Abstract
Study Design Single-center, retrospective study.
Objective Suboptimal concentrations of vitamin D have been linked to hip and knee osteoarthritis in large, population-based cohort studies. We sought to examine the association of vitamin D levels with intervertebral disk disease.
Methods From January 2010 through May 2011, 91 consecutive, eligible adult spine surgery patients who had undergone cervical magnetic resonance imaging (MRI) and preoperative serum 25-hydroxyvitamin D (s25D) measurement were retrospectively included. MRI was read for C2–T1 disk herniation and degeneration (grades I to V). Logistic regressions were performed.
Results Compared with the 384 disks of nondeficient patients, 162 disks of vitamin D-deficient (< 20 ng/mL) patients were more frequently herniated (40% versus 27%, p = 0.004); deficiency was not predictive of individual disk grade (unadjusted odds ratio [uOR] = 0.98, p = 0.817). On regression analysis, deficiency was associated with increased number of herniations per patient (uOR = 2.17, 95% confidence interval [CI] = 1.22 to 3.87, p = 0.009; adjusted odds ratio [aOR] = 2.12, 95% CI = 1.11 to 4.03, p = 0.023). When disks were analyzed individually, and levels (e.g., C5 to C6), additionally controlled for, deficiency correlated with greater likelihood of herniation per disk (uOR = 1.81, 95% CI = 1.22 to 2.66, p = 0.003; aOR = 2.06, 95% CI = 1.25 to 3.41, p = 0.005).
Conclusion Among adults undergoing spine surgery at our institution, vitamin D deficiency was associated with cervical disk herniation. Considering the current epidemics of vitamin D insufficiency and neck pain, further investigation is warranted, as these data were retrospectively collected and subject to sampling bias.
Keywords: vitamin D, hypovitaminosis D, cervical spine, disk herniation, disk disease
Introduction
Vitamin D metabolites modulate the in vitro activity of chondrocyte matrix metalloproteinase (MMP)-3, which plays an essential role in the degradation of articular cartilage.1 2 3 Clinically, suboptimal serum concentrations of the hormone have been linked to hip and knee osteoarthritis (OA) in large, population-based cohort studies.3 4 5 6 To our knowledge, no analogous relationship has been demonstrated for intervertebral disk pathology. However, MMP-3 and MMP-3-activated collagenases hydrolyze components of the disk, and vitamin D has been shown to diminish the expression of proinflammatory cytokines by cells of the human annulus fibrosus.7 8 Thus, vitamin D deficiency may conceivably predispose to neck and back pain via disk degeneration, herniation with neural compression, and chemical radiculitis.7 9 10
Numerous studies have drawn attention to the high global prevalence of hypovitaminosis D, which may exceed 1 billion.11 Despite the aforementioned in vitro studies, the extent to which vitamin D deficiency contributes to the pathogenesis of certain musculoskeletal conditions such as OA and degenerative disk disease is not yet clearly elucidated. Back and neck pain, in particular, afflict a high proportion of adults at some point during their lives and annually incur well over 100 billion dollars in health care expenditure and wage loss in the United States alone.12 13 14 With the present study, we sought to characterize the relationship between vitamin D deficiency and degenerative vertebral pathology—specifically, disk degeneration and herniation. We hypothesized that vitamin D-deficient patients would have a higher prevalence of disk herniation and/or more advanced degenerative disk disease than those with adequate levels of the hormone.
