Abstract
Degeneration of the intervertebral disk and its treatments are currently intensely investigated topics. Back pain is a condition whose chronic and debilitating nature combined with its prevalence make it a major health issue of substantial socioeconomic importance. Although researchers, and even sometimes clinicians, focus on the degenerated disk as the problem, to most patients, pain is the factor that limits their function and impacts their well-being. The purpose of this review is to delineate the changes associated with disk degeneration and to outline mechanisms by which they could be the source of back pain. Although the healthy disk is only innervated in the external layer of its annulus fibrosus, adjacent structures are plentiful with nociceptive receptors. Stimulation of such structures as a consequence of processes initiated by disk degeneration is explored. The concept of discogenic pain and possible mechanisms such as neoinnervation and solute transport are discussed. Finally, how such pain mechanisms may relate to current and proposed treatment strategies is discussed.
Keywords: intervertebral disk, degeneration, low back pain, nociception
Back pain remains one of the most common musculoskeletal ailments with a point prevalence of 12 to 30%.1 In 7 to 10% of patients, this can develop into chronic pain.2,3 Although there can be many different causes of low back pain, intervertebral disk (IVD) degeneration is generally accepted to be one of its major causes. There is a strong correlation of degeneration severity to pain,4 and many of the genetic influences on back pain are the same genetic influences affecting disk degeneration.5 Nevertheless, only a few mechanisms by which the degenerated disk induces pain are proven, and much still remains to be established. Perhaps because of this, our ability to diagnose it as the pain generator also remains elusive. In this review, the focus will be on degeneration-related mechanisms of pain, both indirect and direct as well as their implications for treatments.
Indirect Pain
With degeneration, there are many alterations to the IVD. Some of these changes may affect neighboring structures thus eliciting pain indirectly. Such mechanisms can be divided into those caused by anatomical or functional disk alterations (Fig. 1). Of the former, disk prolapse is most common, reported in almost 25% of asymptomatic individuals and increasing with age.6,7 When painful, the most common type of pain is radicular, caused by irritation of a spinal nerve or its root. This irritation may be caused by inflammation of the roots (most common in extruded or sequestered herniation) and/or compression of the root ganglion or its blood supply. In addition to pain in their extremities, some patients may also experience axial back pain, which may arise through various mechanisms. With extruded prolapse, there may be nerve root sleeve irritation stimulating nociceptive receptors in the dura. In contained prolapses, there may be stretching of the overlying posterior longitudinal ligament. However, it must be noted that prolapse rarely occurs without some degree of disk degeneration, which can be the generator of axial pain itself (see below). Another pain mechanism via compromised nerves due to anatomical changes of the degenerated disk is stenosis. With more severe degeneration, significant height loss and listhesis can occur, resulting in foraminal or central stenosis (i.e., compression of nerve root or spinal cord, respectively, by displaced bony structures). Such mechanisms may not always be obvious on imaging but may become more evident with certain body positions and movements.8,9
Fig. 1.
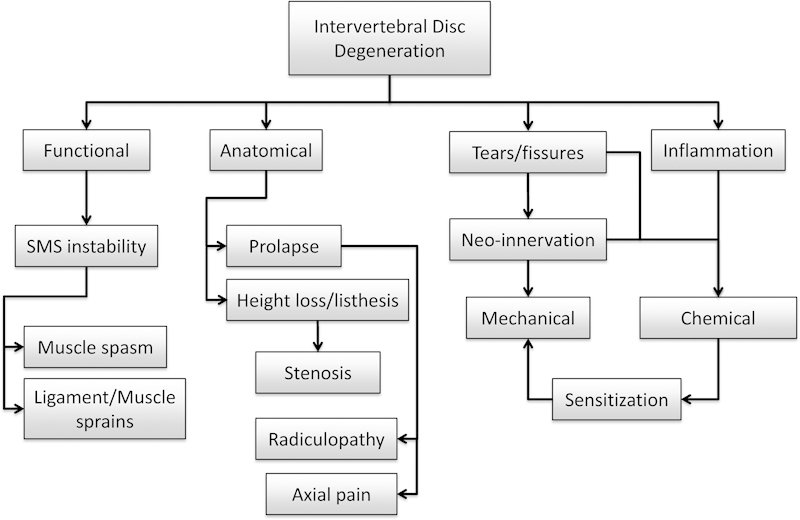
Schematic of pertinent changes to the intervertebral disk during degeneration and the possible biomechanical mechanisms of pain generated thereof. Abbreviation: SMS, spinal motion segment.
