Abstract
Although degeneration of the intervertebral disk has historically been described as a misbalance between anabolic and catabolic factors, the role of inflammatory mediators has long been neglected. However, past research clearly indicates that inflammatory mediators such as interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor-α are expressed at higher levels in “diseased” intervertebral disks. Both disk cells as well as invading macrophages can be the source of the detected cytokines. Importantly, occurrence of inflammatory mediators in the disk can worsen the progress of degeneration by inducing the expression of matrix degrading enzymes as well as by inhibiting extracellular matrix synthesis. In addition, inflammatory mediators play a crucial role in pain development during intervertebral disk herniation (i.e., sciatica) and disk degeneration (i.e., discogenic pain). This review provides information on the most relevant inflammatory mediators during different types of disk diseases and explains how these factors can induce disk degeneration and the development of discogenic and sciatic/radiculopathic pain.
Keywords: inflammatory mediators, intervertebral disk degeneration, degenerative disk disease, discogenic pain, cytokines, innervation
The annulus fibrosus (AF) of a mature lumbar intervertebral disk is rich in collagen type I and contains up to 25 lamellae, with the collagen fibers aligned parallel to one another traversing between adjacent vertebrae at an angle of ~60 degrees to the axis of the spine. This lamellar arrangement is ideal for resisting the tensile forces induced by both bending and twisting of the spine.1 In contrast to the highly organized AF, the nucleus pulposus (NP) is rich in collagen type II fibers that are arranged in a random fashion to entrap the highly anionic proteoglycan, aggrecan, which confers the swelling properties important for resistance to compression.2 The high proteoglycan content of the NP is thought to be a major factor in preventing nerve ingrowth into the disk, which is largely aneural and avascular in a healthy young spine.3
Disk degeneration, which starts in the young adult and progresses with advancing age,4,5 has been proposed to be due to a decrease in nutrition as a result of progressive mineralization of the cartilage end plate, through which the majority of nutrients gain access to the intervertebral disk. During degeneration, phenotypic changes of the resident cells result in reduced proteoglycan synthesis as well as a switch in collagen synthesis with less collagen type II and more collagen type I and III and an increase in matrix metalloproteinase (MMP) synthesis/activity.6,7 Degradation of the extracellular matrix and proteoglycan loss from the NP result in a decrease in weight-bearing capacity and in a loss of disk height. In the final stages of disk degradation, fissures appear in the annular ring, allowing extrusion of the NP and generation of pain due to compression of nerves.8 However, pain can also occur in the early stages of disk degeneration and in the absence of nerve compression. This so-called discogenic pain (or painful degenerative disk disease) seems to be related to the occurrence of inflammatory mediators.
The importance of inflammatory mediators in pain development has been demonstrated by clinical studies as well as by laboratory investigation.9 The expression of inflammatory mediators in the degenerated/herniated disks as well as their involvement in the process of disk degeneration (i.e., in the induction of catabolic processes) has been investigated in detail during the past decade. It was shown that intervertebral disk cells, and also monocytes or macrophages that invade the degenerated disk (i.e., CD68-positive cells), can secrete cytokines.10,11 Identification of the relevance of these mediators in the development of discogenic back pain has led to an improved understanding of the molecular mechanisms of back pain, thus creating new possibilities of more targeted therapeutic intervention.
Expression and Regulation of Inflammatory Mediators in the Intervertebral Disk
Different methods have been used in the past to identify the expression and regulation of candidate inflammatory mediators in the intervertebral disk. To identify how degeneration or disk-related disease influences the level of inflammation, gene and protein expression analysis in healthy and degenerated/diseased tissue has been performed. Clearly, the highest level of relevance is obtained with human disk tissue. However, performing comparative analyses in humans is hindered by difficulties in:
Discriminating between disk degeneration and normal processes of aging
Distinguishing between symptomatic and asymptotic degenerated disks
Creating identical groups (with regard to age, level and degree of degeneration, disease)
Distinguishing between NP and AF in degenerated disks (clinical material)
Obtaining healthy control tissue
Therefore, animal models with a high level of controllability are useful to identify candidates and mechanisms of inflammation, thus gaining knowledge that can then be verified in human disk tissue/cells.
Expression of Inflammatory Mediators: Animal Models
In several studies, the expression of inflammatory mediators has been compared between healthy and degenerated disks, using specific degeneration models in rabbits or rats.12,13,14 Historically, research has focused primarily on interleukin (IL)-1β and tumor necrosis factor (TNF)-α, which were the first inflammatory mediators described in the disk, but additional candidates such as IL-6 and IL-8 are now coming into focus.
A typical method of inducing disk degeneration in vivo is stab incision (either through the bony end plate or through the AF) in rabbits, rats or pigs, which has been shown to induce expression of IL-1, IL-8, IL-10, or TNF-α,13,14,15 with higher and more prolonged effects after repeated injury.13
Apart from in vivo models, experiments on the role of inflammatory mediators during disk degeneration have also been performed in whole-organ culture models. End plate fracture due to excessive loading in rabbit disks as well as injurious mechanical loading of bovine disks induces degenerative processes that are characterized by an inflammatory component (TNF-α, IL-1β, IL-6, IL-8, monocyte chemotactic protein-1).16,17
In addition, in vivo models that simulate radiculopathic pain by placing autologous NP material on the dorsal root ganglion have been developed and extensively used over the past years (see Inflammatory Mediators and Nerve Fibers—Discogenic Pain).
Expression of Inflammatory Mediators: Human Models
Changes in the expression of inflammatory mediators with degeneration, aging, or the occurrence of certain diseases (e.g., herniation) were investigated in the past. However, analysis of human disks is often hampered by the appropriate selection of tissue. Furthermore, it has to be noted that detection of increased levels of a certain mediator does not allow for conclusive statements with regard to its relevance in vivo (e.g., with regard to pain development). Importantly, most studies on human biopsies from patients with disk degeneration without herniation (i.e., painful degenerative disk disease) did not analyze follow-up pain reduction in the operated patient (i.e., it is unclear whether the removed and analyzed tissue was indeed the pain source). In the future, these aspects will need to be considered if inhibition of intervertebral disk inflammation should be used as a therapeutic option.
The primary approach of identifying key players during inflammatory disk disease is to analyze gene or protein expression of disk tissues with different pathologies, either from autopsies or biopsies. Summarizing existing studies is rendered challenging as multiple different pathology comparisons have been undertaken. However, research activities of the past clearly demonstrate increased levels of IL-1β, IL-8, and TNF-α during various pathologies, indicating that these three factors may play an essential role during disk degeneration and discogenic pain (Table 1).
