Abstract
Multiple myeloma is characterized by periods of remission followed by relapse, and eventually the disease becomes refractory to treatment. While patients with multiple myeloma frequently receive multiple lines of treatment, antimyeloma agents are associated with a number of toxicities that can impact their use and influence future treatment options. Patients with relapsed and/or refractory multiple myeloma are particularly challenging to treat due to the advanced state of their disease, typically greater resistance to treatment, and the presence of disease- and treatment-related comorbidities. An understanding of the safety profile of the therapeutic agents used in treating multiple myeloma is thus crucial for appropriate patient management. Single-agent carfilzomib has been approved in the United States for the treatment of patients with relapsed and refractory multiple myeloma, and has been shown to be efficacious and well-tolerated in this setting. This review examines the frequency of common and significant hematologic and nonhematologic adverse events following administration of single-agent carfilzomib in four phase II trials in relapsed and/or refractory multiple myeloma, and provides practical recommendations for their management.
Keywords: carfilzomib, drug toxicity, multiple myeloma, proteasome inhibitors
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy (after non-Hodgkin’s lymphoma) and accounts for 1% of all cancer cases in the United States [Siegel et al. 2013b]. Nearly all patients with MM relapse and eventually become refractory to treatment, and these patients are particularly challenging to treat due to a number of patient- and disease-related factors, including patient comorbidities, biological heterogeneity, and toxicities from prior treatments.
MM typically occurs in an older population (75−79% of patients are over 70 years old [NCCN, 2013]) and, thus, patients generally have a number of preexisting comorbidities. For example, approximately two-thirds of patients with MM have baseline cardiac risk factors (e.g. hypertension, diabetes, hyperlipidemia, coronary artery disease, congestive heart failure) or a prior history of a cardiac event [Kistler et al. 2012; Lonial et al. 2011], and up to 50% of patients with MM also have renal dysfunction [Clark et al. 1999; Faiman et al. 2011]. Hematologic complications also commonly arise in MM; up to 70% of patients with MM are anemic at the time of diagnosis [Steurer et al. 2004; Niesvizky and Badros, 2010]. Myelosuppression can lead to increased incidences of hematologic adverse events (AEs) and infections, including upper respiratory tract infections and pneumonia [Dimopoulos and Terpos, 2010; Mateos, 2010], which can, in turn, lead to dyspnea and worsening immunoparesis.
The choice of treatment can also have an impact on patient toxicities. Anti-MM agents (including the proteasome inhibitor bortezomib and immunomodulatory drugs [IMiDs] lenalidomide, pomalidomide, and thalidomide) are associated with a number of toxicities that can affect their use in patients with relapsed and/or refractory MM. For example, IMiDs and bortezomib are associated with myelosuppression and dyspnea [Weber et al. 2007; Dimopoulos et al. 2007; Richardson et al. 2006a, 2006b; Celgene Corporation, Inc., 2013], and bortezomib and thalidomide are associated with peripheral neuropathy (PN) [Jagannath et al. 2004; Richardson et al. 2003; Richardson et al. 2005; Glasmacher et al. 2006]. IMiDs are also linked to an increased risk of venous thromboembolism [Glasmacher et al. 2006; Knight et al. 2006; Rajkumar and Blood, 2006; Celgene Corporation, Inc., 2013] and incidence of second primary cancers [Badros, 2012; Dimopoulos et al. 2012]. There is, therefore, an enduring clinical need to develop new anti-MM treatments with reduced toxicity.
Carfilzomib is a proteasome inhibitor that was recently approved in the United States as a single agent for the treatment of patients with relapsed and refractory MM. Carfilzomib binds selectively and irreversibly to its target and exhibits durable proteasome inhibition with few off-target effects [Kuhn et al. 2007; Demo et al. 2007]. As nonproteasomal inhibition has been linked to the neurotoxicity of bortezomib, the selectivity of carfilzomib may explain the reduced incidence of PN in carfilzomib-treated patients relative to those treated with bortezomib [Arastu-Kapur et al. 2011].
In the pivotal phase II trial PX-171-003-A1 [ClinicalTrials.gov identifier: NCT00511238], single-agent carfilzomib demonstrated robust and durable activity with an overall response rate of 23%, median duration of response of 7.8 months, and median overall survival of 15.4 months in patients with relapsed and refractory MM [Siegel et al. 2012]. These efficacy results, along with safety results from PX-171-003-A1 and three other phase II trials (PX-171-003-A0 [ClinicalTrials.gov identifier: NCT00511238], PX-171-004 [ClinicalTrials.gov identifier: NCT00530816], and PX-171-005 [ClinicalTrials.gov identifier: NCT00721734]) supported the accelerated US Food and Drug Administration (FDA) approval of carfilzomib in July 2012 [Onyx Pharmaceuticals, Inc., 2012].
Given the complexities of managing patients with relapsed and/or refractory MM, an understanding of the side-effect profile associated with carfilzomib treatment is important to tailor its use to a patient’s individual clinical situation. This review will summarize the safety profile of single-agent carfilzomib in relapsed and/or refractory MM, as determined in a cross-trial safety analysis of the four phase II trials that supported the FDA approval, and will provide practical recommendations for clinicians to manage potential side effects associated with carfilzomib use.