Most of the mechanisms of indirect pain arising from anatomical changes of a degenerating disk are clear, whereas those caused by functional alterations are less so. Kirkaldy-Willis and Farfan first proposed that in early or mild disk degeneration, the spinal motion segment (SMS) becomes less stable before further degenerative changes stabilize the segment with disease progression.10 They hypothesized that this instability of the SMS was a source for back pain. However, there are two aspects of this hypothesis, which remain controversial. Changes in SMS mechanical function with mild disk degeneration have been explored both ex vivo and in silico. Ex vivo, some studies confirmed the hypothesis,11,12,13 whereas others, including a comprehensive study with over 200 cadaveric motion segments, demonstrated the opposite (i.e., decreasing range of motion and/or increasing stiffness).14,15 Using a phenomenological finite element (FE) approach, the main characteristics of disk degeneration and their severity were combined to build a variety of motion segment models.16 Under simulated loading corresponding to daily activities, a tendency to increase stiffness with progressing overall degeneration was demonstrated. Similar results were also obtained using a motion segment FE model with a mechanistic-based disk model, which took into account the biochemical, collagen architectural, and disk height changes associated with early disk degeneration (Rijsbergen in preparation). But again, other FE models have exhibited contrasting destabilizing behavior.17 Nevertheless, even if early degenerative changes did lead to segment instability, how could it result in back pain? With a more lax or compliant interbody structure, the other passive and active spinal elements may be required to compensate. This could lead to ligament/muscle sprains or muscle spasms. Selective local analgesic administration has indicated that this may occur in a minority of patients, but controlled studies with independently correlated indicators of pain are lacking.
Finally, an alternative mechanism of pain may be disk degeneration-induced zygapophyseal joint degeneration. Morphological and functional changes to the disk may result in facet joint overloading and damage leading to progressive loss of cartilage and osteoarthritis. Although a causal relationship has not been demonstrated, disk degeneration and facet joint arthritis are associated both in the same patient and spinal level,18,19 and their relationship is currently a topic of debate.20
Direct Pain
Although causal evidence for many indirect back pain mechanisms in disk degeneration is still not plentiful, associative evidence exists and the postulated mechanisms are rather straightforward to comprehend. This is less so with direct mechanisms of discogenic pain (i.e., pain believed to arise from the disk itself, as a result of disk degeneration).
Innervation and Nociception in the IVD
Innervation of the lumbar intervertebral disk arises from two nerve plexi that accompany the anterior and posterior longitudinal ligaments. The anterior plexus is composed of nerves derived from the sympathetic trunk and gray rami communicans and supplies the anterior and lateral annulus fibrosus (AF). The posterior plexus supplies the posterior and lateral aspects of the AF and derives its nerves from the sinuvertebral nerves, formed by both somatic and autonomic roots. This plexus also receives branches from multiple levels, which perhaps is the reason that axial back pain may be so difficult to assign to a specific level. In addition to the disk, this plexus also innervates the posterior longitudinal ligament, the dural sac, and the posterior aspect of the vertebra (also see article by Hiyama et al in this issue).21,22
In the healthy disk, nerve fibers are only found in the outer few lamellae penetrating up to approximately 3 mm into the AF.23,24,25,26,27 The inner AF and the central nucleus pulposus (NP) are not innervated.23,24,25,28 This is perhaps because nerves require accompanying blood vessels for nutrition and the inner AF and NP is virtually avascular due to: (1) collapse of vessels from interstitial hydrostatic pressures greater than systolic blood pressure,29 and (2) vascular ingrowth inhibition (as well as nerve ingrowth) by an extracellular matrix rich in proteoglycans containing glycosaminoglycans.30,31 The nerve fibers that are found in the peripheral AF are small in diameter (1 to 3 μm) and contain substance P (a nociceptive neurotransmitter),27,28,32 consistent with myelinated Aδ fibers that transmit sharp pain and unmyelinated C-type fibers that transmit dull pain.32,33 The fibers may end in various types of mechanoreceptors. The most commonly found in the IVD are consistent with the morphology of encapsulated Ruffini endings and Golgi tendon organs. Some unencapsulated Pacini corpuscles and abundant free nerve endings have also been reported.24,34 Free terminals were most often reported within the AF, but partial and fully encapsulated mechanoreceptors were confined to the AF surface.23 Whereas Golgi tendon organs, Ruffini endings, and Pacinian corpuscles are believed to be active in proprioception, free nerve endings are believed to be active in nociception.24 In addition to the disk itself, the vertebral end plate, particularly the central portion overlying the NP, is well innervated, similar to the outer AF (also see article by Lotz et al, in this issue).35,36