Table 1. Expression of inflammatory mediators in human intervertebral disk tissue.
| Mediator | A/B | Diseasea | Method | Comparison | NP/AF | Reference |
|---|---|---|---|---|---|---|
| IL-1β↑ | A + B A + B A + B A + B B |
DDD + H DDD DDD + H H H |
PCR, IHC PCR, IHC FC IHC PCR |
Degree of DD Degree of DD DDD/H vs. control H vs. control Degree of DD |
NP + AF NP + AF Disk Disk NP |
18 19 20 21 31 |
| IL-2↑ | A + B | DDD + H | FC | DDD/H vs. control | Disk | 20 |
| IL-4↑ | A + B | DDD + H | FC | DDD/H vs. control | Disk | 20 |
| IL-6↑ | B B B |
H + S + T H DDD + H |
E E E |
H vs. S/T Levels of cytokines DDD vs. H |
Disk Disk NP |
12,13 26 33 |
| IL-8↑ | B B B B B |
DDD H H DDD + H H + S |
WB PCR E E E |
DDD vs. H Levels of cytokines Levels of cytokines DDD vs. H H vs. S |
NP Disk CSF NP NP |
32 34 27 33 25 |
| IL-17↑ | A + B | DDD/H | IHC | DDD/H vs. control | NP + AF | 11 |
| Interferon-γ↑ | B | DDD + S | E | DDD vs. S | Disk | 28 |
| Leukotriene ↑ | B | H | E | Types of H | Disk | 35 |
| Monocyte chemotactic protein-1↑ | B B |
H H + S |
E E |
Levels of cytokines H vs. S |
Disk NP |
26 25 |
| NO↑ | B | H + S/T | E | H vs. S/T | Disk | 12,13 |
| Prostaglandin 2↑ | B B |
H H |
E E |
H vs. control Types of H |
Disk Disk |
12,13 36 |
| TNF-α↑ | A + B A + B B A + B B A + B A + B B |
DDD + H DDD + H DDD DDD + H H DDD/H DDD H |
PCR, IHC PCR, IHC WB IHC PCR FC IHC PCR |
Degree of DD Age + Degree of DD DDD vs. H Age + Symptoms Levels of cytokines DDD/H vs. control DDD vs. control Degree of DD |
NP + AF NP + AF NP NP + AF Disk Disk AF NP |
18 29 32 30 34 20 22 31 |
| Thromboxane ↑ | B | H | E | Types of H | Disk | 35 |
Abbreviations: A, autopsy; AF, annulus fibrosus; B, biopsy; CSF, cerebrospinal fluid; DD, disk degeneration; DDD, degenerative disk disease (i.e., discogenic pain); E, enzyme-linked immunosorbent assay; FC, flow cytometry; H, herniation/sequestration; IHC, immunohistochemistry; IL, interleukin; NO, nitric oxide; NP, nucleus pulposus; PCR, polymerase chain reaction; S, scoliosis; T, trauma; TNF-α, tumor necrosis factor-α; WB, Western blot.
Disease in case of biopsy.
Degeneration/Herniation Versus Controls
In degenerated/herniated disks, IL-1β and TNF-α expression is elevated,18,19,20,21 with IL-1β expression being more elevated than TNF-α expression.18,19 In addition, levels of IL-2, IL-4, IL-10, IL-12, and IL-17 are increased in degenerated/herniated samples relative to healthy controls.11,20 Interestingly, high-intensity zones in the AF, which can be detected by T2-weighted magnetic resonance imaging in painful degenerated disks, contain higher levels of TNF-α than surrounding AF tissue or control AF tissue.22
Instead of analyzing the expression of inflammatory mediators in the tissue, it is also possible to analyze their secretion upon placement of tissue into culture medium. Using this approach, Kang et al were able to demonstrate that herniated lumbar and cervical disks release higher amounts of NO, prostaglandin 2, and IL-6 than scoliotic/traumatic control disks (IL-1α, IL-1β, and TNF-α were under the detection limit).23,24 Similarly, herniated disks released more IL-8 and monocyte chemotactic protein-1 than scoliotic disks.25 Furthermore, herniated disks show high levels of IL-6 and IL-8 compared with other inflammatory mediators, as demonstrated by diskographic lavage of herniated disks or enzyme-linked immunosorbent assay analysis of cerebrospinal fluid.26,27 Furthermore, diskographic lavage indicated that disks with painful degenerative disk disease are characterized by high levels of interferon-γ compared with scoliotic control disks.28
Process of Aging/Degeneration
TNF-α and IL-1β mRNA and protein levels have also been shown to be upregulated with increasing age/degeneration, with higher levels in the NP compared with the AF as well as higher levels in symptomatic versus asymptomatic disks.29,30,31
Herniation Versus Painful Degenerative Disk Disease
When comparing NP material from disk herniation to painful degenerative disk disease, protein expression of TNF-α and IL-8 is increased in the degenerative disk disease group, and both groups had similar levels of IL-1β, IL-6, and IL-12.32 Therefore, TNF-α and IL-8 might be promising candidates to treat patients with discogenic back pain on a molecular level (e.g., with inhibitors or receptor antagonists). In a similar study, Burke et al found that cultured biopsies (mostly NP material) from patients suffering from painful degenerative disk disease release higher levels of IL-6 and IL-8 than biopsies from patients with sciatica/herniation.33 In fact, IL-8 has not only been shown to be highly expressed in herniated disks (IL-8 > TNF-α >> IL-1α and IL-10), but also seems to be associated with the development of short-term radicular pain.34
Contained Herniation Versus Noncontained Herniation
Noncontained herniated disks possess higher levels of leukotriene B4 and thromboxane B2 than contained herniated disks.35 Furthermore, the level of prostaglandin 2 is highest in sequestered disks, followed by extruded and finally protruded disks.36
Regulation of Inflammatory Mediators
As demonstrated previously, the degree of degeneration clearly influences the levels of inflammatory mediators in the intervertebral disk. The exact mechanism is unclear, but it may be related to the accumulation of specific matrix fragmentation products.37 However, multiple other internal and external cues can influence disk inflammation (e.g., heredity, inflammation, mechanical loading, oxygenation, or the presence of other cell types).