Recommended carfilzomib dosing and administration
Carfilzomib is approved in the United States for the treatment of patients with MM who have received at least two prior therapies, including bortezomib and an IMiD, and have demonstrated disease progression on or within 60 days of completion of the last therapy [Onyx Pharmaceuticals, Inc., 2012]. Intravenous carfilzomib should be administered over 2–10 minutes on days 1, 2, 8, 9, 15, and 16 of each 28-day cycle, for up to 12 cycles. The recommended dose for cycle 1 is 20 mg/m2/day, with a target dose of 27 mg/m2/day in cycle 2 and subsequent cycles if treatment is tolerated. It is also recommended to hydrate patients prior to and following administration of carfilzomib and premedicate with dexamethasone prior to all cycle 1 doses, during the first cycle of dose escalation, and if infusion reaction symptoms develop or reappear.
Cross-trial safety analysis of phase II single-agent carfilzomib trials
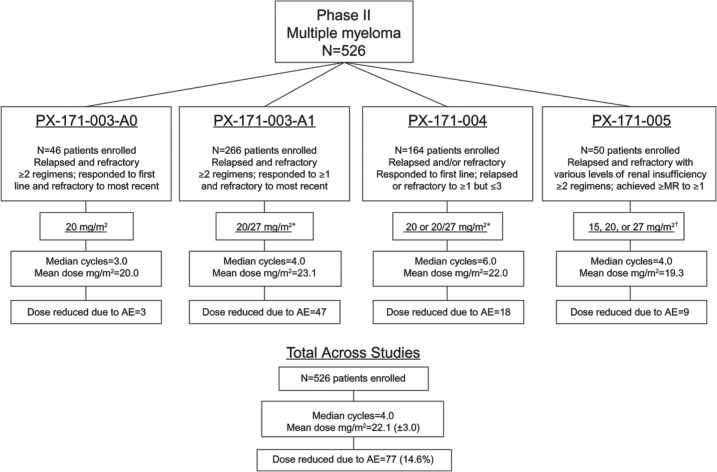
A cross-trial analysis examined the safety profile of single-agent carfilzomib in 526 patients with relapsed and/or refractory MM in the four phase II trials upon which US approval was based (Figure 1) [Siegel et al. 2013a]. A total of 278 patients (53%) received the labeled dose regimen of 20/27 mg/m2. Patients in these trials were heavily pretreated, with a median of four prior regimens.
Figure 1.
Baseline patient characteristics and treatment-emergent AEs were pooled and analyzed from the trials depicted (PX-171-003-A0, PX-171-003-A1, PX-171-004, and PX-171-005). (Adapted with permission from Siegel et al. [2013a].)
AE, adverse event; MR, minimal response.
*20 mg/m2 in cycle 1, 27 mg/m2 thereafter.
†Increased each cycle as tolerated.
Overall, the most common AEs of any grade were fatigue (55.5%), anemia (46.8%), and nausea (44.9%) (Table 1). Grade 3/4 nonhematologic AEs were uncommon (<10% grade 3 and <1% grade 4). Of the 526 patients included in the analysis, only 14.6% required a dose reduction and 22.6% required a dose delay due to an AE. Among patients who discontinued, 14.8% discontinued treatment due to an AE.
Table 1.
Treatment-emergent AEs in the cross-trial safety analysis (N = 526).
| AE, n (%) | All grades | Grades 3/4 | SAE |
|---|---|---|---|
| Fatigue | 292 (55.5) | 40 (7.6) | 0 |
| Nausea | 236 (44.9) | 7 (1.3) | 0 |
| Dyspnea | 182 (34.6) | 26 (4.9) | 11 (2.1) |
| Diarrhea | 172 (32.7) | 5 (1.0) | 3 (0.6) |
| Pyrexia | 160 (30.4) | 9 (1.7) | 18 (3.4) |
| Upper respiratory tract infection | 149 (28.3) | 17 (3.2) | 5 (1.0) |
| Headache | 145 (27.6) | 7 (1.3) | 0 |
| Cough | 137 (26.0) | 1 (0.2) | 1 (0.2) |
| Increased serum creatinine | 127 (24.1) | 14 (2.7) | 7 (1.3) |
| Peripheral edema | 126 (24.0) | 3 (0.6) | 0 |
| Vomiting | 117 (22.2) | 5 (1.0) | 2 (0.4) |
| Constipation | 110 (20.9) | 1 (0.2) | 0 |
| Back pain | 106 (20.2) | 15 (2.9) | 1 (0.2) |
| Pneumonia* | 67 (12.7) | 55 (10.5) | 52 (9.9) |
| Anemia | 246 (46.8) | 118 (22.4) | 7 (1.3) |
| Thrombocytopenia | 191 (36.3) | 123 (23.4) | 6 (1.1) |
| Lymphopenia | 126 (24.0) | 95 (18.1) | 0 |
| Neutropenia | 109 (20.7) | 54 (10.3) | 2 (0.4) |
| Leukopenia | 71 (13.5) | 28 (5.3) | 0 |
AE, adverse event; SAE, serious adverse event.
One grade 5 event of pneumonia in 003-A1.
Adapted with permission from Siegel et al. [2013a].
