Abstract
T-1-rho (T1ρ) magnetic resonance imaging (MRI) and disc height ratio (DHR) are potential biomarkers of degenerative disk disease (DDD) related to biochemical composition and morphology of the intervertebral disk (IVD), respectively. To objectively detect DDD at an early stage, the hypothesis was tested that the average T1ρ relaxation time of the nucleus pulposus (NP) correlates with the disk height of degenerate IVDs, measured by MRI. Studies were performed on a 3-T Siemens Tim Trio clinical MRI scanner (Siemens Healthcare, Malvern, Pennsylvania, United States) on patients being treated for low back pain whose disks were categorized into (1) painful and (2) nonpainful subgroups based on provocative diskography and (3) age-matched healthy controls. Painful disks presented both low DHR and T1ρ values, nonpainful disks measured the highest DHR and extended to a higher range of T1ρ, and control disks presented a midrange DHR with the highest T1ρ values. T1ρ MRI evaluated in the NP of IVDs may be useful to establish a threshold (120 milliseconds here) above which indicates a healthy disk, and disks measuring low NP T1ρ (50 to 120 milliseconds here) would require disk height analysis to further categorize the disk. Combining T1ρ MRI and disk height analysis may hold promise in predicting painful disks without provocative diskography, and predictive models should be developed.
Keywords: T1rho, MRI, disk degeneration, disk pain, biomarker, disk height
Degenerated disks are marked by structural failure and mechanical dysfunction caused by a cascade of biochemical degradation changes that additionally can result in neurochemical rendering of a disk to painful status.1 Healthy disks rely on diffusion to transport nutrients and waste products between the surrounding blood vessels and the ordered collagen fibers of the annulus fibrosus (AF) to the central gel-like nucleus pulposus (NP).2 Age-related degradation is marked by a loss of the gel-like consistency of the NP,3 including decreased proteoglycan (PG) content and decreased water concentration.4 PG is made up of a protein core connected to sugars, the glycosaminoglycans, which a negative fixed charge.5 Glycosaminoglycans attract sodium ions, which contribute to the osmolarity of the extracellular matrix. Concurrent with the breakdown of PG is a loss of ions, leading to a loss of hydration in the NP. Lower osmotic pressure in the NP also increases the load on the annulus, which leads to further degradation of the collagen because high intradiscal pressure is a prerequisite for the normal mechanical function of the disk under physiologic conditions.4,6 Eventually, collagen of the AF breaks down, and the later stages of degenerative disk disease (DDD) is characterized by an NP indistinguishable from the AF and a collapsed disk space.7
The disease pathway for DDD suggests at least three detectable markers of disk quality: biochemical composition of the NP, osmotic pressure, and disk height. Early changes in PG content of the NP are detectable with T-1-rho (T1ρ) magnetic resonance imaging (MRI).8,9,10,11,12 T1ρ MRI is an alternative to conventional T1 and T2 MRI in which a long-duration, low-power radiofrequency referred to as spin-lock pulse is applied to the magnetization in the transverse plane. The magnetization is spin-locked and undergoes relaxation in the presence of a radiofrequency field (B1) in the rotating frame, a situation similar to that of the longitudinal magnetization in the main magnetic (B0) field. The spin-locked magnetization will relax with a time constant T1ρ, called the spin-lattice relaxation in the rotating frame, during the B1 field, which attenuates the effect of signal loss mechanisms (i.e., dipolar relaxation, static dipolar coupling, chemical exchange, and background gradients) on the MRI signal.13 For this reason, T1ρ is always greater than T2. In a typical T1ρ mapping experiment, the duration of the spin-lock pulse is incremented while the amplitude of spin-lock pulse (γB1 ∼ 0.1-few kHz) is fixed. There has been considerable amount of work on biological tissues using T1ρ dealing with tumors, muscle, myocardium, blood flow, and cartilage.13 The T1ρ relaxation time constant probes the time scale of the diffusion of macromolecules and is lower in the NP of DDD patients.8,9 T1ρ has been shown to correlate strongly with cadaveric PG content and pressure in the NP.14,15
Late-stage morphologic degradation of the intervertebral disk (IVDs) can be detected with conventional T1- and T2-weighted weighted MRI, where IVDs can be distinguished from bone, and disk space can be measured. Hence, to explore the feedback between macromolecular and morphologic degradation, the hypothesis was tested that the average T1ρ relaxation time measured in the NP correlates with the disk height normalized by the width of the disk. Therefore, this study uniquely combines in vivo assessment of biochemistry (i.e., PG content using T1ρ relaxation time parameter) and morphology (i.e., disk height-to-width ratio).
