Oncol 31: 456–461 [DOI] [PubMed] [Google Scholar]
- Reshef R., Luger S., Hexner E., Loren A., Frey N., Nasta S., et al. (2012) Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med 367: 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba R., Couriel D., Giralt S., Rondon G., Okoroji G., Rashid A., et al. (2012) Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant 47: 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick J., Taylor P., Holtick U., Makar Y., Douglas K., Berlin G., et al. (2008) U.K. consensus statement on the use of extracorporeal photophoresis for treatment of cutaneous T-cell lymphoma and chronic-graft-versus-host disease. Br J Dermatol 158: 659–678 [DOI] [PubMed] [Google Scholar]
- Socie G., Stone J., Wingard J., Weisdorf D., Henslee-Downey P., Bredeson C., et al. (1999) Long term survival and late deaths after allogenic bone marrow transplantation. N Engl J Med 341: 14–21 [DOI] [PubMed] [Google Scholar]
- Stadler M., Ahlborn R., Kamal H., Diedrich H., Buchholz S., Eder M., et al. (2009) Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood 114: 3718–3719 [DOI] [PubMed] [Google Scholar]
- Stewart B., Storer B., Storek J., Deeg H., Storb R., Hansen J., et al. (2004) Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood 104: 3501–3506 [DOI] [PubMed] [Google Scholar]
- Van Lint M., Milone G., Leotta S., Uderzo C., Scime R., Dallorso S., et al. (2006) Treatment of acute graft-versus-host disease with prednisolone significant survival advantage for day +5 responders and no advantage for non-responders receiving anti-thymocyte globulin. Blood 107: 4177–4181 [DOI] [PubMed] [Google Scholar]
- Van Lint M., Uderzo C., Locasciulli A., Majolino I., Scimie R., Locatelli F., et al. (1998) Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomised trial from the Italian Group for Bone Marrow Transplantation. Blood 92: 2288–2293 [PubMed] [Google Scholar]
- Vogelsang G., Farmer E., Hess A., Altamonte V., Beschorner W., Jabs D., et al. (1992) Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med 326: 1055–1058 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu K., Xu L., Liu D., Han W., Chen H., et al. (2011) Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoetic stem cell transplantation. Transplant Proc 43: 1928–1933 [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu L., Liu D., Chen H., Chen Y., Han W., et al. (2009) First-line therapy for chronic graft versus host disease that includes low-dose methotrexate is associated with a high response rate. Biol Blood Bone Marrow Transplant 15: 505–511 [DOI] [PubMed] [Google Scholar]
- Weisdorf D., Haake R., Blazar B., Miller W., McGlave P., Ramsay N., et al. (1990) Treatment of moderate/severe acute graft-versus-host disease after allogenic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 75: 1024–1030 [PubMed] [Google Scholar]
- Wolf D., von Lilienfeld-Toal M., Wolf A., Schleuning M., von Bergwelt-Baildon M., Held S., et al. (2012) Novel treatment concepts for graft-versus-host disease. Blood 119: 16–25 [DOI] [PubMed] [Google Scholar]