Hematologic AEs
The most common grade ≥3 AEs were primarily hematologic, and included thrombocytopenia (23.4%), anemia (22.4%), lymphopenia (18.1%), and pneumonia (10.5%). Discontinuations and dose reductions due to hematologic AEs were rare (≤1.1% for any thrombocytopenia, lymphopenia, neutropenia, or anemia AE). Hematologic AEs following single-agent carfilzomib treatment occurred at similar or lower rates compared with those reported for other agents [Siegel et al. 2013a; Richardson et al. 2011; Mikhael et al. 2009; van de Donk et al. 2011].
Platelet counts were observed to be cyclic with carfilzomib treatment, typically decreasing during the dosing cycle and returning to baseline by day 1 of the next cycle, and there was no indication of cumulative or grade 4 thrombocytopenia [Siegel et al. 2013a]. Neutrophil counts were also observed to be cyclic following carfilzomib treatment [Harvey and McCulloch, 2013].
Febrile neutropenia was uncommon (1.1%) [Siegel et al. 2013a], and patients with normal neutrophil counts at baseline rarely developed grade 3 or 4 neutropenia (7/187 patients [3.7%]). Approximately half of the patients in the cross-trial safety analysis experienced anemia [Siegel et al. 2013a]. Grade 3/4 anemia was reported in 22.4% of patients; however, no patient with normal hemoglobin at baseline shifted to grade 3 or 4 anemia.
Cardiac/pulmonary AEs
Although 74% of patients had a history of a past cardiac event and 70% had cardiac risk factors at baseline in the cross-trial safety analysis, cardiac AEs occurred infrequently with single-agent carfilzomib treatment [Siegel et al. 2013a]. A total of 22% of patients reported ≥1 grouped cardiac AE, a rate similar to that reported for other MM therapies [Hazarika et al. 2008; Richardson et al. 2005]. Aggregated cardiac-failure events (including congestive heart failure [CHF], pulmonary edema, and decreased ejection fraction) were reported in 38 patients (7.2%), regardless of causality [Lonial et al. 2013]. Importantly, the mortality rate was the same (7%) in patients who had baseline cardiac risk factors as it was for patients without these risk factors [Siegel et al. 2013a]. It should be noted that patients with New York Heart Association Class III–IV heart failure or recent myocardial infarction/unstable angina were excluded from the phase II studies. These patients may be at a greater risk for cardiac complications; however, it is important to note that carfilzomib is not contraindicated in this population [Onyx Pharmaceuticals, Inc., 2012].
Dyspnea, a common complication from the disease itself and from other MM treatments, was reported in 42.2% of patients; however, most incidences were grade 1 or 2 and transient and resolved without dose reduction or discontinuation.
Renal AEs
Although 23.8% of patients had moderate to severe renal dysfunction (creatinine clearance [CrCl] <50 ml/min) and 39.4% had mild renal dysfunction (CrCl ≥50 to <80 ml/min) at baseline, grade 3 or 4 acute renal failure Standardized MedDRA Query (SMQ) AEs were rare, and the majority (86.8%) of evaluable patients (n = 515) did not have worsening of renal function while receiving single-agent carfilzomib. Among patients who reported any renal event (n = 174), half required no change in carfilzomib treatment, 10.9% required a dose reduction, and 12.1% discontinued treatment. The remaining 23.6% and 3.4% of patients had missed or delayed doses, respectively.
While three of the studies included in the cross-trial safety analysis limited enrollment to patients with serum creatinine <2 mg/dl and an estimated glomerular filtration rate ≥30 ml/min, the PX-171-005 study examined the use of carfilzomib in patients with varying degrees of renal function (ranging from normal renal function to chronic dialysis). An analysis of the patients in PX-171-005 noted no differences in tolerability or pharmacokinetics among patients with varying levels of renal impairment, indicating that dose reductions are not necessary in patients with renal dysfunction, including patients on dialysis [Badros et al. 2013]. In these patients, the dose was safely escalated to the target dose (27 mg/m2) that is used in patients with normal renal function (estimated glomerular filtration rate ≥30 ml/min). Carfilzomib did not appear to be associated with clinically relevant nephrotoxicity, and most patients who experienced irreversible worsening of renal function had clear evidence of progressive MM [Badros et al. 2013].
Peripheral neuropathy
Although 71.9% of patients had active PN at baseline, 87.3% (330/378) did not report AEs related to PN while receiving single-agent carfilzomib (Table 2). PN AEs were mostly grade 1 or 2 and rarely resulted in dose modifications or discontinuations [Martin et al. 2012]. Newly developed PN was reported infrequently. These results compare favorably with other currently approved MM therapies [Mohty et al. 2010; Moreau et al. 2011; Cavaletti and Jakubowiak, 2010].
Table 2.