In addition to accurate detection of DDD, prognosis of individual IVDs of patients with noticeable lower back pain is important for prescribing treatment options in the correct disks.1 Due to the lack of a proper gold standard, the presence of pain in each disk is currently determined by provocative diskography. The technique relies on the patients' subjective perception of pain to increasing intradiscal pressure created by injection of fluid into the disk. Diskography can provide a more objective criterion for IVD quality via a measurement of disk opening pressure when an injected fluid matches the internal osmotic pressure of the disk nucleus. However, diskography is invasive, and we believe a more reliable, objective, and noninvasive determinant of pain in degenerate disks will improve patient care. Toward this goal, we evaluated the predictability of IVD pain in patients with low back pain (LBP) by fusing T1ρ with disk height measurements.
Materials and Methods
Volunteers
This study is part of an ongoing project whose long-term goal is to evaluate T1ρ MRI as a quantitative, noninvasive biomarker of disk degeneration in patients with LBP. Volunteers were recruited from three cohorts: patients being treated for LBP (n = 12, 49 levels, mean age 44 ± 6 years, range 30 to 53) whose disks were categorized into painful and nonpainful subgroups based on provocative diskography, and age-matched control subjects not being treated for back pain (n = 11, 44 levels, mean age 43 ± 17, range 22 to 76). All cohorts received conventional and T1ρ MRI of their lumbar spine, including L2-L3 to L5-S1. The patients with LBP received multilevel provocative diskography before their MRI.
Measurement of Disk Pain and Pressure
Pain was determined in the LBP cohort by the patient's perception during a diskography procedure.16,17 Diskography pressure data were obtained, following the placement of 22-gauge needles into the center of the L2-L3 through L5-S1 disks, using the IntelliSystem (Merit Medical, South Jordan, Utah, United States) with digital pressure display. Iohexol-Omnipaque 300 (GE Healthcare, Princeton, New Jersey, United States), a low osmolar, nonionic, iodinated contrast agent was injected into each disk under continuous fluoroscopic imaging. Opening pressure was recorded as the pressure when fluid first enters the NP. All diskographies were performed by the same physician.
Assessment of T1ρ
Imaging was performed with approval from the university's Institutional Review Board with the subject's informed consent. T1ρ MRI was performed on a 3-T clinical scanner (Siemens Healthcare, Malvern, Pennsylvania, United States) using the vendor-supplied spine array coil. A 3D T1ρ pulse sequence was used to generate five data sets by varying the duration of spin lock.18 Other parameters were: echo time/repetition time/flip angle = 3 ms/6 ms/20 degrees, field of view = 20 × 20 cm, acquisition matrix = 256 × 128 × 16, interpolated to 256 × 256 × 16, slab thickness = 80 mm, in-plane resolution = 0.8 × 0.8 mm2, bandwidth = 130 Hz/pixel, centric k-space encoding, and the spin-locking amplitude (γB1) was fixed at 500 Hz. Total imaging time was under 20 minutes. T1ρ-weighted images were corrected for in-plane rigid body motion. T1ρ parametric maps were generated by fitting images pixelwise to the linearized monoexponential decay equation and thresholded using a Pearson correlation coefficient of R2 = 0.95 to remove misfit pixels of background noise. The average T1ρ of the NP (NP-T1ρ) was measured in the middle one-third of each disk manually by a single user with custom in-house software written in MATLAB version 20011b (MatLab, Natick, Massachusetts, United States). Conventional T1-weighted fluid-attenuated inversion recovery (FLAIR) sagittal images were acquired with the same slice parameters as T1ρ MRI images to measure disk height ratio and to ensure accurate postprocessing and segmentation.