Disk Degeneration
In early disk degeneration, there are subtle changes to the matrix of the NP and inner AF.37,38 This is believed to be a result of a shift in the balance of anabolic and catabolic activities from maintenance to that of more proteolytic activity.39,40 Furthermore, there is evidence to support that an inflammatory process mediates this matrix breakdown.41,42 It has been demonstrated that disk cells are capable of producing proinflammatory cytokines such as interleukin (IL)-1,41 IL-4, IL-6,43 IL-8,44,45 IL-12, IL-17, interferon-γ,43 and tumor necrosis factor-α (TNFα).46,47,48 These themselves can be nociceptive triggers or mimic noxious effects,49,50 but also downstream of these signaling molecules, other agents such as nitric oxide (NO), leukotrienes, and prostaglandin E are powerful direct nociceptive stimuli.45,51 Additionally, by-products of disk cell metabolism such as lactic acid may also be noxious (for a more complete discussion of nociceptive signaling, see article by Hiyama et al, in this issue).52,53
One possible mechanism of discogenic pain generation is that such noxious stimuli would reach the nociceptive receptors in the outer AF and osseous end plates. These solutes range in size from very small (e.g., NO) to those on the order of several hundred (e.g., prostaglandins) and tens of thousands of daltons for cytokines. In vitro measurements have shown that solutes of limited size (~400 Da) have a diffusivity of ~60 μm2/s.54,55,56,57 In a computational model, it was calculated that with one diurnal cycle, these solutes could be transported from the NP center to the end plates by diffusion alone.58 Thus, such solutes could freely diffuse from their production source within the disk to nociceptive receptors in the external AF and end plates. In addition, inner and, to a lesser extent, outer AF cells have been shown to produce proinflammatory cytokines IL-1 and TNFα,48 of which particularly the latter cytokine has been shown to be directly involved in pain signaling by sensitization.59 Although few studies have directly compared cytokine and inflammatory mediator expression between symptomatic and nonsymptomatic degenerated disks, comparison of symptomatic degenerated to symptomatic herniated disks suggests that similar mediators may be responsible for pain generation in both conditions.44 However, only TNFα production has been shown to be higher and more strongly correlated to degeneration in disks from symptomatic compared with nonsymptomatic patients with similar degrees of degeneration.47 Moreover, the concentrations of NP-derived factors reaching the end plate or outer annulus by diffusion would be quite low, in particular for generally larger cytokines, and the temporal stimulation pattern quite constant, which is not always consistent with the nature of axial back pain (but could be responsible for sensitization; see below). Thus, although plausible, the direct role of preinflammatory cytokines and mediators in degenerated disk pain generation remains mechanistically unproven.
With moderate to advanced disk degeneration, there is general fibrosis of the NP and disorganization of the AF.37,60 The AF also becomes stiffer and weaker with age.61 Associated with aging and degeneration, various forms of AF tears develop.37 Similar to engineering crack propagation, these are probably the result of coalescing of micro-clefts and -tears that developed earlier. Eventually these AF tears may reach the external AF, with or without actual prolapse. In addition to AF tears, the end plate can also become compromised through cracks and fissures. This is true for both the osseous and cartilaginous end plates.60 In a study of human osseous end plate opening sizes, it was shown that the number of larger openings, corresponding to cracks and fissures, increases with degeneration grade,62 and this was also reflected by increased osseous end plate permeability with advanced degeneration,63 as well as increased diffusion through the end plate64 (for a more detailed review of the end plate mechanisms of pain generation, see the article by Lotz et al, in this issue). Thus, with such structural defects, the transport of noxious stimuli and nociceptive modulators from within the disk to nociceptive receptors in the AF periphery and end plates would be enhanced.