Materials and Methods
Patient Selection
The serum 25-hydroxyvitamin D (s25D) concentrations of adults undergoing orthopedic spine surgery at our institution have been routinely measured since 2010 with the DiaSorin LIAISON (Diasorin Inc., Stillwater, Minnesota, United States). Following institutional review board approval, operative reports of five surgeons were monitored from January 2010 through May 2011. Consecutive adults (≥ 18 years) with cervical magnetic resonance imaging (MRI) and preoperative s25D measurement were identified. Of note, several patients whose preoperative s25D measurement was obtained prior to a thoracic and/or lumbar fusion were included; these patients underwent cervical spine MRI for a variety of reasons. For example, they may have had concurrent neck pain, or the surgeon may have been considering including the cervical spine in their fusion construct. Exclusion criteria were prior vitamin D supplementation, active infection,15 active inflammatory spondyloarthropathies,15 16 cervical neoplasm, congenital autofusion, radiotherapy, and surgery. Of 900 adults who underwent any surgery during the study timeframe, 91 consecutive, eligible patients were included. The low inclusion rate is largely derived from the temporal disparity with which each surgeon began routinely ordering s25D during 2010. The present time frame extends back to January 2010 for all surgeons for uniformity and to ensure that all measured patients were captured.
Radiologic Evaluation
In accordance with published reliability studies, MRI was evaluated for C2–T1 disk degeneration and herniation.17 18 19 Degeneration was rated on a spectrum from normal structure and signal intensity (grade I) to disk collapse (grade V) and summed per patient (Fig. 1).18 This grading scheme was devised by Miyazaki et al and is described in detail in their original work.18 In the present study, the first author read each patient's MRI on two occasions and came to a consensus for each parameter. These data were utilized for analysis. Another author then independently reviewed the MRI twice; intraobserver agreement κ statistics were calculated per disk between these two readings. Interobserver κ values were derived using the secondary reader's second and primary reader's consensus evaluations. Both authors were uninvolved in the care of the analyzed cohort and blinded to s25D levels during imaging assessment. The interobserver κ statistics for grade and herniation were 0.59 and 0.66, respectively. Those relating to intraobserver reliability were 0.83 and 0.84. Values from 0.41 to 0.60 have been described as moderate; those between 0.61 and 0.80, substantial; and 0.81 to 0.99, approaching absolute agreement.20 The observers agreed on the presence or absence of herniation for the overwhelming majority of disks, 465 of 546 (85%).
Fig. 1.
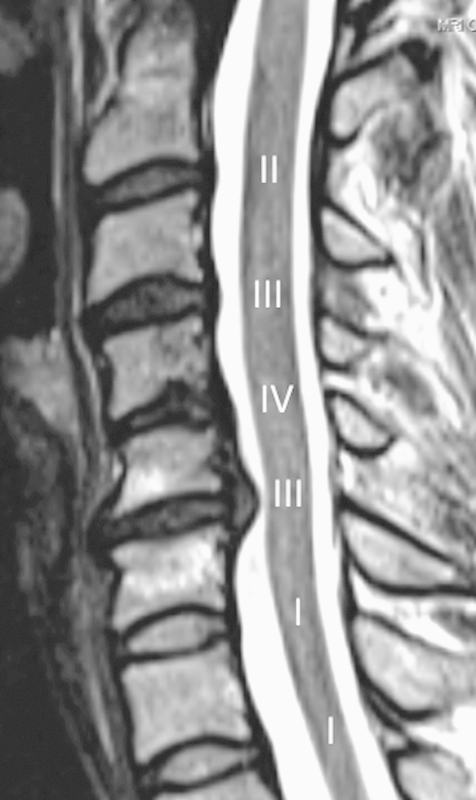
Sagittal T2-weighted scan shows disk herniation at C5–C6. C2–T1 disk grades are depicted, overlying the adjacent cord. This patient underwent anterior cervical disk replacement at C5–C6.
Statistical Analysis
Averages are reported as means with standard deviations. Fisher exact and Mann-Whitney U tests were employed to compare subgroups. Vitamin D deficiency was defined as s25D below 20 ng/mL.11 The following factors were controlled for in multivariate regression models: s25D measurement season, skin tone, age, gender, body mass index, active smoking within 2 months, pack-year smoking history, multivitamin use, native patient latitude, American Society of Anesthesiologists (ASA) status grade, symptom-generating spinal injury, and patient-reported visual analog scale (VAS) pain scores (scored 0 to 10).