Heredity
There is growing evidence that demonstrates a strong involvement of heredity in the development of disk degeneration (e.g., due to polymorphisms in the genes encoding disk-typical extracellular matrix proteins such as collagen I, collagen IX, collagen XI, or aggrecan).38,39,40 However, recent studies also demonstrate that single nucleotide polymorphisms of IL-1β as well as of the IL-1 receptor antagonist may play a role in lumbar disk herniation and degeneration.41,42,43 Furthermore, the importance of the IL-1A gene in the pathophysiology of Modic changes and disk signal intensity on magnetic resonance imaging has been demonstrated.44,45 IL-6 as well as IL-10 single nucleotide polymorphisms also seem to be involved in the development of disk degeneration and disk disease-related sciatica (for IL-6).46,47,48
Inflammation
Apart from heredity, one of the major internal cues controlling inflammation is the occurrence of inflammatory mediators themselves. Use of various in vitro models has clearly demonstrated that exposure of intervertebral disk cells to inflammatory cytokines cause upregulation of multiple inflammatory mediators. Treatment of disk cells with IL-1α or IL-1β leads to an increase in mRNA and/or protein expression of inducible nitric oxide synthase (iNOS)/nitric oxide, IL-1β, IL-6, IL-8, IL-20, prostaglandin 2, and TNF-α12,49,50,51,52,53,54,55,56,57 in cells from different species (human,50,51,52,53,54,55,56,57 porcine,53 bovine,49 rabbit12). Similarly, exposing disk cells to TNF-α induced expression of IL-1β, IL-6, nitric oxide, prostaglandin 2, and TNF-α,56,58,59 and IL-17 treatment induced nitric oxide and prostaglandin 2 synthesis.59 Studer et al stimulated human NP cells with IL-6 and found an increase in prostaglandin 2 synthesis and cyclooxygenase-2 mRNA expression, which was even stronger if cotreatment with IL-1β or TNF-α was performed.60 The cytokine IL-20 also induces expression of IL-1β, IL-6, and monocyte chemotactic protein-1 in isolated human disk cells.57 Importantly, the response of disk cells to the inflammatory environment seems to be determined by the cellular localization (NP, AF) as well as by the degree of degeneration.12,54,55
Mechanical Loading
The effects of mechanical loading on intervertebral disk anabolism and catabolism have been investigated over the past decade.61,62 However, only few studies investigated the role of mechanical loading on disk inflammation. Application of asymmetric wedge compression on bovine caudal disks resulted in an upregulation of IL-1β and IL-6 mRNA expression, which coincided with a shift toward catabolism.17 Excessive torsional loading in an in vivo rat tail model resulted in a slight increase of IL-1β and TNF-α mRNA expression in the AF.63 In a rabbit disk explant model, Dudli et al were able to demonstrate that end plate fracture due to mechanical loading (but not the load impact itself) results in an increase in mRNA expression of TNF-α, IL-6, IL-8, and monocyte chemotactic protein-1 early after the event.16 Nitric oxide production was increased after application of high hydrostatic pressure in NP/AF cells or after exposure of AF cells to 5% cyclic tensile strain.64,65 Although the above-mentioned studies applied mechanical loading in a noninflammatory environment, Miyamoto et al and Sowa et al applied cyclic strain under inflammatory conditions.12,66 When cyclic strain was applied to rat NP and AF cells, mechanical loading alone resulted in an increase in prostaglandin 2, which was synergistically enhanced under inflammatory conditions. In contrast, tensile stress (6%; 0.05 Hz) protected rabbit AF cells from the detrimental effects of IL-1β stimulation.67 Importantly, the beneficial effect of cyclic strain on AF cells isolated from healthy tissue (e.g., reduction in iNOS) is lost in cells from degenerative disks.12 These, findings illustrate that type, magnitude, frequency, and duration as well as health status of the tissue determine whether the applied mechanical load has inflammatory or anti-inflammatory/protective effects.
Oxygenation
Variations in oxygen levels have long been known to influence disk metabolism.7 However, little is known about the effect of oxygen on disk inflammation. It has been demonstrated recently that exposure of disk cells (isolated from human degenerated tissue) to hyperbaric oxygen (100% O2; 2.5 atm) reduced levels of IL-1β and nitric oxide, possibly by inhibiting the mitogen-activated protein kinase (MAPK) pathway.68 Comparable results were found in a rodent in vivo study, with reduced levels of IL-1β, prostaglandin 2, and iNOS upon hyperbaric oxygen treatment.69 On the other hand, oxidative/nitrosative stress (i.e., application of peroxynitrite) increased expression of IL-1β, IL-6, and IL-8 due to nuclear translocation of nuclear factor kappa-B (NF-κB).70
Exposure to Macrophages and Notochordal Cells
Macrophages have been shown to infiltrate degenerated intervertebral disks.71 Disk cell-macrophage interaction leads to increased levels of IL-6, IL-8, iNOS, and prostaglandin 2,72,73,74,75,76,77,78 but contradictory results were found for TNF-α and IL-1β, with some studies showing increased expression upon coculture,12,13 and others showed no alteration and generally low levels.75 Interestingly, notochordal cells or notochordal cell-conditioned medium seems to protect AF cells from increased inflammation upon exposure to macrophages (THP-1 cells)76 or after stimulation with IL-1β.79
Inflammatory Mediators and Proteinases—Disk Degeneration
Proteases are responsible for fragmentation and breakdown of important components of the extracellular matrix, including aggrecan and collagen in the degenerating disk, but they also have significant roles in normal remodeling of the disk.80,81,82 Matrix metalloproteinases (MMP1, -2, -3, -7, -9, -13), aggrecanases/a Disintegrin and Metalloproteinase with Thrombospondin Motifs (ADAMTS4, -5), and cathepsins (cathepsins K, D, and L) are all upregulated in the disk during degeneration.83,84,85 Aggrecan proteolysis by MMPs, however, has been shown to be mainly a process of normal turnover in the disk, in which cathepsin K also seems to have a significant role.86 HtrA1(High Temperature Requirement Factor A1) is a serine protease that plays a central role in the pathology of arthritic diseases such as osteoarthritis,87 but also participates in disk degeneration, during which its levels are elevated.88