However, these structural defects also have another mechanism to generate discogenic pain. Unlike healthy disks, degenerated disks can be innervated, even in their central NP.33,65,66 These nerve fibers are positive for substance P and have nerve-ending morphologies consistent with nociception. They are also more common in painful degenerated disks. They are often accompanied by vasculature and seem to propagate along cracks and fissures. It is hypothesized that these cracks/fissures create an environment that is favorable to neoinnervation and angiogenesis. Biomechanically, the crack/fissure relieves the stresses within the surrounding disk matrix and the pressure within the crack is substantially reduced. The inner surface of cracks/fissures is reported to be depleted of proteoglycans,67 leaving the remaining collagen, the surface of which is conducive to cell adhesion and chemotaxis. Also because the AF does have the capacity to heal small tears, reactive granulation tissue, which is inductive for angiogenesis and neoinnervation, has been reported in radial fissures.66 Thus, without the factors that are believed to inhibit nerve ingrowth in healthy disks, these crack/fissures may allow innervation in degenerated disk.
Although the clinical efficacy of diskography and its potential complications are a topic of intense debate, the behavior of positively painful disks and their classification are quite illustrative for understanding pain generation in degenerated disks.68 If there is immediate onset of pain when contrast media reaches the outer AF or the injection pressure is still below 100 kPa over the opening pressure, the disk can be classified as “chemically” sensitive. If concordant pain is provoked at pressures between 100 and 350 kPa above opening pressure, the disk can be classified as “mechanically” sensitive.
Mechanically sensitized disk are believed to be painful because they exceed the tissue stretch threshold for nociception. This may be due to stretch at the outer AF or end plates, but also internally in neoinnervated disks. In intact disks, the exact amount of strain in the AF is controversial. FE models have predicted strains as high as 20 to 50%,69,70 but in vitro measurements only as high as 8%,71 which is more in line with the elongation at failure of 4 to 13% reported for individual AF collagen fiber bundles.72,73 Such lower AF strains are believed to be below the threshold required to illicit pain and AF collagen failure. However, with AF or end plate structural failure, strains may be greater, similar to when posterior elements are removed (18% strain),71 and thus sufficient to stimulate pain. Although NP compression within degenerated disks is drastically lower due to loss of proteoglycans and fibrosis,74 particularly high gradients of stress have been reported internally within the AF with degeneration.75 With ingrowth of nerves into the AF, these gradients may directly stimulate pain.
Chemically sensitized disks are believed to be painful either because of direct stimulation of nociceptive receptors (either at AF periphery or ingrown) by chemically noxious stimuli (produced by inflammatory processes during degeneration) transported by flow of contrast medium injected during diskography or because of sensitization by chronic exposure to such stimuli. In the latter, in response to nociceptive stimulation by degeneration products, the somatosensory system increases its sensitivity resulting in an amplified response to normally innocuous stimuli. Hence, lower concentrations of such noxious degeneration products or lower levels of tissue strain (e.g., by low diskography pressures) that would not normally result in pain generation have now, through this process, become painful.
It should be noted that this classification of pain generation in degenerated disks is purely theoretical, and most likely, pain mechanisms are not one or the other, but rather mixed in some form. However, the combination of neoinnervation within the disk via cracks/fissures, nociceptive stimuli produced during degeneration, and the correlation of discogenic pain with degenerated disks exhibiting AF tears and end plate lesions supports the presented mechanisms.76,77
Implication for Treatments
Many of the current surgical treatments used for discogenic pain (e.g., fusion and total disk replacement) are based on the commonly accepted rationale that the degenerating disk is the source of pain, and thus the disk should be removed. Although these treatments are reported to offer some pain relief, their effectiveness is still debated, and they are not unequivocally recommended. They are also not without substantial and significant complications (e.g., adjacent segment disease)78 or are of limited longevity. Furthermore, their less than desirable efficacy may be attributable to deficiencies in accurately diagnosing the specific mechanism of back pain. For example, in chemically sensitized disks, posterolateral fusion alone may not be successful because the sensitized disk (i.e., the source of pain) remains.