Skin tone was categorized as light (self-reported “Caucasian” or “Asian”) or dark (“Hispanic” or “African American”), in accordance with a published analysis of hypovitaminosis D among 723 orthopedic surgery patients.21 ASA grades stood as an index of comorbidity, and VAS reflected overall pain intensity. Covariates were deemed clinically relevant a priori.3 5 6 11 16 For instance, excess adipose tissue and advanced age limit bioavailability and biosynthesis of vitamin D, respectively.11 The Neck and Oswestry Disability Index scores were pooled but eventually disregarded due to their suboptimal completion rate (79 of 91, 87%) and correlation with VAS (100% completion; Spearman ρ = +0.57, p < 0.001).22 23 Two patients (12 of 546 disks, 2.2%) who failed to complete questionnaires were excluded from multivariate analysis.
Linear regression was utilized to assess the relationships between deficiency or age and the number of herniations per patient or summed degeneration score of each patient. Taking on the characteristics of their respective patient, disks were then analyzed as separate entities; binary logistic regression was used to assess the association of deficiency with herniation, and linear regression was employed to do so for deficiency and individual disk grade. Binary logistic regression was also utilized in examining the association between herniation and individual disk grade. Cervical levels (e.g., C2–C3 to C7–T1) were additionally controlled for when separately analyzing disks to account for the varying biokinematics thereof. If dependent variables (degeneration or herniation) were insignificantly associated with s25D deficiency on univariate regression, multivariate analysis was forgone. No significant p value reported herein became insignificant or vice versa with robust regression modeling. The two-tailed threshold of significance was p < 0.05.
Results
Characteristics of the study cohort are summarized in Table 1. Operations consisted of 57 anterior and 2 posterior cervical fusions, 3 cervical disk replacements, 19 cervical decompressions, and 12 thoracic and/or lumbar fusions. Two underwent combined cervical fusion and arthroplasty. Sixty-three operations were indicated, at least in part, for 99 cervical herniations. In total, 74 patients (81%) had 166 herniated cervical disks (30%), and the average summed degeneration score was 20 ± 4.0. Grades III (39%) and IV (39%) predominated. Twenty-seven patients (30%) were vitamin D-deficient.
Table 1. Total cohort and patients with and without herniationa .
| Total | Herniation | No herniation | |
|---|---|---|---|
| Patients, n (%) | 91 (100.0) | 74 (81.3) | 17 (18.7) |
| Vitamin D deficient, n (%)b | 27 (29.7) | 24 (32.4) | 3 (17.6) |
| Women, n (%) | 37 (40.7) | 29 (39.2) | 8 (47.1) |
| Age, mean (SD), y | 52.1 (13.2) | 52.8 (11.2) | 49.2 (20.0) |
| Light-skinned, n (%)c | 87 (95.6) | 70 (94.6) | 17 (100.0) |
| Body mass index, mean (SD), kg/m2 | 29.8 (5.9) | 30.5 (5.9)d | 26.4 (4.4)d |
| Multivitamin use, n (%) | 16 (17.6) | 13 (17.6) | 3 (17.6) |
| Current smoker, n (%)e | 21 (23.1) | 17 (18.7) | 4 (23.5) |
| Smoking history, mean (SD), PYf | 13.9 (19.2) | 15.0 (19.4) | 8.8 (18.3) |
| Native latitude, mean (SD), n | 38.1 (2.0) | 38.3 (1.4) | 37.2 (3.6) |
| Prior spinal injury, n (%) | 37 (40.7) | 29 (39.2) | 8 (47.1) |
| VAS pain, mean (SD), score | 6.7 (2.5) | 6.6 (2.6) | 7.0 (2.1) |
| ASA status I, n (%) | 6 (6.6) | 4 (5.4) | 1 (6.3) |
| ASA status II, n (%) | 61 (67.0) | 50 (67.6) | 11 (68.8) |
| ASA status III, n (%) | 24 (26.4) | 20 (27.0) | 4 (25.0) |
Abbreviations: ASA, American Society of Anesthesiologists; PY, pack-years; SD, standard deviation; VAS, visual analog scale.