Inflammatory cytokines are known to regulate protease production and the direct effects of specific cytokines have been studied in 2-D and 3-D cultures of isolated NP and AF cells. Treatment of disk cells with IL-1β increased expression of MMP1, MMP2, MMP3, MMP9, MMP13, syndecan 4, iNOS, ADAMTS4, and ADAMTS5 and decreased levels of aggrecan, collagen type I, collagen type II, SOX6, and SOX9,19,50,51,52,56,89,90,91,92 with generally higher effects in NP cells than AF cells.19 IL-1α treatment was also shown to increase protein levels of MMP1 and MMP3 in human NP cells.53
Similar to IL-1β, exposure of isolated disk cells or entire motion segments to TNF-α increased expression of MMP1, MMP3, MMP9, MMP13, ADAMTS4, and ADAMTS556,93,94 (probably via the NF-κB and MAPK pathway95) and decreased expression and synthesis of aggrecan and collagen type II.93 Compared with IL-1β treatment, effects of TNF-α on MMP3 and MMP9 were less pronounced.56 MMP2 activity was stimulated in bovine NP cultures, and no changes on gene and protein expression levels were noted.96
Exposure to IL-6 increased MMP3, decreased collagen and aggrecan expression, and inhibited proteoglycan synthesis, and this effect was potentiated by IL-1β and/or TNF-α.60
Based on the described studies, inflammatory mediators clearly influence catabolism in the disk, primarily via alteration in (1) the expression and activity of MMPs and ADAMTs and (2) proteoglycan synthesis and degradation. However, intervertebral disk catabolism can also be influenced by mechanical loading, with effects depending on the magnitude, frequency, and duration of the applied load.61 Unphysiologic mechanical stress (i.e., static or hyperphysiologic loading) is not only able to induce catabolic effects but also can cause programmed cell death in disk cells.97 An important interplay seems to exist between various environmental factors in the disk as it has been most recently described that inflammatory mediators such as nitric oxide and IL-1β are also able to negatively influence cell viability, especially if other limiting factors such as serum deprivation are applied.98,99,100,101,102
Inflammatory Mediators and Nerve Fibers—Discogenic Pain
Innervation of the Intervertebral Disk
Under normal conditions, the terminals of both sympathetic and sensory nerves, including a subset of nociceptive fibers that sense pain, penetrate only the outer third of the AF as illustrated in Fig. 1103,104 The sensory nerve fibers originate from the dorsal root ganglion of the spine.105,106 It has been proposed that cells in degenerating disks can synthesize and release “neurogenic” factors that attract a subset of peptidergic small neurons into the disk.107 Freemont and colleagues have reported that deep nerve growth into the inner third of human lumbar disks was present in 57% of disks collected from patients with chronic low back pain compared with 25% of disks harvested postmortem from subjects without a history of back pain.108 Some of the fibers penetrating deep into disk tissue were identified as immunopositive for the neuropeptide substance P, which is commonly expressed by small unmyelinated nociceptive nerve fibers involved in pain sensation. The nerve fibers in the healthy disk display the same histologic profile as the ones found in painful disks, indicating that the difference is not due to the type of nerve fibers innervating a painful disk but rather to the pattern and quantity of the innervating nerves. Painful disks have a higher number and density of nerve fibers penetrating deep into the disk (Fig. 1).107 Radial fissures, proteoglycan loss, and reduced pressure in the NP are often seen in innervated disks.3,109,110 Given the proposed role of proteoglycans as a barrier to vascular and neural ingrowth into the NP, it has been proposed that excessive metalloproteinase activity and matrix degradation is a stimulus for vascular and neural ingrowth into the disk.3 The increased penetration of nerve fibers in degenerated disks could also result from elevated expression of nerve growth factor, which is upregulated in disk cells by proinflammatory cytokines such as TNF-α and IL-1β.108,111,112,113 Richardson et al have demonstrated that cells from degenerated disks express factors stimulating neurite outgrowth, from an SH-SY5Y neuroblastoma cell line. Furthermore, addition of anti-nerve growth factor resulted in decreased neurite expressing cells, and addition of anti-brain-derived neurotrophic factor resulted in both decreased neurite length and fewer neurite-expressing cells.114 As another trigger of nerve growth factor production is ischemia in the cardiovascular system (reviewed by Nico et al115), it is reasonable to speculate that the hostile environment in the degenerated disk with low oxygen and glucose levels alone could induce nerve growth factor production, which in the presence of cytokines would be further enhanced (Fig. 1).116 To date, little is known about the link between mechanical forces and the release of chemical mediators, but it is speculated that adverse mechanical forces could result in cytokine and nerve growth factor production, recruiting and activating nociceptive fibers deep in intervertebral disks and thus generating “discogenic pain.”
Fig. 1.
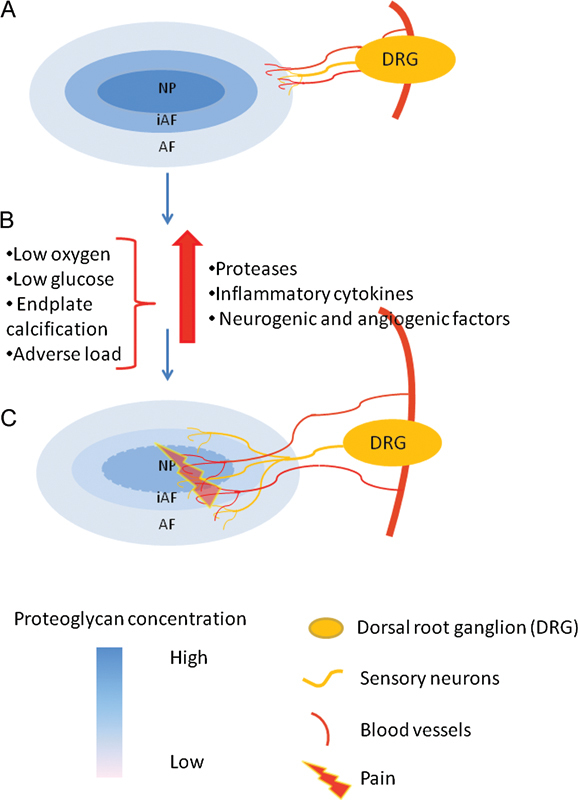
Schematic representation of innervations and vascularization pattern in healthy and degenerate intervertebral disk. (A) Nerve fibers and blood vessels penetrate only the outer third of the AF in healthy disks with a high proteoglycan content. (B) Low oxygen levels, low glucose concentrations, end plate calcification, and adverse load contribute to an increased production of proteases, cytokines, and neurogenic and angiogenic factors resulting in matrix degradation and attraction of nerve fibers and blood vessels. (C) Nerve fibers and blood vessels penetrate deep into the degenerated disk as a result of the low proteoglycan content and the increased production of angiogenic and neurogenic mediators. Abbreviations: AF, annulus fibrosus; DRG, dorsal root ganglion; iAF, inner annulus fibrosus; NP, nucleus pulposus.