Various treatments have also been developed based on the mechanisms of discogenic pain, some of which have been outlined in this review. In some cohort studies, intradiscal electrothermal therapy was reported to have success rates as high as 75%.79,80 The mechanism of action was believed to be a sort of thermal annealing of AF damage as well as thermal disruption of nerve fibers. However, ex vivo studies did not demonstrate such morphological changes,81 and later blinded, randomized placebo-controlled trials showed little benefit.82,83 Intradiscal steroid injections were developed to suppress the inflammatory processes in disk degeneration and thus to inhibit the production of noxious agents that would chemically sensitize the degenerating disk. However, three studies did not show any prolonged pain relief,84,85,86 but interestingly, there was significant short-term pain relief for those patients with documented subchondral end plate and vertebral marrow changes consistent with inflammation (i.e., type I Modic changes; also see article by Lotz et al, in this issue). Intradiscal radiofrequency thermocoagulation or intradiscal biacuplasty is another procedure to ablate nerves within the AF. This technique was shown to indeed increase temperatures in a significant portion of the AF.87 However, randomized controlled trials did not show substantial benefits for treatment of axial back pain.88,89 Finally, two striking but unreplicated recent reports have been about the use of methylene blue for the treatment of discogenic pain. Methylene blue is a neurolytic agent that can block nerve conduction or destroy nerve endings. It also is an inhibitor of NO production. Hence, it was introduced into the disk to devitalize the nerves, which had grown in along the AF cracks/fissures.90 In a double-blind randomized placebo-controlled trial, incredible long-term improvement in pain was reported. In these patients, 19% reported complete loss of pain and another 72% reported only slight pain that no longer required medication.91 Furthermore, the rate of complications was small, although the leakage of such a neurotoxic agent near the spinal cord remains an understandable concern. Since then, only a small series study was reported that did not corroborate these findings.92 Although the jury is still out on the true efficacy of this treatment, the general approach to treat discogenic pain based on a mechanistic approach is certainly worthy of further investigation.
Interestingly, there has been much excitement and research on biological methods to regenerate degenerated disks, showing promising results in vivo of several growth factors such as osteogenesis protein 1 and growth and differentiating factor 5 and small molecules such as simvastatin.93,94,95,96 However, these studies all concerned acute models of disk degeneration and may not reflect the actual conditions of the human degenerated disk that are very likely to affect regeneration. In addition, pain has never been an outcome parameter in these studies. We must keep in mind that it will simply not be enough to restore some of the matrix and biomechanical function of the disk. We will also need to stop the inflammatory processes, repair internal disruptions, and restore the lack of innervation within the disk. Only then will disk regeneration become an effective treatment for discogenic low back pain.
Acknowledgments
The authors received some financial research support by: the project P2.01 IDiDAS of the research program of the BioMedical Materials institute, cofunded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation; the European Community's Seventh Framework Program (FP7, 2007-2013) under grant agreement no. HEALTH-F2-2008-201626 (GENODISC); the project no. SRN 2011_11 from AOSpine International; and the Dutch Arthritis Foundation.
Footnotes
Disclosures Keita Ito, None Laura Creemers, None
References
- 1.Anema JR, Schellart AJM, Cassidy JD, Loisel P, Veerman TJ, van der Beek AJ. Can cross country differences in return-to-work after chronic occupational back pain be explained? An exploratory analysis on disability policies in a six country cohort study. J Occup Rehabil. 2009;19:419–426. doi: 10.1007/s10926-009-9202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker A, Held H, Redaelli M. et al. Implementation of a guideline for low back pain management in primary care: a cost-effectiveness analysis. Spine (Phila Pa 1976) 2012;37:701–710. doi: 10.1097/BRS.0b013e31822b01bd. [DOI] [PubMed] [Google Scholar]
- 3.Von Korff M. Studying the natural history of back pain. Spine (Phila Pa 1976) 1994;19(18, Suppl):2041S–2046S. doi: 10.1097/00007632-199409151-00005. [DOI] [PubMed] [Google Scholar]