Summary of PN in the cross-trial safety analysis.
| 003-A0 | 003-A1 | 004 | 005 | All patients | |
|---|---|---|---|---|---|
| (n = 46) | (n = 266) | (n = 164) | (n = 50) | (N = 526) | |
| Any PN, n (%) | 7 (15.2) | 33 (12.4) | 26 (15.9) | 7 (14.0) | 73 (13.9) |
| Neuropathy peripheral | 4 (8.7) | 17 (6.4) | 11 (6.7) | 5 (10.0) | 37 (7.0) |
| Neuropathy | 3 (6.5) | 9 (3.4) | 7 (4.3) | 3 (6.0) | 22 (4.2) |
| Peripheral sensory neuropathy | 2 (4.3) | 11 (4.1) | 8 (4.9) | 0 | 21 (4.0) |
| Peripheral motor neuropathy | 0 | 2 (0.8) | 0 | 0 | 2 (0.4) |
| Action taken due to a PN AE* | |||||
| Dose reduced | 0/7 | 1/33 | 1/26 | 2/7 | 4/73 |
| Permanently discontinued | 0/7 | 0/33 | 0/26 | 1/7 | 1/74 |
| No change required | 7/7 | 31/33 | 24/26 | 4/7 | 66/73 |
PN, peripheral neuropathy.
Represents number of patients with at least 1 occurrence.
Adapted with permission from Siegel et al. [2013a].
Other AEs
Upper respiratory tract infection and pneumonia were often seen with carfilzomib treatment (reported in 28.3% and 12.7% of patients, respectively). Gastrointestinal AEs such as nausea, diarrhea, vomiting, and constipation were common, but rarely led to treatment discontinuation or dose reductions. Overall in the cross-trial safety analysis, the majority of transaminase elevation events were grade 1 or 2 and did not result in dose reduction or discontinuation.
Overall summary of cross-trial safety analysis
Taken together, the results of the cross-trial safety analysis demonstrated that single-agent carfilzomib has an acceptable safety profile in heavily pretreated patients with relapsed and/or refractory MM, including patients with preexisting comorbidities.
Clinical management of AEs
When beginning treatment, the safety profile of single-agent carfilzomib should be evaluated in conjunction with a patient’s preexisting comorbidities and toxicities from prior therapeutic regimens. Treatment-emergent AEs can be managed by following basic treatment recommendations and the use of appropriate prophylactic measures.
Management of hematologic AEs
In patients with MM, the replacement of bone marrow by malignant plasma cells frequently leads to cytopenia, including thrombocytopenia, neutropenia, and anemia. In general, support with transfusion and/or hematologic growth factor may be needed during anti-MM treatment.
Thrombocytopenia occurring during treatment with single-agent carfilzomib is managed with dose reduction and platelet transfusion (if the platelet count falls below 25,000/mm3) (Table 3). In the event of grade 4 thrombocytopenia, it is recommended that carfilzomib dose be held until the platelet count rises above 25,000/mm3. If platelet count is recovered before the next scheduled dose, carfilzomib can be re-administered at the same dose level; however, if platelet count is 25,000 to <50,000/mm3 (grade 3 thrombocytopenia), dose should be reduced by one dose level (e.g., from the target dose of 27 mg/m2 to 20 mg/m2 or from 20 mg/m2 to 15 mg/m2) [Onyx Pharmaceuticals, Inc., 2012]. If the reduced dose is tolerated, treatment may be escalated to the previous dose at the discretion of the physician, and should be considered as the patient’s bone marrow reserve improves following treatment. It is our experience that in extreme circumstances, patients who are profoundly cytopenic due to extensive bone marrow involvement may be managed with platelet transfusions so that maximally effective carfilzomib dosing may still be maintained. Improved tolerability can be expected in responding patients with subsequent cycles of treatment.
Table 3.
Recommendations for the management of hematologic AEs.
| AE | Treatment recommendation |
|---|---|
| Thrombocytopenia |
|
| Neutropenia |
|
| Anemia |
|
AE, adverse event; ANC, absolute neutrophil count; G-CSF, granulocyte-colony stimulating factor.
Grade 3 or 4 neutropenia is frequently treated with granulocyte-colony stimulating factor [Palumbo et al. 2012]. In general, it is recommended to hold the carfilzomib dose until absolute neutrophil count (ANC) is restored to ≥1000/mm3 [Onyx Pharmaceuticals, Inc., 2012]. If the ANC is fully recovered before the next scheduled dose, carfilzomib may be re-administered at the same dose level if the patient fully recovers, or may be administered at a reduced dose if the ANC is recovered to between 1000 and 1500/mm3 (grade 2 neutropenia); treatment may be re-escalated to the previous dose as clinically appropriate. In our center’s experience, aggressive growth factor support in patients with profound bone marrow involvement may allow maximal carfilzomib dosing to be maintained, which can result in greater efficacy and tolerability in subsequent cycles as patients respond to treatment.
Grade 1 or 2 anemia rarely requires transfusions and may be managed with erythropoiesis stimulating agents to stimulate red blood cell production [Faiman et al. 2011; Niesvizky and Badros, 2010]. For hemoglobin ≤10 g/dl, 40,000 units subcutaneous epoetin alfa weekly or 300 µg subcutaneous darbepoetin alfa every 2 weeks or 500 µg every month may be administered. Transfusions are typically needed for grade ≥3 anemia, and dose modifications or holds may also be required. To avoid dose interruptions, carfilzomib can be administered followed by transfusion, as needed, for hemoglobin <8 g/dl.