Vascularization
Nerves and blood vessels often comigrate into the disk. Nerve growth factor affects not only nerve cells but also can function as a chemotactic agent for endothelia cells resulting in neovascularization, reviewed by Nico et al.115 The endothelia cells of the ingrowing blood vessels express both proteinases and nerve growth factor. In addition, disk cells exposed to TNF-α and IL-1β respond with increased expression of vascular endothelial growth factor, nerve growth factor, and brain-derived neurotrophic factor, which may further promote innervations. Nerve growth factor functions in concert with vascular endothelial growth factor and shares the Ras/ERK and P13K/Akt intracellular signaling pathways affecting survival and proliferation of nerve and endothelial cells. Santos et al suggest that nerve growth factor might be involved in capillary sprouting by inducing vascular endothelial growth factor, which would promote endothelial cell growth, remodeling of the extracellular matrix, and functional maturation of new forming vessels.117
Animal Models of Pain
The effects of disk herniation and consequent induction of pain can be simulated by placing autologous NP material on the dorsal root ganglion. Originally, this model has been used to demonstrate the inflammatory properties of the NP.118,119 In the years thereafter, researchers used this model to identify inflammatory mediators that induce radiculopathic pain, either in a direct or an indirect approach. Using a direct approach, Cuéllar et al performed epidural lavage between 3 and 24 hours after inducing herniation and found an increase in epidural IL-6, TNF-α, and interferon-γ.120 In addition, several studies investigated pain behavior (e.g., via von Frey filament testing) after application of inflammatory mediators expected to induce pain behavior upon contact with dorsal root ganglions and compared this with effects seen after application of autologous NP. In this context, TNF-α proved to induce pain behavior similar to NP treatment,121,122 and application of antibodies to IL-1β, TNF-α, or high mobility group protein B1 as well as application of NF-κB decoy (i.e., NP + treatment) reduced pain behavior (compared with NP alone).123,124,125
Conclusion
The term discogenic low back pain was coined more than 30 years ago to describe pain associated with disk degeneration and still today represents an enormous socioeconomic cost to the health care systems in developed countries. Despite the burden of disease, there are no effective diagnostic tools to identify the early degenerative changes in intervertebral disks that result in pain. It is clear from the literature that disk cells in vitro have the ability to release cytokines and neurogenic and angiogenic factors if they are exposed to an inflammatory milieu. It is also well described that proteases, cytokines, and neurogenic and angiogenic factors along with nerve fibers and blood vessels are present in human degenerated disks and that cytokines as well as NP matrix applied to dorsal root ganglions induce pain in rodents. However, the early events leading up to degeneration and pain are not well understood. It is to date unclear what initiates the production of cytokines and pain mediators in vivo. Nutrient deprivation due to end plate calcification is one suggested factor. A few reports indicate that altered mechanical loading of the disks result in protease production, which by the generation of matrix fragments activating Toll-like receptors could result in cytokine production, but this still needs to be proven. There is also some evidence that adverse load by itself induces production of cytokines, along with pain and angiogenic mediators. In addition to biological and biochemical factors, social and psychological factors are known to play an important role in the generation and perception of pain, presenting an additional challenge for the treating physician in selecting patients who will respond to treatment. It is important to focus future research on the initial events leading to degeneration and pain. This will facilitate the development of biomarkers and allow for more specific therapeutic interventions preventing degradation of the disk matrix and relieving pain, thus avoiding invasive and often dangerous surgical procedures. It will also aid in distinguishing between the contribution of psychosocial and biochemical factors, allowing relevant intervention to be offered and decreasing the risk for failed treatment.
Funding
This work was supported by grants from the Swiss National Science Foundation (310030_130813 / 45244402), AOSpine (SRN 02/103 & SRN_2011_04_104981), as well as by CIHR MOP-119564.
Footnotes
Disclosures Karin Wuertz, Research Support: AOSpine, SNF Lisbet Haglund, Research Support: AOSpine, CIHR
References
- 1.Hukins D, Meakin J R. Relationship between structure and mechanical function of the tissues of the intervertebral joint. Amer Zool. 2000;40:42–52. [Google Scholar]
- 2.Urban J P. [Role of mechanical constraints in the maintenance and degradation of articular cartilage] Rev Prat. 2000;50(16)(, Suppl):9–12. [PubMed] [Google Scholar]
- 3.Johnson W E, Caterson B, Eisenstein S M, Hynds D L, Snow D M, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 4.Roughley P J. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou J, Steffen T, Nelson F. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan W C Sze K L Samartzis D Leung V Y Chan D Structure and biology of the intervertebral disk in health and disease Orthop Clin North Am 201142447–464., vii [DOI] [PubMed] [Google Scholar]
- 7.Urban J P, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt K F, Nerlich A G. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Beaudreuil J, Dieude P, Poiraudeau S, Revel M. Disabling chronic low back pain with Modic type 1 MRI signal: acute reduction in pain with intradiscal corticotherapy. Ann Phys Rehabil Med. 2012;55:139–147. doi: 10.1016/j.rehab.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo Y, Uchida K, Kobayashi S. et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008;9:285–295. doi: 10.3171/SPI/2008/9/9/285. [DOI] [PubMed] [Google Scholar]
- 11.Shamji M F, Setton L A, Jarvis W. et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowa G A, Coelho J P, Vo N V, Pacek C, Westrick E, Kang J D. Cells from degenerative intervertebral discs demonstrate unfavorable responses to mechanical and inflammatory stimuli: a pilot study. Am J Phys Med Rehabil. 2012;91:846–855. doi: 10.1097/PHM.0b013e31825f145a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich J A, Liebenberg E C, Thuillier D U, Lotz J C. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–2819. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau M A, Ulrich J A, Bass E C, Rodriguez A G, Liu J J, Lotz J C. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976) 2007;32:17–24. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 15.Holm S, Mackiewicz Z, Holm A K. et al. Pro-inflammatory, pleiotropic, and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental porcine intervertebral disk degeneration. Vet Pathol. 2009;46:1292–1300. doi: 10.1354/vp.07-VP-0179-K-FL. [DOI] [PubMed] [Google Scholar]