- 4.Cheung KM, Karppinen J, Chan D. et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 5.Battié MC, Videman T, Levalahti E, Gill K, Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131:272–280. doi: 10.1016/j.pain.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 7.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 8.Blankenbaker DG, Haughton VM, Rogers BP, Meyerand ME, Fine JP. Axial rotation of the lumbar spinal motion segments correlated with concordant pain on discography: a preliminary study. AJR Am J Roentgenol. 2006;186:795–799. doi: 10.2214/AJR.04.1629. [DOI] [PubMed] [Google Scholar]
- 9.Karadimas EJ, Siddiqui M, Smith FW, Wardlaw D. Positional MRI changes in supine versus sitting postures in patients with degenerative lumbar spine. J Spinal Disord Tech. 2006;19:495–500. doi: 10.1097/01.bsd.0000211213.98070.c2. [DOI] [PubMed] [Google Scholar]
- 10.Kirkaldy-Willis WH, Farfan HF. Instability of the lumbar spine. Clin Orthop Relat Res. 1982;(165):110–123. [PubMed] [Google Scholar]
- 11.Fujiwara A, Lim TH, An HS. et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000;25:3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Krismer M, Haid C, Ogon M, Behensky H, Wimmer C. [Biomechanics of lumbar instability] Orthopade. 1997;26:516–520. doi: 10.1007/PL00003406. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka N, An HS, Lim TH, Fujiwara A, Jeon CH, Haughton VM. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1:47–56. doi: 10.1016/s1529-9430(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 14.Kettler A, Rohlmann F, Ring C, Mack C, Wilke H-J. Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. Eur Spine J. 2011;20:578–584. doi: 10.1007/s00586-010-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976) 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Galbusera F, Schmidt H, Neidlinger-Wilke C, Gottschalk A, Wilke H-J. The mechanical response of the lumbar spine to different combinations of disc degenerative changes investigated using randomized poroelastic finite element models. Eur Spine J. 2011;20:563–571. doi: 10.1007/s00586-010-1586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohlmann A, Zander T, Schmidt H, Wilke H-J, Bergmann G. Analysis of the influence of disc degeneration on the mechanical behaviour of a lumbar motion segment using the finite element method. J Biomech. 2006;39:2484–2490. doi: 10.1016/j.jbiomech.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Gries NC, Berlemann U, Moore RJ, Vernon-Roberts B. Early histologic changes in lower lumbar discs and facet joints and their correlation. Eur Spine J. 2000;9:23–29. doi: 10.1007/s005860050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum. 2007;37:69–80. doi: 10.1016/j.semarthrit.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Suri P, Miyakoshi A, Hunter DJ. et al. Does lumbar spinal degeneration begin with the anterior structures? A study of the observed epidemiology in a community-based population. BMC Musculoskelet Disord. 2011;12:202. doi: 10.1186/1471-2474-12-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282–296. doi: 10.1002/aja.1001880307. [DOI] [PubMed] [Google Scholar]
- 22.Bogduk N. Oxford, UK: Elsevier Health Sciences; 2005. Clinical Anatomy of the Lumbar Spine and Sacrum. [Google Scholar]
- 23.Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication) Acta Anat (Basel) 1959;38:96–113. [PubMed] [Google Scholar]
- 24.Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976) 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Fagan AB, Sarvestani G, Moore RJ, Fraser RD, Vernon-Roberts B, Blumbergs PC. Innervation of anulus tears: an experimental animal study. Spine (Phila Pa 1976) 2010;35:1200–1205. doi: 10.1097/BRS.0b013e3181c02812. [DOI] [PubMed] [Google Scholar]
- 26.Palmgren TT, Grönblad MM, Virri JJ, Kääpä EE, Karaharju EE. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976) 1999;24:2075–2079. doi: 10.1097/00007632-199910150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ashton IK, Roberts S, Jaffray DC, Polak JM, Eisenstein SM. Neuropeptides in the human intervertebral disc. J Orthop Res. 1994;12:186–192. doi: 10.1002/jor.1100120206. [DOI] [PubMed] [Google Scholar]
- 28.Konttinen YT, Grönblad M, Antti-Poika I. et al. Neuroimmunohistochemical analysis of peridiscal nociceptive neural elements. Spine (Phila Pa 1976) 1990;15:383–386. doi: 10.1097/00007632-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 1999;24:755–762. doi: 10.1097/00007632-199904150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Johnson WEB, Caterson B, Eisenstein SM, Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine (Phila Pa 1976) 2005;30:1139–1147. doi: 10.1097/01.brs.0000162624.95262.73. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WEB, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 32.Cavanaugh JM, Kallakuri S, Ozaktay AC. Innervation of the rabbit lumbar intervertebral disc and posterior longitudinal ligament. Spine (Phila Pa 1976) 1995;20:2080–2085. doi: 10.1097/00007632-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy PW, Carruthers B, Martin D, Petts P. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine (Phila Pa 1976) 1991;16:653–655. doi: 10.1097/00007632-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Dimitroulias A, Tsonidis C, Natsis K. et al. An immunohistochemical study of mechanoreceptors in lumbar spine intervertebral discs. J Clin Neurosci. 2010;17:742–745. doi: 10.1016/j.jocn.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Brown MF, Hukkanen MV, McCarthy ID. et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- 36.Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976) 2003;28:2570–2576. doi: 10.1097/01.BRS.0000096942.29660.B1. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Antoniou J, Steffen T, Nelson F. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 40.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 41.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuertz K Vo N Kletsas D Boos N Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases Eur Cell Mater 201223103–119., discussion 119-120 [DOI] [PubMed] [Google Scholar]
- 43.Shamji MF, Setton LA, Jarvis W. et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 45.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Evans CH. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1995;20:2373–2378. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 46.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine (Phila Pa 1976) 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 47.Weiler C Nerlich AG Bachmeier BE Boos N Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls Spine (Phila Pa 1976) 20053044–53., discussion 54 [DOI] [PubMed] [Google Scholar]
- 48.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine (Phila Pa 1976) 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 50.Aoki Y, Rydevik B, Kikuchi S, Olmarker K. Local application of disc-related cytokines on spinal nerve roots. Spine (Phila Pa 1976) 2002;27:1614–1617. doi: 10.1097/00007632-200208010-00004. [DOI] [PubMed] [Google Scholar]
- 51.Brisby H, Byröd G, Olmarker K, Miller VM, Aoki Y, Rydevik B. Nitric oxide as a mediator of nucleus pulposus-induced effects on spinal nerve roots. J Orthop Res. 2000;18:815–820. doi: 10.1002/jor.1100180520. [DOI] [PubMed] [Google Scholar]
- 52.Keshari KR, Lotz JC, Link TM, Hu S, Majumdar S, Kurhanewicz J. Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine (Phila Pa 1976) 2008;33:312–317. doi: 10.1097/BRS.0b013e31816201c3. [DOI] [PubMed] [Google Scholar]
- 53.Hwang SW, Oh U. Current concepts of nociception: nociceptive molecular sensors in sensory neurons. Curr Opin Anaesthesiol. 2007;20:427–434. doi: 10.1097/ACO.0b013e3282eff91c. [DOI] [PubMed] [Google Scholar]
- 54.Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10:365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torzilli PA, Adams TC, Mis RJ. Transient solute diffusion in articular cartilage. J Biomech. 1987;20:203–214. doi: 10.1016/0021-9290(87)90311-3. [DOI] [PubMed] [Google Scholar]
- 56.Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine (Phila Pa 1976) 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 57.Quinn TM, Morel V, Meister JJ. Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech. 2001;34:1463–1469. doi: 10.1016/s0021-9290(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37:213–221. doi: 10.1016/s0021-9290(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 59.Leung L, Cahill CM. TNF-alpha and neuropathic pain—a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 61.Ebara S, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1996;21:452–461. doi: 10.1097/00007632-199602150-00009. [DOI] [PubMed] [Google Scholar]
- 62.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976) 2005;30:167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez AG, Slichter CK, Acosta FL. et al. Human disc nucleus properties and vertebral endplate permeability. Spine (Phila Pa 1976) 2011;36:512–520. doi: 10.1097/BRS.0b013e3181f72b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine (Phila Pa 1976) 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 65.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 66.Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87:62–67. [PubMed] [Google Scholar]
- 67.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 68.Derby R Howard MW Grant JM Lettice JJ Van Peteghem PK Ryan DP The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes Spine (Phila Pa 1976) 199924364–371., discussion 371-372 [DOI] [PubMed] [Google Scholar]
- 69.Iatridis JC, MaClean JJ, Ryan DA. Mechanical damage to the intervertebral disc annulus fibrosus subjected to tensile loading. J Biomech. 2005;38:557–565. doi: 10.1016/j.jbiomech.2004.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt H, Kettler A, Heuer F, Simon U, Claes L, Wilke HJ. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine (Phila Pa 1976) 2007;32:748–755. doi: 10.1097/01.brs.0000259059.90430.c2. [DOI] [PubMed] [Google Scholar]