Management of nonhematologic AEs
Dosing guidelines from the carfilzomib prescribing information recommend that patients are administered 250−500 ml of saline prior to receiving single-agent carfilzomib in cycle 1, which can help prevent or reduce fatigue and other potential AEs such as renal toxicity and tumor lysis syndrome (Table 4) [Onyx Pharmaceuticals, Inc., 2012]. As needed, the same amount of saline may be administered following carfilzomib dosing, and intravenous hydration may be continued in subsequent cycles. However, patients should be monitored for fluid overload which can contribute to serious cardiac events and pulmonary complications, including cough and dyspnea.
Table 4.
Recommendations for the management of nonhematologic AEs.
| AE | Treatment recommendation |
|---|---|
| Fatigue |
|
| Infections |
|
| Gastrointestinal AEs |
|
AE, adverse event.
The frequency and severity of gastrointestinal AEs can vary significantly among patients. In general, nausea was mild and manageable in the four clinical trials. While the majority of patients did not require it, premedication with an antinausea medication, such as 8 mg oral ondansetron 30 minutes prior to carfilzomib dosing, may be recommended.
Antiviral and/or antibacterial prophylaxis are recommended, particularly for patients with a history of herpes virus infections or who are at risk for certain infections, such as urinary tract infections or pneumonia.
Management of AEs of additional clinical interest
Although occurring infrequently with carfilzomib treatment, cardiac, pulmonary, renal, and hepatic AEs have the potential to be serious. It is important to identify patients with prior history or who are at risk for cardiac events or renal or hepatic dysfunction for appropriate management of their treatment.
Cardiac AEs can be a serious complication; care should be taken for appropriate patient selection and evaluation before and during treatment (Table 5). Patients with cardiac risk factors should be cleared by a cardiologist prior to receiving treatment and closely monitored for fluid overload. Patients receiving hypertensive medication may need adjustments in their medication to manage their blood pressure while receiving carfilzomib. In the event of grade 3 or 4 cardiac events, carfilzomib should be withheld until recovery; carfilzomib may be resumed at the physician’s discretion based on a benefit:risk assessment [Onyx Pharmaceuticals, Inc., 2012]. In our center’s experience, most patients with shortness of breath as their primary manifestation of potential cardiac disease do not typically demonstrate a decrease in ejection fraction or other evidence of myocardial dysfunction. In these patients, we restart carfilzomib as soon as symptoms improve. In the rare patient who exhibits worsening myocardial function, we typically wait for echocardiographic evidence of improved function before rechallenging with carfilzomib.
Table 5.
Recommendations for the management of AEs of additional clinical interest.
| AE | Treatment recommendation |
|---|---|
| Cardiac and pulmonary AEs |
|
| Renal AEs |
|
| Hepatic AEs |
|
| PN |
|
| Infusion reactions |
|
AE, adverse event; PN, peripheral neuropathy.
It is important to monitor and manage dyspnea immediately; carfilzomib treatment should be interrupted until symptoms resolve or return to baseline [Onyx Pharmaceuticals, Inc., 2012]. There are concerns that dyspnea may develop due to fluid overload rather than drug toxicity. Hydration has been previously recommended with carfilzomib treatment due to concerns of acute deterioration of renal function. If a patient is not expected to tolerate aggressive hydration, however, serum creatinine may be monitored and, if stable, hydration may be decreased or discontinued. In general, overly aggressive hydration should be avoided and patients should be monitored for signs and symptoms of fluid overload, including weight gain.
A baseline evaluation of renal function is recommended prior to starting carfilzomib treatment, and creatinine clearance should be monitored throughout treatment. Patients with renal dysfunction, including patients on dialysis, do not typically require dose modifications. Transient increases in serum creatinine are frequently observed between consecutive carfilzomib doses and are easily managed with oral and intravenous hydration.
Grade 3 or 4 renal AEs are uncommon with carfilzomib treatment, but can be reversed with dose modifications or by discontinuing treatment. Carfilzomib should be held in patients with serum creatinine equal to or greater than 2 × baseline until renal function has recovered to grade 1 (≤1.5 × baseline [DCTD, NCI, NIH and DHHS, 2003]) or to baseline; dosing may be resumed at the previous dose level if the event was deemed unrelated to carfilzomib treatment, or may be resumed at a reduced dose if the event was considered to be related to treatment [Onyx Pharmaceuticals, Inc., 2012].
As with renal impairment, hepatic impairment has not typically been observed in response to carfilzomib treatment; however, mild to moderate elevations in hepatic enzymes are observed occasionally. In these cases, a review of the patient’s concomitant medications is recommended and, if deemed necessary, the dose of carfilzomib may be reduced. In cases of grade 3 or 4 elevations of transaminases, bilirubin, or other liver abnormalities, carfilzomib should be held until the abnormalities are resolved or return to baseline; carfilzomib may be resumed at the discretion of the physician [Onyx Pharmaceuticals, Inc., 2012].
PN is rarely observed with single-agent carfilzomib use in the clinic, and evidence suggests that there is no cumulative neurotoxicity. In the event of grade 3 or 4 PN, carfilzomib dosing should be withheld until the PN is resolved or returns to baseline; dosing may be resumed at the dose used prior to the event or at a reduced dose at the discretion of the clinician [Onyx Pharmaceuticals, Inc., 2012].