Compared with Fisher exact and Mann-Whitney U tests.
Serum 25-hydroxyvitamin D concentration below 20 ng/mL.
Self-reported “Caucasian” and “Asian.”
Denotes p < 0.01. No other pair of variables was significantly different.
Within 2 mo prior to documentation.
Smoking history not quantified by 1 patient in each subgroup.
Deficiency was positively associated with the number of herniations per patient (unadjusted odds ratio [uOR] = 2.17, 95% confidence interval [CI] = 1.22 to 3.87, p = 0.009; adjusted odds ratio [aOR] = 2.12, 95% CI = 1.11 to 4.03, p = 0.023) but not the summed degeneration score (uOR = 0.89, p = 0.896). Increasing age was strongly correlated with overall degeneration (uOR = 1.24, p < 0.001) but not herniations per subject (uOR = 1.01, p = 0.450; aOR = 1.00, p = 0.903). None of the remaining covariates was significantly associated with herniation quantity, including VAS pain (aOR = 0.95, p = 0.393).
Compared with the 384 disks of nondeficient patients, the 162 disks of vitamin D-deficient patients were more frequently herniated (40% versus 27%, p = 0.004; Table 2). By disk, deficiency was correlated with increased likelihood of herniation (uOR = 1.81, 95% CI = 1.22 to 2.66, p = 0.003; aOR = 2.06, p = 0.005; Table 3). Levels C4–C5, C5–C6, and C6–C7 were potentially more accurate determinants of herniation, as suggested by their larger odds ratio values (Table 3). Deficiency was not associated with individual disk degeneration severity (uOR = 0.98, p = 0.817). Disk grades lost their association with herniation when spinal levels alone were included as covariates (uOR = 1.38, p = 0.002; aOR = 1.08, p = 0.529).
Table 2. Comparison of vitamin D-deficient and nondeficient statesa .
| Herniation, n (%) | Deficientb | Nondeficient | OR | 95% CI | p value |
|---|---|---|---|---|---|
| Patients | 24 (88.9) | 50 (78.1) | 2.24 | 0.59–8.54 | 0.439 |
| Total levels | 64 (39.5) | 102 (26.6) | 1.81 | 1.22–2.66 | 0.004 |
| C2–C3 level | 6 (22.2) | 12 (18.8) | 1.52 | 0.51–4.56 | 0.999 |
| C3–C4 level | 11 (40.7) | 14 (21.9) | 2.46 | 0.93–6.48 | 0.096 |
| C4–C5 level | 15 (55.6) | 16 (25.0) | 3.75 | 1.46–9.67 | 0.009 |
| C5–C6 level | 16 (59.3) | 31 (48.4) | 1.55 | 0.62–3.85 | 0.489 |
| C6–C7 level | 15 (55.6) | 27 (42.2) | 1.71 | 0.69–4.24 | 0.341 |
| C7–T1 level | 1 (3.7) | 2 (3.1) | 1.19 | 0.10–13.7 | 0.999 |
Abbreviations: CI, confidence interval; OR indicates unadjusted odds ratio.
Deficient and nondeficient states are compared with Fisher exact test.
Serum 25-hydroxyvitamin D concentration below 20 ng/mL.