- 16.Dudli S, Haschtmann D, Ferguson S J. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res. 2012;30:809–816. doi: 10.1002/jor.21573. [DOI] [PubMed] [Google Scholar]
- 17.Walter B A, Korecki C L, Purmessur D, Roughley P J, Michalek A J, Iatridis J C. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011;19:1011–1018. doi: 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Maitre C L, Hoyland J A, Freemont A J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Maitre C L, Freemont A J, Hoyland J A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akyol S, Eraslan B S, Etyemez H, Tanriverdi T, Hanci M. Catabolic cytokine expressions in patients with degenerative disc disease. Turk Neurosurg. 2010;20:492–499. doi: 10.5137/1019-5149.JTN.3394-10.1. [DOI] [PubMed] [Google Scholar]
- 21.Grönblad M, Virri J, Tolonen J. et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine (Phila Pa 1976) 1994;19:2744–2751. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Dongfeng R, Hou S, Wu W. et al. The expression of tumor necrosis factor-α and CD68 in high-intensity zone of lumbar intervertebral disc on magnetic resonance image in the patients with low back pain. Spine (Phila Pa 1976) 2011;36:E429–E433. doi: 10.1097/BRS.0b013e3181dfce9e. [DOI] [PubMed] [Google Scholar]
- 23.Kang J D, Georgescu H I, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson W F III, Evans C H. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kang J D, Georgescu H I, McIntyre-Larkin L, Stefanovic-Racic M, Evans C H. Herniated cervical intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 1995;20:2373–2378. doi: 10.1097/00007632-199511001-00001. [DOI] [PubMed] [Google Scholar]
- 25.Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2002;27:1402–1407. doi: 10.1097/00007632-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Gajendran V K, Reuter M W, Golish S R, Hanna L S, Scuderi G J. Is the fibronectin-aggrecan complex present in cervical disk disease? PM R. 2011;3:1030–1034. doi: 10.1016/j.pmrj.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11:62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuellar J M, Golish S R, Reuter M W. et al. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 2010;10:212–218. doi: 10.1016/j.spinee.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Bachmeier B E, Nerlich A G, Weiler C, Paesold G, Jochum M, Boos N. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- 30.Weiler C Nerlich A G Bachmeier B E Boos N Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls Spine (Phila Pa 1976) 20053044–53., discussion 54 [DOI] [PubMed] [Google Scholar]
- 31.Park J Y, Kuh S U, Park H S, Kim K S. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. J Spinal Disord Tech. 2011;24:352–357. doi: 10.1097/BSD.0b013e3181fee4df. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Moon C S, Sul D. et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem. 2009;42:1504–1511. doi: 10.1016/j.clinbiochem.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 34.Ahn S H, Cho Y W, Ahn M W, Jang S H, Sohn Y K, Kim H S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976) 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Nygaard O P, Mellgren S I, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine (Phila Pa 1976) 1997;22:2484–2488. doi: 10.1097/00007632-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell J L O'Donnell A L Prostaglandin E2 content in herniated lumbar disc disease Spine (Phila Pa 1976) 1996211653–1655., discussion 1655-1656 [DOI] [PubMed] [Google Scholar]
- 37.Quero L Leonardi M Nerlich A Boos N Wuertz K Fragmentation of hyaluronic acid stimulates expression of proinflammatory cytokines and matrix degrading enzymes in human intervertebral disc cells Paper presented at: International Society for the Study of the Lumbar Spine; April 13-17, 2010; Auckland, New Zealand
- 38.Kalb S, Martirosyan N L, Kalani M Y, Broc G G, Theodore N. Genetics of the degenerated intervertebral disc. World Neurosurg. 2012;77:491–501. doi: 10.1016/j.wneu.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Eyre D R, Matsui Y, Wu J J. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30(Pt 6):844–848. doi: 10.1042/bst0300844. [DOI] [PubMed] [Google Scholar]
- 40.Kao P Y Chan D Samartzis D Sham P C Song Y Q Genetics of lumbar disk degeneration: technology, study designs, and risk factors Orthop Clin North Am 201142479–486., vii [DOI] [PubMed] [Google Scholar]
- 41.Paz Aparicio J, Fernández Bances I, López-Anglada Fernández E. et al. The IL-1β (+3953 T/C) gene polymorphism associates to symptomatic lumbar disc herniation. Eur Spine J. 2011;20 03:383–389. doi: 10.1007/s00586-011-1915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D H, Lee S H, Kim K T, Yu S D. Association of interleukin-1 receptor antagonist gene polymorphism with response to conservative treatment of lumbar herniated nucleus pulposus. Spine (Phila Pa 1976) 2010;35:1527–1531. doi: 10.1097/BRS.0b013e3181e4efb6. [DOI] [PubMed] [Google Scholar]
- 43.Solovieva S, Kouhia S, Leino-Arjas P. et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626–633. doi: 10.1097/01.ede.0000135179.04563.35. [DOI] [PubMed] [Google Scholar]
- 44.Karppinen J, Solovieva S, Luoma K, Raininko R, Leino-Arjas P, Riihimäki H. Modic changes and interleukin 1 gene locus polymorphisms in occupational cohort of middle-aged men. Eur Spine J. 2009;18:1963–1970. doi: 10.1007/s00586-009-1139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Videman T, Saarela J, Kaprio J. et al. Associations of 25 structural, degradative, and inflammatory candidate genes with lumbar disc desiccation, bulging, and height narrowing. Arthritis Rheum. 2009;60:470–481. doi: 10.1002/art.24268. [DOI] [PubMed] [Google Scholar]
- 46.Kelempisioti A, Eskola P J, Okuloff A. et al. Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC Med Genet. 2011;12:153. doi: 10.1186/1471-2350-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin W P, Lin J H, Chen X W. et al. Interleukin-10 promoter polymorphisms associated with susceptibility to lumbar disc degeneration in a Chinese cohort. Genet Mol Res. 2011;10:1719–1727. doi: 10.4238/vol10-3gmr1283. [DOI] [PubMed] [Google Scholar]
- 48.Noponen-Hietala N, Virtanen I, Karttunen R. et al. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 49.Smith L J, Chiaro J A, Nerurkar N L. et al. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cell Mater. 2011;22:291–301. doi: 10.22203/ecm.v022a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wuertz K, Quero L, Sekiguchi M. et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine (Phila Pa 1976) 2011;36:E1373–E1384. doi: 10.1097/BRS.0b013e318221e655. [DOI] [PubMed] [Google Scholar]
- 51.Klawitter M, Quero L, Klasen J. et al. Triptolide exhibits anti-inflammatory, anti-catabolic as well as anabolic effects and suppresses TLR expression and MAPK activity in IL-1β treated human intervertebral disc cells. Eur Spine J. 2012;21 06:S850–S859. doi: 10.1007/s00586-011-1919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klawitter M, Quero L, Klasen J. et al. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm (Lond) 2012;9:29. doi: 10.1186/1476-9255-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buser Z, Liu J, Thorne K J, Coughlin D, Lotz J C. Inflammatory response of intervertebral disc cells is reduced by fibrin sealant scaffold in vitro. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1503. [DOI] [PubMed] [Google Scholar]