Infusion reactions were observed in early clinical trials and were characterized by symptoms including fever, chills, myalgia, facial swelling or flushing, vomiting, weakness, hypotension, chest tightness, or shortness of breath. Prophylactic premedication with very low-dose dexamethasone (4 mg) prior to carfilzomib in cycles 1 and 2 has decreased the incidence and severity of infusion reactions. If the patient tolerates the drug, dexamethasone prophylaxis may be stopped beyond cycle 1. However, if infusion reactions appear in subsequent cycles within 24−48 hours after receiving carfilzomib, dexamethasone should be reintroduced.
Future directions and conclusion
Additional studies, including randomized phase III trials, are needed to fully understand which patient- and disease-related factors contribute to the AEs reported in patients treated with carfilzomib, particularly cardiac and pulmonary complications. Serial echocardiograms are being conducted in a number of ongoing carfilzomib studies, including as a substudy in the randomized phase III trial ENDEAVOR [ClinicalTrials.gov identifier: NCT01568866], which is comparing carfilzomib and dexamethasone versus bortezomib and dexamethasone. These assessments will provide important information on the pathophysiology of cardiac events during carfilzomib treatment, and their comparative rates in relationship to bortezomib treatment.
It is noteworthy that carfilzomib is generally well tolerated when doses twice the amount of those discussed in this manuscript (up to 56 mg/m2) are infused over 30 minutes [Papadopoulos et al. 2011]. Ongoing trials are further investigating administration of such higher doses, including ENDEAVOR, which is examining a target dose of 56 mg/m2 carfilzomib in combination with dexamethasone in patients with relapsed MM. In addition, trials are underway investigating carfilzomib in combination with other agents and in earlier lines of treatment. The combination of carfilzomib with lenalidomide and dexamethasone, in particular, has shown promising results and appears to be well tolerated [Wang et al. 2013]. This combination is being further explored in patients with relapsed MM in the randomized phase III trial ASPIRE [ClinicalTrials.gov identifier: NCT01080391], and in patients with newly diagnosed MM in phase I and II trials [Jakubowiak et al. 2013; Korde et al. 2013].
These and other studies of carfilzomib-based combination regimens [Bringhen et al. 2013; Jakubowiak et al. 2013; Moreau et al. 2013] are expected to provide important information that will expand upon the known safety profile of carfilzomib and, when combined with additional efficacy data, will provide a clearer view of the risk:benefit profile of the compound. Although carfilzomib is currently approved as a single agent in patients with relapsed and refractory MM, it is anticipated that in the future carfilzomib will be used routinely in combination regimens and in earlier lines of treatment. In addition, oral proteasome inhibitors in clinical development are promising future treatment options. These include ixazomib, a reversible boronate proteasome inhibitor similar to bortezomib [Kumar et al. 2012], and oprozomib, an epoxyketone proteasome inhibitor which, like carfilzomib, binds selectively and irreversibly to its target [Kaufman et al. 2013].
In conclusion, patients with MM, particularly those in the relapsed and/or refractory setting, are likely to have comorbidities and risk factors for AEs due to a number of patient- and disease-related factors including age, prior treatment received, and effects of the disease itself. These include high rates of renal dysfunction and cardiac risk factors prior to treatment, and increased rates of hematologic complications and PN stemming from disease- and treatment-induced effects.
Given the frequency of these complications at baseline, an awareness and understanding of potential side effects with carfilzomib treatment is important for managing patients with advanced MM. Carfilzomib treatment has been well tolerated in the majority of patients, and administration of prophylactic measures and careful monitoring of patients throughout the entire course of treatment can help reduce the frequency and severity of AEs. Prevention of serious complications (and subsequent dose interruptions) will allow for carfilzomib to be administered at the target dose, so that patients can receive the maximal clinical benefit from its use. Ongoing studies will help further define the optimal treatment regimen for patients and will offer additional insight into the safety profile of carfilzomib.
Acknowledgments
The authors would like to thank all of the patients and their families who contributed to these studies. The authors also acknowledge the critical review of the manuscript by Thomas Renau, PhD and A. Peter Morello, PhD, CMPP (Onyx Pharmaceuticals, Inc.). Medical writing and editorial assistance was provided by Cheryl Chun, PhD, (BlueMomentum, a division of KnowledgePoint360 Group, San Bruno, CA) and funded by Onyx Pharmaceuticals, Inc.
Footnotes
Funding: Medical writing and editorial assistance was funded by Onyx Pharmaceuticals, Inc. The studies PX-171-003-A1, PX-171-003-A0, PX-171-004, and PX-171-005 were supported by Onyx Pharmaceuticals, Inc. and the Multiple Myeloma Research Consortium.
Conflict of interest statement: The author has provided/received consultancy, honoraria, and board of directors or advisory committee membership for Millennium and Celgene.