Table 3. Multivariate logistic regression analysis of herniation by diska .
| OR | 95% CI | p value | |
|---|---|---|---|
| Vitamin D deficiencyb | 2.06 | 1.25–3.41 | 0.005 |
| Body mass index | 1.05 | 1.00–1.09 | 0.032 |
| Pack-year smoking history | 1.01 | 1.00–1.03 | 0.037 |
| C4–C5 level | 2.28 | 1.12–4.66 | 0.023 |
| C5–C6 level | 5.41 | 2.68–10.94 | <0.001 |
| C6–C7 level | 4.23 | 2.10–8.53 | <0.001 |
| C7–T1 level | 0.14 | 0.04–0.50 | 0.002 |
Abbreviations: CI, confidence interval; OR indicates unadjusted odds ratio.
Only statistically significant independent variables are depicted.
Serum 25-hydroxyvitamin D concentration below 20 ng/mL.
Discussion
In the present study, preoperative vitamin D deficiency in adult spine surgery patients at a single institution was positively associated with the number of cervical disk herniations per patient (aOR = 2.12, p = 0.023). No relationship was discerned between deficiency and summed disk degeneration score (uOR = 0.89, p = 0.896). When disks were analyzed as distinct entities, lower levels of vitamin D were associated with a greater likelihood of herniation (uOR = 1.81, p = 0.004; aOR = 2.06, p = 0.005). Cervical spinal level appeared to account for any association between herniation and degeneration.
In chondrocyte models, vitamin D negatively modulates MMP-3, and it has been theorized that s25D insufficiency may drive cartilage remodeling in favor of degradation.1 2 3 Lane et al demonstrated that low s25D conferred increased risk of incident hip OA in elderly women.3 Low vitamin D predicted progression of knee OA in the Framingham Study.4 More recently, s25D positively correlated with quantitative tibial cartilage volume on MRI among adults in Tasmania; values below 50 nmol/L were associated with tibiofemoral joint space narrowing.5 Despite vitamin D's theoretical ability to diminish hydrolysis of proteoglycan,1 2 3 a major constituent of the nucleus pulposus,7 deficiency was not associated with degeneration here. This may be attributed to the general acellularity, tenuous nutrient supply, and immunoprivilege of the nucleus, which likely renders it relatively insensitive to fluctuating s25D.
The annulus fibrosus is, in contrast, directly exposed to vitamin D metabolites in the cerebrospinal fluid and more vascularized than the nucleus.7 8 Having localized the vitamin D receptor within human annulus cells, Gruber et al observed dramatic downregulation of monocyte chemoattractant protein-1, interferon-γ, and interleukin (IL)-8 upon administration of 24,25- or 1,25-dihydroxyvitamin D, calcitriol.8 Each of these proinflammatory factors has been isolated from herniated disk tissue, and each enhances chemotaxis or activity of monocytes.8 In a human tumor necrosis factor (TNF) murine model of arthritis, vitamin D receptor knockout engendered a proinflammatory macrophage influx to the diseased joints, exacerbating cartilage erosion.24 Lack of vitamin D receptor signaling also augmented monocyte expression and serum levels of TNF and IL-1.24 Bolstering these results, calcitriol has been shown to attenuate TNF production by T cells from rheumatoid patients and mononuclear cell secretion of IL-1β and TNF,25 26 both of which have been widely implicated in disk disease pathogenesis.7 8 9
When chondrocyte-like cells of nondegenerated human disks are challenged with IL-1β, MMP-3, and collagenase (MMP-13) are upregulated.9 Aggrecan is simultaneously downregulated, representing an imbalance between catabolism and anabolism. In degenerated specimens, IL-1β amplifies its own expression, MMPs are upregulated, and the expression of aggrecan and collagen I and II decline. This IL-1β-induced phenotype can be partially reversed in human chondrocytes by transforming growth factor-β,27 the chondrocyte-specific effects of which are modulated by vitamin D.28 Although Le Maitre et al did not study herniated tissue, the generated metabolic shift is likely more detrimental to the annulus than nucleus.9 The former is predominantly collagenous, with a highly organized, lamellar architecture. We believe this catabolic cascade is a potential, biological etiology of the association demonstrated between deficiency and herniation, which by definition entails annular rupture. In turn, our observations may also add weight to previous genetic studies that have linked vitamin D receptor polymorphisms with lumbar disk disease and a vitamin D receptor phenotype signature to arthritis resistance.29 30