- 54.Kang J D, Stefanovic-Racic M, McIntyre L A, Georgescu H I, Evans C H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 55.LeMaitre C L Williamson B Ross R Freemont A J Hoyland J A Response of human intervertebral disc cells to IL-1 Paper presented at: The Society for Back Pain Research, 2003
- 56.Millward-Sadler S J, Costello P W, Freemont A J, Hoyland J A. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11:R65. doi: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu Y H, Li H H, Hsieh M Y. et al. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54:2722–2733. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- 58.Sinclair S M, Shamji M F, Chen J. et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 2011;36:1190–1196. doi: 10.1097/BRS.0b013e3181ebdb43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabr M A, Jing L, Helbling A R. et al. Interleukin-17 synergizes with IFNγ or TNFα to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J Orthop Res. 2011;29:1–7. doi: 10.1002/jor.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Studer R K, Vo N, Sowa G, Ondeck C, Kang J. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-α. Spine (Phila Pa 1976) 2011;36:593–599. doi: 10.1097/BRS.0b013e3181da38d5. [DOI] [PubMed] [Google Scholar]
- 61.Chan S C, Ferguson S J, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011;20:1796–1812. doi: 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Setton L A, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88 02:52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 63.Barbir A, Godburn K E, Michalek A J, Lai A, Monsey R D, Iatridis J C. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine (Phila Pa 1976) 2011;36:607–614. doi: 10.1097/BRS.0b013e3181d9b58b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu G Z, Ishihara H, Osada R, Kimura T, Tsuji H. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976) 2001;26:134–141. doi: 10.1097/00007632-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 65.Rannou F, Richette P, Benallaoua M. et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90:148–157. doi: 10.1002/jcb.10608. [DOI] [PubMed] [Google Scholar]
- 66.Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine (Phila Pa 1976) 2006;31:4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- 67.Sowa G, Agarwal S. Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil. 2008;87:537–544. doi: 10.1097/PHM.0b013e31816197ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu C C, Lin S S, Yuan L J. et al. Hyperbaric oxygen treatment suppresses MAPK signaling and mitochondrial apoptotic pathway in degenerated human intervertebral disc cells. J Orthop Res. 2013;31:204–209. doi: 10.1002/jor.22209. [DOI] [PubMed] [Google Scholar]
- 69.Wang I C, Liu H T, Yu C M. et al. Effect of hyperbaric oxygenation on intervertebral disc degeneration: an in vivo study with Sprague-Dawley rats. Spine (Phila Pa 1976) 2013;38:E137–E142. doi: 10.1097/BRS.0b013e31827bf6bf. [DOI] [PubMed] [Google Scholar]
- 70.Poveda L, Hottiger M, Boos N, Wuertz K. Peroxynitrite induces gene expression in intervertebral disc cells. Spine (Phila Pa 1976) 2009;34:1127–1133. doi: 10.1097/BRS.0b013e31819f2330. [DOI] [PubMed] [Google Scholar]
- 71.Peng B, Hao J, Hou S. et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976) 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 72.Takada T, Nishida K, Maeno K. et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 2012;64:2601–2610. doi: 10.1002/art.34456. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto J, Maeno K, Takada T. et al. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J Orthop Res. 2013;31:608–615. doi: 10.1002/jor.22274. [DOI] [PubMed] [Google Scholar]
- 74.Takada T Nishida K Doita M Miyamoto H Kurosaka M Interleukin-6 production is upregulated by interaction between disc tissue and macrophages Spine (Phila Pa 1976) 2004291089–1092., discussion 1093 [DOI] [PubMed] [Google Scholar]
- 75.Hamamoto H, Miyamoto H, Doita M, Takada T, Nishida K, Kurosaka M. Capability of nondegenerated and degenerated discs in producing inflammatory agents with or without macrophage interaction. Spine (Phila Pa 1976) 2012;37:161–167. doi: 10.1097/BRS.0b013e31821a874b. [DOI] [PubMed] [Google Scholar]
- 76.Kim J H, Moon H J, Lee J H, Kim J H, Kwon T H, Park Y K. Rabbit notochordal cells modulate the expression of inflammatory mediators by human annulus fibrosus cells cocultured with activated macrophage-like THP-1 cells. Spine (Phila Pa 1976) 2012;37:1856–1864. doi: 10.1097/BRS.0b013e3182579434. [DOI] [PubMed] [Google Scholar]
- 77.Kim J H, Studer R K, Vo N V, Sowa G A, Kang J D. p38 MAPK inhibition selectively mitigates inflammatory mediators and VEGF production in AF cells co-cultured with activated macrophage-like THP-1 cells. Osteoarthritis Cartilage. 2009;17:1662–1669. doi: 10.1016/j.joca.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Kim J H, Studer R K, Sowa G A, Vo N V, Kang J D. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976) 2008;33:2253–2259. doi: 10.1097/BRS.0b013e318182c35f. [DOI] [PubMed] [Google Scholar]
- 79.Moon H J, Joe H, Kwon T H, Choi H K, Park Y K, Kim J H. Notochordal cells influence gene expression of inflammatory mediators of annulus fibrosus cells in proinflammatory cytokines stimulation. J Korean Neurosurg Soc. 2010;48:1–7. doi: 10.3340/jkns.2010.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Maitre C L, Pockert A, Buttle D J, Freemont A J, Hoyland J A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 81.Struglics A, Hansson M. MMP proteolysis of the human extracellular matrix protein aggrecan is mainly a process of normal turnover. Biochem J. 2012;446:213–223. doi: 10.1042/BJ20120274. [DOI] [PubMed] [Google Scholar]
- 82.Feng H, Danfelter M, Strömqvist B, Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88 02:25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 83.Bachmeier B E, Nerlich A, Mittermaier N. et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18:1573–1586. doi: 10.1007/s00586-009-1031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberts S, Caterson B, Menage J, Evans E H, Jaffray D C, Eisenstein S M. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 85.Ariga K, Yonenobu K, Nakase T. et al. Localization of cathepsins D, K, and L in degenerated human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2666–2672. doi: 10.1097/00007632-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 86.Gruber H E, Ingram J A, Hoelscher G L, Zinchenko N, Norton H J, Hanley E N Jr. Constitutive expression of cathepsin K in the human intervertebral disc: new insight into disc extracellular matrix remodeling via cathepsin K and receptor activator of nuclear factor-κB ligand. Arthritis Res Ther. 2011;13:R140. doi: 10.1186/ar3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grau S, Richards P J, Kerr B. et al. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 88.Tiaden A N, Klawitter M, Lux V. et al. Detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287:21335–21345. doi: 10.1074/jbc.M112.341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu Z G, Xu N, Wang W B, Pan S H, Li K S, Liu J K. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin Med J (Engl) 2009;122:2483–2488. [PubMed] [Google Scholar]