Measurement of Disk Height Ratio
When measuring the height of IVDs for comparison of disease progression between patients, the height must be normalized to compensate for absolute differences between patients. We defined disk height as a ratio (DHR) of the average height to the width of the disk to account for variations in sizes in the population. A semiautomated computer program was developed for segmenting disks from surrounding tissue to report T1ρ values. This strategy used T1-FLAIR images of the midslice in a 3D sagittal data set, which provided better contrast between end plate and vertebral body and between disk and spinal fluid for the computer algorithm than T2-weighted MRI. Furthermore, we noted that the annulus signal was greater in the T1-weighted MRI compared with corresponding T2 MRI, thus providing a more accurate measure of the disk width. This is especially beneficial during DHR measurements of healthy disks, where the intact collagen architecture reduces T2 due to a strong dipolar interaction among collagen-bound water.19
The first step in the disk height measurement algorithm was to provide manual segmentation of the IVDs from the images. An operator viewed each T1-weighted MRI slice of interest at 2× or higher zoom and traced out a polygon region of interest (ROI) for each of the five lumbar disks. The mask overlay of the disk ROIs and the segmented IVDs are shown in Fig. 1a, b.
Fig. 1.

(a) T1-weighted magnetic resonance imaging with manually segmented disks shown in yellow. (b) Regions of interest corresponding to segmented intervertebral disks. (c) The region corresponding to a single disk. (d) The best-fit ellipse is overlaid on the disk in black. The major axis of the ellipse, employed as a profile to measure disk width, is shown in blue. The “rake” profiles normal to the major axis, used to compute average disk height, are shown in red.
Given these manually defined ROIs, we employed an automated algorithm, shown in Fig. 1c-e, to measure the dimensions of each IVD as follows: First, an ellipse with central second moments matching those of the ROI are fitted to the disk. Next, the length of the ROI along the major axis of this ellipse is measured and defined as the “width” of the disk (shown in blue in Fig. 1d). A series of closely spaced “rakes” are computed normal to the major axis, along a line extending beyond the end points of the axis (red lines in Fig. 1d). The disk is measured along each of these rakes, and the average of all nonzero values is defined as the height. Finally, the DHR is computed by dividing the average height by the width; this quantity is the value examined for correlation with pain and T1ρ relaxation time. The DHR measurement algorithm was written in MATLAB. This method can more accurately account for variations in height across the disk compared with similar but more rudimentary approaches.20,21
Statistical Analysis
Statistical analysis was performed in IBM SPSS Statistics 20.0 (IBM SPSS Inc., Chicago, Illinois, United States). Correlations between DHR and the average NP-T1ρ and between DHR and disk opening pressure from diskography were performed using Pearson correlation analysis. One-way analysis of variance (ANOVA) was performed to determine differences between painful, nonpainful, and healthy DHR and T1ρ values. Receiver operating characteristic (ROC) analysis was performed using a continuous scale of T1ρ and DHR, where confidence in their predictive power was included (JROCFIT 1.0.2). A p value of less than 0.05 was considered to be statistically significant in all analyses.
Results
Representative DHR and T1ρ parametric maps of a patient with LBP and a normal control subject are shown (Fig. 2). Note the conspicuity of the IVD compared with surrounding bone and fluid in the spinal canal in the T1-weighted MRI images (left panel of Fig. 2a, b). This facilitated user-guided semiautomated segmentation of the IVDs from these images. Lower T1ρ values (40 to 70 milliseconds) indicating lower PG content was observed in the NP region of both painful disks Fig. 2a (right panel) suggesting early DDD in this patient.9,10 A moderate correlation is observed between DHR and NP-T1ρ when considering disks from all cohorts (Fig. 3). Further accounting for painful, nonpainful, and control status, disks seemed to separate into clusters. Painful disks (represented with X in Fig. 3) present both low DHR and NP-T1ρ. Nonpainful disks (O in Fig. 3) measure the highest DHR and extend to a higher range of NP-T1ρ than painful disks. Control disks (Δ in Fig. 3) have a midrange DHR with the highest NP-T1ρ values.
Fig. 2.