- 71.Heuer F, Schmidt H, Wilke HJ. The relation between intervertebral disc bulging and annular fiber associated strains for simple and complex loading. J Biomech. 2008;41:1086–1094. doi: 10.1016/j.jbiomech.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Skaggs DL, Weidenbaum M, Iatridis JC, Ratcliffe A, Mow VC. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1994;19:1310–1319. doi: 10.1097/00007632-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Holzapfel GA, Schulze-Bauer CAJ, Feigl G, Regitnig P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005;3:125–140. doi: 10.1007/s10237-004-0053-8. [DOI] [PubMed] [Google Scholar]
- 74.Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976) 1999;24:2468–2474. doi: 10.1097/00007632-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 75.Adams MA, McNally DS, Dolan P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 76.Videman T, Nurminen M. The occurrence of anular tears and their relation to lifetime back pain history: a cadaveric study using barium sulfate discography. Spine (Phila Pa 1976) 2004;29:2668–2676. doi: 10.1097/01.brs.0000146461.27105.2b. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Videman T, Battié MC. ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine (Phila Pa 1976) 2012;37:1490–1496. doi: 10.1097/BRS.0b013e3182608ac4. [DOI] [PubMed] [Google Scholar]
- 78.Harrop JS, Youssef JA, Maltenfort M. et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701–1707. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 79.Assietti R, Morosi M, Migliaccio G, Meani L, Block JE. Treatment of discogenic low back pain with Intradiscal Electrothermal Therapy (IDET): 24 months follow-up in 50 consecutive patients. Acta Neurochir Suppl (Wien) 2011;108:103–105. doi: 10.1007/978-3-211-99370-5_15. [DOI] [PubMed] [Google Scholar]
- 80.Maurer P, Block JE, Squillante D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. J Spinal Disord Tech. 2008;21:55–62. doi: 10.1097/BSD.0b013e31812f4f29. [DOI] [PubMed] [Google Scholar]
- 81.Kleinstueck FS Diederich CJ Nau WH et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine Spine (Phila Pa 1976) 2003281700–1708., discussion 1709 [DOI] [PubMed] [Google Scholar]
- 82.Pauza KJ, Howell S, Dreyfuss P, Peloza JH, Dawson K, Bogduk N. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27–35. doi: 10.1016/j.spinee.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 83.Freeman BJC Fraser RD Cain CMJ Hall DJ Chapple DCL A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain Spine 2005302369–2377., discussion 2378 [DOI] [PubMed] [Google Scholar]
- 84.Simmons JW, McMillin JN, Emery SF, Kimmich SJ. Intradiscal steroids. A prospective double-blind clinical trial. Spine (Phila Pa 1976) 1992;17(6, Suppl):S172–S175. [PubMed] [Google Scholar]
- 85.Buttermann GR. The effect of spinal steroid injections for degenerative disc disease. Spine J. 2004;4:495–505. doi: 10.1016/j.spinee.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 86.Khot A Bowditch M Powell J Sharp D The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial Spine (Phila Pa 1976) 200429833–836., discussion 837 [DOI] [PubMed] [Google Scholar]
- 87.Petersohn JD, Conquergood LR, Leung M. Acute histologic effects and thermal distribution profile of disc biacuplasty using a novel water-cooled bipolar electrode system in an in vivo porcine model. Pain Med. 2008;9:26–32. doi: 10.1111/j.1526-4637.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 88.Kvarstein G, Måwe L, Indahl A. et al. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy—a 12-month follow-up. Pain. 2009;145:279–286. doi: 10.1016/j.pain.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Kapural L, Vrooman B, Sarwar S. et al. A randomized, placebo-controlled trial of transdiscal radiofrequency, biacuplasty for treatment of discogenic lower back pain. Pain Med. 2013;14:362–373. doi: 10.1111/pme.12023. [DOI] [PubMed] [Google Scholar]
- 90.Peng B, Zhang Y, Hou S, Wu W, Fu X. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33–38. doi: 10.1007/s00586-006-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peng B, Pang X, Wu Y, Zhao C, Song X. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010;149:124–129. doi: 10.1016/j.pain.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 92.Gupta GG, Radhakrishna MM, Chankowsky JJ, Asenjo JFJ. Methylene blue in the treatment of discogenic low back pain. Pain Physician. 2012;15:333–338. [PubMed] [Google Scholar]
- 93.Masuda K, Imai Y, Okuma M. et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976) 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 94.Imai Y Miyamoto K An HS Thonar EJ-MA Andersson GBJ Masuda K Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells Spine (Phila Pa 1976) 2007321303–1309., discussion 1310 [DOI] [PubMed] [Google Scholar]
- 95.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976) 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, Wang L, Park JB. et al. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res Ther. 2009;11:R172. doi: 10.1186/ar2861. [DOI] [PMC free article] [PubMed] [Google Scholar]