Despite their novelty, our results have inherent limitations. Foremost, the demonstrated associations are based on retrospectively collected data. A modest sample size was analyzed, and the numerous exclusion criteria hamper generalizability. The issue of selection or sampling bias must also be addressed. The analyzed cohort comprised a subset of the patient population of highly subspecialized spine surgeons at a single tertiary-referral, academic medical center. Thus, the prevalence of disk pathology was undoubtedly increased in comparison to that of the general population. Such prominent sampling bias not only further limits extrapolation of results, it also augments the likelihood of identifying statistically significant associations. It should be underscored, then, that the authors have made no calls for vitamin D supplementation to prevent herniation or screening of those with spinal pathology; such actions would be premature. Although supplementation is largely efficacious, it is not without risk (e.g., hypercalcemia and nephrolithiasis),11 and widespread screening is not without cost. Last, it is impossible to know whether herniation-related symptomatology, a potentially confounding factor, was adequately controlled for. Intuitively, debilitating spinal pathology limits sunlight exposure necessary for vitamin D biosynthesis.11 16
In a population-based, cross-sectional survey of 1,131 Canadian adults, the lifetime prevalence of neck and low back pain was 67% and 84%, respectively.13 31 In the United States, as few as 5% of patients who experience an episode of back pain incur ∼75% of over 100 billion dollars in total annual cost, with lost wages and surgery factoring prominently.14 Moreover, in recent years, Medicare recipients underwent increasingly complex surgery for lumbar stenosis, entailing heightened risk of short-term mortality and resource utilization.32 These statistics underscore a need to more fully elucidate the pathophysiology of intervertebral disk disease such that future physicians may be equipped to intervene before costly surgery is necessitated.
In summary, our study demonstrates that vitamin D deficiency was associated with cervical disk herniation in a population of adults undergoing spine surgery. Although the study design and sampling bias preclude inference of causality, the biological effects of vitamin D provide ample rationale for the associations drawn from these data. Considering the profound socioeconomic burden of disk disease and the ubiquity of hypovitaminosis D, longitudinal clinical studies and further basic science investigation are warranted to more clearly delineate the effects of vitamin D deficiency on the aging spine.
Funding
No funds were received in support of this work.
Footnotes
Disclosures None
References
- 1.Dean D D, Boyan B D, Muniz O E, Howell D S, Schwartz Z. Vitamin D metabolites regulate matrix vesicle metalloproteinase content in a cell maturation-dependent manner. Calcif Tissue Int. 1996;59:109–116. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz J P, Schwartz Z, Sylvia V L, Dean D D, Calderon F, Boyan B D. Vitamin D3 regulation of stromelysin-1 (MMP-3) in chondrocyte cultures is mediated by protein kinase C. J Cell Physiol. 1996;168:570–579. doi: 10.1002/(SICI)1097-4652(199609)168:3<570::AID-JCP9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Lane N E, Gore L R, Cummings S R. et al. Study of Osteoporotic Fractures Research Group . Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Arthritis Rheum. 1999;42:854–860. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.McAlindon T E, Felson D T, Zhang Y. et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ding C, Cicuttini F, Parameswaran V, Burgess J, Quinn S, Jones G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009;60:1381–1389. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 6.Chaganti R K, Parimi N, Cawthon P, Dam T L, Nevitt M C, Lane N E. Association of 25-hydroxyvitamin D with prevalent osteoarthritis of the hip in elderly men: the osteoporotic fractures in men study. Arthritis Rheum. 2010;62:511–514. doi: 10.1002/art.27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts S, Caterson B, Menage J, Evans E H, Jaffray D C, Eisenstein S M. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Gruber H E, Hoelscher G, Ingram J A, Chow Y, Loeffler B, Hanley E N Jr. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine. 2008;33:755–765. doi: 10.1097/BRS.0b013e3181695d59. [DOI] [PubMed] [Google Scholar]