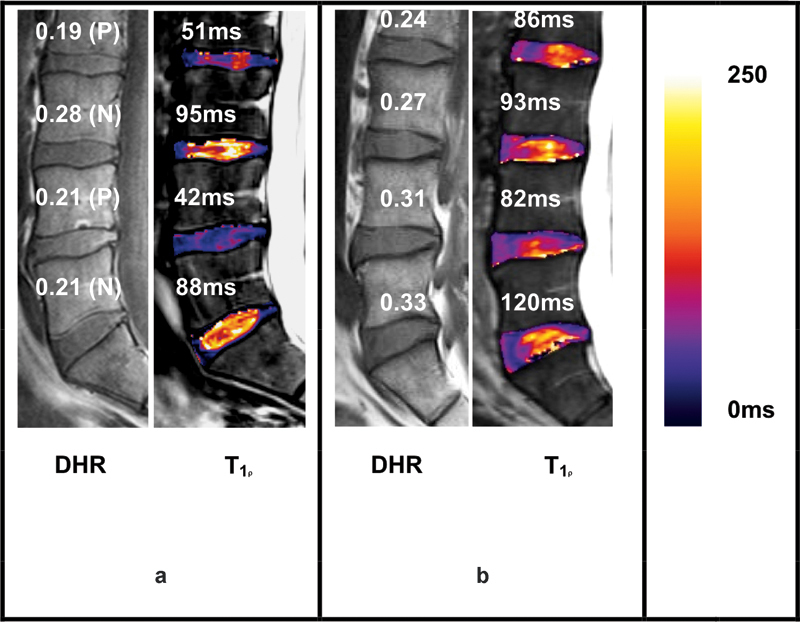
Representative T-1-rho (T1ρ) maps (in color) corresponding to T1-weighted magnetic resonance images (grayscale) of a patient with low back pain (a) and an asymptomatic normal volunteer (b). Listed above each disk are its disk height ratio (DHR) and presence of pain (P) or no pain (N) as determined by diskography and corresponding average T1ρ value in the middle third of the disk (nucleus pulposus region). The color scale on the right indicates T1ρ values from 0 to 250 milliseconds.
Fig. 3.
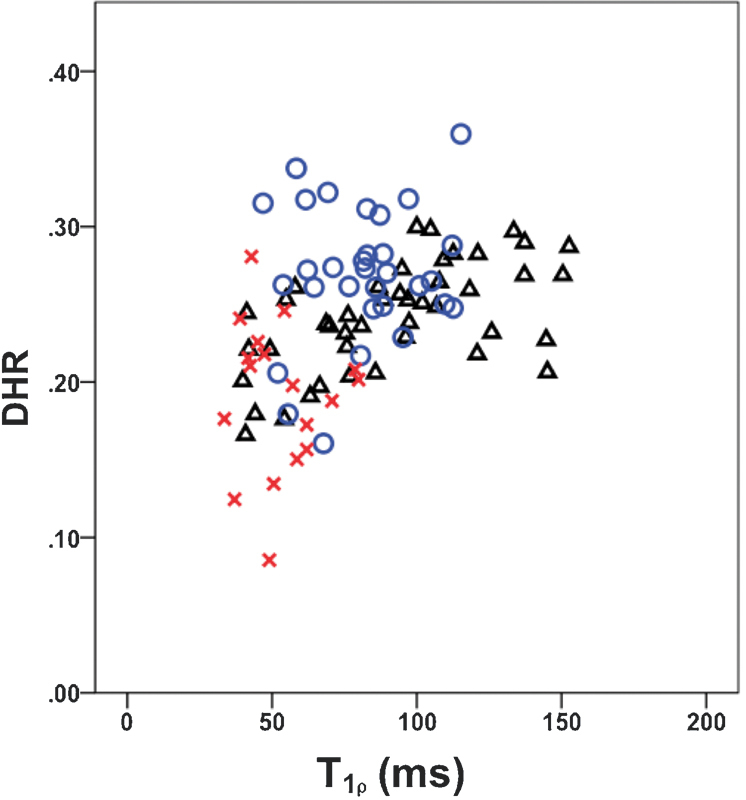
Correlation between disk height ratio (DHR) and average T-1-rho of the nucleus pulposus. Painful disks (X, n = 18), disks without pain (O, n = 31) in the low back pain cohort and in control disks (Δ, n = 44).