References
- Arastu-Kapur S., Anderl J., Kraus M., Parlati F., Shenk K., Lee S., et al. (2011) Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res 17: 2734–2743 [DOI] [PubMed] [Google Scholar]
- Badros A. (2012) Lenalidomide in myeloma - a high-maintenance friend. N Engl J Med 366: 1836–1838 [DOI] [PubMed] [Google Scholar]
- Badros A., Vij R., Martin T., Zonder J., Kunkel L., Wang Z., et al. (2013) Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia 27: 1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringhen S., Federica C., Petrucci M., Gray F., Federico V., Conticello C., et al. (2013) Carfilzomib, cyclophosphamide and dexamethasone(CCD) for newly diagnosed multiple myeloma (MM) patients: initial results of a multicenter, open label phase II study. Haematologica 98(Suppl. 1): abstract S578. [Google Scholar]
- Cavaletti G., Jakubowiak A. (2010) Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma 51: 1178–1187 [DOI] [PubMed] [Google Scholar]
- Celgene Corporation, Inc (2013) POMYLAST® Prescribing Information. Celgene Corporation, Inc, Summit, NJ [Google Scholar]
- Clark A., Shetty A., Soutar R. (1999) Renal failure and multiple myeloma: Pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev 13: 79–90 [DOI] [PubMed] [Google Scholar]
- DCTD, NCI, NIH and DHHS (2003) Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, 31 March 2003. Available at: http://ctep.cancer.gov (published online 9 August 2006).
- Demo S., Kirk C., Aujay M., Buchholz T., Dajee M., Ho M., et al. (2007) Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res 67: 6383–6391 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Richardson P., Brandenburg N., Yu Z., Weber D., Niesvizky R., et al. (2012) A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood 119: 2764–2767 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Spencer A., Attal M., Prince H., Harousseau J., Dmoszynska A., et al. (2007) Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357: 2123–2132 [DOI] [PubMed] [Google Scholar]
- Dimopoulos M., Terpos E. (2010) Multiple myeloma. Ann Oncol 21: vii143-vii150 [DOI] [PubMed] [Google Scholar]
- Faiman B., Mangan P., Spong J., Tariman J. for the International Myeloma Foundation Nurse Leadership Board (2011) Renal complications in multiple myeloma and related disorders: survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs 15(Suppl.): 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasmacher A., Hahn C., Hoffmann F., Naumann R., Goldschmidt H., von Lilienfeld-Toal M., et al. (2006) A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol 132: 584–593 [DOI] [PubMed] [Google Scholar]
- Harvey R., McCulloch L. (2013) Carfilzomib safety overview from 4 phase 2 studies. Presented at the Hematology/Oncology Pharmacy Association 9th Annual Conference (Los Angeles, CA, 20–23 March 2013), Poster 10. [Google Scholar]
- Hazarika M., Rock E., Williams G., Dagher R., Sridhara R., Booth B., et al. (2008) Lenalidomide in combination with dexamethasone for the treatment of multiple myeloma after one prior therapy. Oncologist 13: 1120–1127 [DOI] [PubMed] [Google Scholar]
- Jagannath S., Barlogie B., Berenson J., Siegel D., Irwin D., Richardson P., et al. (2004) A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127: 165–172 [DOI] [PubMed] [Google Scholar]
- Jakubowiak A., Dytfeld D., Griffith K., Jasielec J., McDonnell K., Lebovic D., et al. (2013) Treatment outcome with the combination of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) for newly diagnosed multiple myeloma (NDMM) after extended follow-up. J Clin Oncol 31(15 Suppl.): abstract 8543. [Google Scholar]
- Kaufman J., Siegel D., Vij R., Ghobrial I., Badros A., Neuman L., et al. (2013) Clinical profile of once-daily, modified-release oprozomib tablets in patients with hematologic malignancies: results of a phase 1b/2 trial. Haematologica 98(Suppl. 1): abstract P233. [Google Scholar]
- Kistler K., Rajangam K., Faich G., Lanes S. (2012) Cardiac event rates in patients with newly diagnosed and relapsed multiple myeloma in US clinical practice. Blood 120: abstract 2916. [Google Scholar]
- Knight R., DeLap R., Zeldis J. (2006) Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med 354: 2079–2080 [DOI] [PubMed] [Google Scholar]
- Korde N., Zingone A., Kwok M., Manasanch E., Wu P., Tageja N., et al. (2013) Phase 2 clinical and correlative study of carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide extended dosing (CRD-R) in newly diagnosed multiple myeloma (MM) patients. Haematologica 98(Suppl. 1): abstract P228. [Google Scholar]
- Kuhn D., Chen Q., Voorhees P., Strader J., Shenk K., Sun C., et al. (2007) Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 110: 3281–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Bensinger W., Reeder C., Zimmerman T., Berenson J., Liu G., et al. (2012) Weekly dosing of the investigational oral proteasome inhibitor MLN9708 in patients (pts) with relapsed/refractory multiple myeloma (MM): A phase I study. J Clin Oncol 30(15 Suppl.): abstract 8034. [Google Scholar]
- Lonial S., Mitsiades C., Richardson P. (2011) Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res 17: 1264–1277 [DOI] [PubMed] [Google Scholar]