- 90.Wang J, Markova D, Anderson D G, Zheng Z, Shapiro I M, Risbud M V. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao C Q, Zhang Y H, Jiang S D, Li H, Jiang L S, Dai L Y. ADAMTS-5 and intervertebral disc degeneration: the results of tissue immunohistochemistry and in vitro cell culture. J Orthop Res. 2011;29:718–725. doi: 10.1002/jor.21285. [DOI] [PubMed] [Google Scholar]
- 92.Shen B, Melrose J, Ghosh P, Taylor F. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- 93.Séguin C A, Pilliar R M, Roughley P J, Kandel R A. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 94.Chen J Jung L Boyd L Setton L A Intervertebral disc cells respond to pro-inflammatory cytokines TNF-alpha in a mouse motion segment culture system Paper presented at: ORS, March 19-22, 2006; Chicago, IL
- 95.Séguin C A, Bojarski M, Pilliar R M, Roughley P J, Kandel R A. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25:409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Séguin C A, Pilliar R M, Madri J A, Kandel R A. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 97.Zhao C Q, Jiang L S, Dai L Y. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079–2088. doi: 10.1007/s10495-006-0290-7. [DOI] [PubMed] [Google Scholar]
- 98.Kohyama K, Saura R, Doita M, Mizuno K. Intervertebral disc cell apoptosis by nitric oxide: biological understanding of intervertebral disc degeneration. Kobe J Med Sci. 2000;46:283–295. [PubMed] [Google Scholar]
- 99.Zhang C C, Zhou J S, Hu J G. et al. Effects of IGF-1 on IL-1β-induced apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep. 2013;7:441–444. doi: 10.3892/mmr.2012.1238. [DOI] [PubMed] [Google Scholar]
- 100.Zhao C Q, Liu D, Li H, Jiang L S, Dai L Y. Interleukin-1beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis. 2007;12:2155–2161. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 101.Cui L Y, Liu S L, Ding Y. et al. IL-1beta sensitizes rat intervertebral disc cells to Fas ligand mediated apoptosis in vitro. Acta Pharmacol Sin. 2007;28:1671–1676. doi: 10.1111/j.1745-7254.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- 102.Duan D Y, Yang S H, Xiong X Q, Shao Z W, Wang H. Interleukin-6 protects annulus fibrosus cell from apoptosis induced by interleukin-1 beta in vitro. Chin Med Sci J. 2006;21:107–110. [PubMed] [Google Scholar]
- 103.McCarthy P W, Carruthers B, Martin D, Petts P. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine (Phila Pa 1976) 1991;16:653–655. doi: 10.1097/00007632-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 104.Weinstein J, Claverie W, Gibson S. The pain of discography. Spine (Phila Pa 1976) 1988;13:1344–1348. doi: 10.1097/00007632-198812000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Fujimoto K, Miyagi M, Ishikawa T. et al. Sensory and autonomic innervation of the cervical intervertebral disc in rats: the pathomechanics of chronic discogenic neck pain. Spine (Phila Pa 1976) 2012;37:1357–1362. doi: 10.1097/BRS.0b013e31824ba710. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Kerns J M, Anderson D G. et al. Sensory neurons and fibers from multiple spinal cord levels innervate the rabbit lumbar disc. Am J Phys Med Rehabil. 2006;85:865–871. doi: 10.1097/01.phm.0000242633.41202.ef. [DOI] [PubMed] [Google Scholar]
- 107.García-Cosamalón J, del Valle M E, Calavia M G. et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Freemont A J, Watkins A, Le Maitre C. et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 109.Adams M A, Stefanakis M, Dolan P. Healing of a painful intervertebral disc should not be confused with reversing disc degeneration: implications for physical therapies for discogenic back pain. Clin Biomech (Bristol, Avon) 2010;25:961–971. doi: 10.1016/j.clinbiomech.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 110.Stefanakis M, Al-Abbasi M, Harding I. et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976) 2012;37:1883–1891. doi: 10.1097/BRS.0b013e318263ba59. [DOI] [PubMed] [Google Scholar]
- 111.Abe Y, Akeda K, An H S. et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976) 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 112.Purmessur D, Freemont A J, Hoyland J A. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Richardson S M, Doyle P, Minogue B M, Gnanalingham K, Hoyland J A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. doi: 10.1186/ar2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson S M, Purmessur D, Baird P, Probyn B, Freemont A J, Hoyland J A. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PLoS ONE. 2012;7:e47735. doi: 10.1371/journal.pone.0047735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75:135–141. doi: 10.1016/j.mvr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 116.Ponnappan R K, Markova D Z, Antonio P J. et al. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res Ther. 2011;13:R171. doi: 10.1186/ar3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos P M, Winterowd J G, Allen G G, Bothwell M A, Rubel E W. Nerve growth factor: increased angiogenesis without improved nerve regeneration. Otolaryngol Head Neck Surg. 1991;105:12–25. doi: 10.1177/019459989110500103. [DOI] [PubMed] [Google Scholar]
- 118.McCarron R F, Wimpee M W, Hudkins P G, Laros G S. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine (Phila Pa 1976) 1987;12:760–764. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 119.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine (Phila Pa 1976) 1993;18:1425–1432. [PubMed] [Google Scholar]
- 120.Cuéllar J M, Borges P M, Cuéllar V G, Yoo A, Scuderi G J, Yeomans D C. Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation. Spine (Phila Pa 1976) 2013;38:17–23. doi: 10.1097/BRS.0b013e3182604baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamashita M, Ohtori S, Koshi T. et al. Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine (Phila Pa 1976) 2008;33:1836–1842. doi: 10.1097/BRS.0b013e31817bab2a. [DOI] [PubMed] [Google Scholar]
- 122.Cuellar J M, Montesano P X, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110:578–587. doi: 10.1016/j.pain.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 123.de Souza Grava A L, Ferrari L F, Defino H L. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. Eur Spine J. 2012;21:537–545. doi: 10.1007/s00586-011-2027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Otoshi K, Kikuchi S, Kato K, Sekiguchi M, Konno S. Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine (Phila Pa 1976) 2011;36:E692–E698. doi: 10.1097/BRS.0b013e3181ecd675. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki M, Inoue G, Gemba T. et al. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18:1001–1007. doi: 10.1007/s00586-009-0940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