- 9.Le Maitre C L, Freemont A J, Hoyland J A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarron R F, Wimpee M W, Hudkins P G, Laros G S. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine. 1987;12:760–764. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Holick M F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Deyo R A, Tsui-Wu Y J. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987;12:264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Côté P, Cassidy J D, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- 14.Katz J N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88 02:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 15.Louw J A, Werbeck A, Louw M E, Kotze T J, Cooper R, Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit Care Med. 1992;20:934–941. doi: 10.1097/00003246-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Welsh P, Peters M J, Sattar N. Is vitamin D in rheumatoid arthritis a magic bullet or a mirage? The need to improve the evidence base prior to calls for supplementation. Arthritis Rheum. 2011;63:1763–1769. doi: 10.1002/art.30341. [DOI] [PubMed] [Google Scholar]
- 17.Fardon D F Milette P C; Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology Spine 200126E93–E113. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki M, Hong S W, Yoon S H, Morishita Y, Wang J C. Reliability of a magnetic resonance imaging-based grading system for cervical intervertebral disc degeneration. J Spinal Disord Tech. 2008;21:288–292. doi: 10.1097/BSD.0b013e31813c0e59. [DOI] [PubMed] [Google Scholar]
- 19.Zook J, Djurasovic M, Crawford C III, Bratcher K, Glassman S, Carreon L. Inter- and intraobserver reliability in radiographic assessment of degenerative disk disease. Orthopedics. 2011;34:275. doi: 10.3928/01477447-20110228-07. [DOI] [PubMed] [Google Scholar]
- 20.Landis J R, Koch G G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 21.Bogunovic L, Kim A D, Beamer B S, Nguyen J, Lane J M. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92:2300–2304. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 23.Fairbank J CT, Pynsent P B. The Oswestry Disability Index. Spine. 2000;25:2940–2952, discussion 2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 24.Zwerina K, Baum W, Axmann R. et al. Vitamin D receptor regulates TNF-mediated arthritis. Ann Rheum Dis. 2011;70:1122–1129. doi: 10.1136/ard.2010.142331. [DOI] [PubMed] [Google Scholar]
- 25.Rausch-Fan X, Leutmezer F, Willheim M. et al. Regulation of cytokine production in human peripheral blood mononuclear cells and allergen-specific th cell clones by 1alpha,25-dihydroxyvitamin D3. Int Arch Allergy Immunol. 2002;128:33–41. doi: 10.1159/000058001. [DOI] [PubMed] [Google Scholar]
- 26.Colin E M, Asmawidjaja P S, van Hamburg J P. et al. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–142. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 27.Sandell L J, Xing X, Franz C, Davies S, Chang L W, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16:1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz Z, Bonewald L F, Caulfield K, Brooks B, Boyan B D. Direct effects of transforming growth factor-beta on chondrocytes are modulated by vitamin D metabolites in a cell maturation-specific manner. Endocrinology. 1993;132:1544–1552. doi: 10.1210/endo.132.4.8462452. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am. 2002;84-A:2022–2028. doi: 10.2106/00004623-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Brenner M, Linge C P, Li W, Gulko P S. Increased synovial expression of nuclear receptors correlates with protection in pristane-induced arthritis: a possible novel genetically regulated homeostatic mechanism. Arthritis Rheum. 2011;63:2918–2929. doi: 10.1002/art.30507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassidy J D, Carroll L J, Côté P. The Saskatchewan health and back pain survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine. 1998;23:1860–1866, discussion 1867. doi: 10.1097/00007632-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 32.Deyo R A, Mirza S K, Martin B I, Kreuter W, Goodman D C, Jarvik J G. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]