- Lonial S., Niesvizky R., McCulloch L., Kanya R., Ravi V., et al. (2013) Cardiac and pulmonary safety analysis of single-agent carfilzomib treated relapsed and/or refractory multiple myeloma. Paper presented at the XIVth International Myeloma Workshop (Kyoto, Japan, April 2013), Abstract 413. [Google Scholar]
- Martin T., Vij R., Badros A., Patel P., McCulloch L., Jagannath S. (2012) Carfilzomib is associated with a low rate of typically mild to moderate, non-dose limiting treatment-emergent peripheral neuropathy. Haematologica 97: abstract 0857. [Google Scholar]
- Mateos M. (2010) Management of treatment-related adverse events in patients with multiple myeloma. Cancer Treat Rev 36: S24-S32 [DOI] [PubMed] [Google Scholar]
- Mikhael J., Belch A., Prince H., Lucio M., Maiolino A., Corso A., et al. (2009) High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol 144: 169–175 [DOI] [PubMed] [Google Scholar]
- Mohty B., El-Cheikh J., Yakoub-Agha I., Moreau P., Harousseau J., Mohty M. (2010) Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica 95: 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Pylypenko H., Grosicki S., Karamanesht I., Leleu X., Grishunina M., et al. (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12: 431–440 [DOI] [PubMed] [Google Scholar]
- Moreau P., Kolb B., Hulin C., Caillot D., Benboubker L., Tiab M., et al. (2013) CMP—carfilzomib (CFZ) plus melphalan-prednisone (MP)—in elderly patients (pts) with newly diagnosed multiple myeloma (NDMM): results of a phase (Ph) I/II trial. Haematologica 98(Suppl. 1): abstract P224. [Google Scholar]
- National Comprehensive Cancer Network (NCCN) (2013) Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple myeloma; Version 2. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (accessed 8 May 2013). [Google Scholar]
- Niesvizky R., Badros A. (2010) Complications of multiple myeloma therapy, part 2: risk reduction and management of venous thromboembolism, osteonecrosis of the jaw, renal complications, and anemia. J Natl Compr Canc Netw 8: S13-S20 [DOI] [PubMed] [Google Scholar]
- Onyx Pharmaceuticals, Inc (2012) KYPROLIS™ Prescribing Information. Onyx Pharmaceuticals, Inc, South San Francisco, CA [Google Scholar]
- Palumbo A., Bladé J., Boccadoro M., Palladino C., Davies F., Dimopoulos M., et al. (2012) How to manage neutropenia in multiple myeloma. Clin Lymphoma Myeloma Leuk 12: 5–11 [DOI] [PubMed] [Google Scholar]
- Papadopoulos K., Lee P., Singhal S., Holahan J., Vesole D., Rosen S., et al. (2011) A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56mg/m2 expansion cohort of PX-171–007. Blood 118(21): abstract 2930. [Google Scholar]
- Rajkumar S., Blood E. (2006) Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med 354: 2079–2080 [PubMed] [Google Scholar]
- Richardson P., Barlogie B., Berenson J., Singhal S., Jagannath S., Irwin D., et al. (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348: 2609-2617 [DOI] [PubMed] [Google Scholar]
- Richardson P., Blood E., Mitsiades C., Jagannath S., Zeldenrust S., Alsina M., et al. (2006a) A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood 108: 3458–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P., Jagannath S., Colson K. (2006b) Optimizing the efficacy and safety of bortezomib in relapsed multiple myeloma. Clin Adv Hematol Oncol 4(Suppl.): 1; discussion 8 [PubMed] [Google Scholar]
- Richardson P., Siegel D., Vij R., Hofmeister C., Jagannath S., Chen C., et al. (2011) Randomized, open label phase 1/2 study of pomalidomide (POM) alone or in combination with low-dose dexamethasone (LoDex) in patients (Pts) with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide (LEN) and bortezomib (BORT): phase 2 results. Blood. 118: abstract 634. [Google Scholar]
- Richardson P., Sonneveld P., Schuster M., Irwin D., Stadtmauer E., Facon T., et al. for the Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352: 2487–2498 [DOI] [PubMed] [Google Scholar]
- Siegel D., Martin T., Wang M., Vij R., Jakubowiak A., Lonial S., et al. (2012) A phase 2 study of single-agent carfilzomib (PX-171–003-A1) in patients with relapsed and refractory multiple myeloma. Blood 120: 2817–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D., Martin T., Nooka A., Harvey D., Vij R., Niesvizky R., et al. (2013a) Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase 2 clinical studies. Haematologica [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2013b) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30 [DOI] [PubMed] [Google Scholar]
- Steurer M., Wagner H., Gastl G. (2004) Prevalence and management of anaemia in haematologic cancer patients receiving cyclic nonplatinum chemotherapy: results of a prospective national chart survey. Wien Klin Wochenschr 116: 367–372 [DOI] [PubMed] [Google Scholar]
- van de Donk N., Lokhorst H., Dimopoulos M., Cavo M., Morgan G., Einsele H., et al. (2011) Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev 37: 266–283 [DOI] [PubMed] [Google Scholar]
- Wang M., Martin T., Bensinger W., Alsina M., Siegel D., Kavalerchik E., et al. (2013) Phase 2 dose-expansion study (PX-171–006) of carfilzomib, lenalidomide and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D., Knight R., Chen C., Spencer S., Yu Z., Zeldis J., et al. (2007) Prolonged overall survival with lenalidomide plus dexamethasone compared with dexamethasone alone in patients with relapsed or refractory multiple myeloma. Blood 110: Abstract 412. [Google Scholar]