In Fig. 4a, DHR can distinguish painful disks (mean DHR = 0.19 ± 0.02, mean ± 95% confidence for n = 18) from nonpainful (mean DHR = 0.27 ± 0.02, n = 31) and control disks (mean DHR = 0.24 ± 0.01, n = 44). There was a significant difference between the DHR of painful versus nonpainful disks (p ≤ 0.001) and painful versus control disks (p ≤ 0.001). DHR was also significantly different between nonpainful disks of patients with LBP and disks from healthy controls (p ≤ 0.05). Fig. 4b shows that T1ρ alone has the ability to distinguish between the three cohorts, with significant differences between painful and nonpainful (34%, p ≤ 0.001) and between painful and control disks (48%, p ≤ 0.001). Table 1 summarizes these results.
Fig. 4.
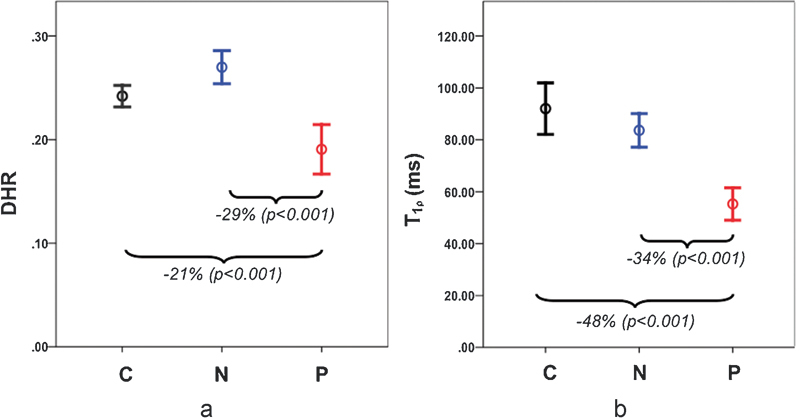
Plot of mean disk height ratio (DHR; a) and average T-1-rho nucleus pulposus (b) for the control (C), low back pain (LBP) nonpainful (N), and LBP painful (P) disk cohorts along with 95% confidence intervals. Significant differences were observed between painful and nonpainful disks and between painful and control disks.
Table 1. ANOVA analysis of DHR for the three cohorts: painful and nonpainful disks from patients with low back pain and control disks.
| DHR | n | Mean | 95% Confidence interval for mean | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Painful | 18 | 0.19 | 0.16 | 0.21 |
| Nonpainful | 31 | 0.27 | 0.25 | 0.28 |
| Control | 44 | 0.24 | 0.23 | 0.25 |
Abbreviations: ANOVA, analysis of variance; DHR, disk height ratio; LBP, low back pain.
Note: Significance was considered p ≤ 0.001.
The ROC analysis using a continuous rating scale of the predictors of disk pain shows the greatest are under the curve (AUC) with a combination of DHR and T1ρ compared with each measure alone (Table 2). The high AUC of DHR alone is most likely due to a selection bias of our patient cohort (i.e., patients with LBP were chosen because they had a high proportion of degenerated disks).
Table 2. Results of ROC analyses of T1ρ and DHR.
| Test variables | ROC area under curve | Asymptotic significancea | Asymptotic 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| DHR | 0.89 | 0.001 | 0.80 | 0.98 |
| T1ρ | 0.90 | 0.001 | 0.81 | 0.99 |
| T1ρ-DHR combined | 0.95 | 0.001 | 0.89 | 1.00 |
Abbreviations: DHR, disk height ratio; ROC, receiver operating characteristic; T1ρ, T-1-rho.
Null hypothesis: true area = 0.5.
Discussion
The experiments presented here combine biochemical assessment (by T1ρ MRI) with morphologic parameter (disk height) and related these to the presence of disk pain for the first time. Although it is a reasonable assumption that disk height and disk degeneration may be related, previous studies have demonstrated contrary results. But these studies were either limited to cadaveric specimens,22 where fluid pressures and disk volume may be altered from that in vivo,23 or performed on a large population of asymptomatic subjects uncontrolled for DDD.24
The T1ρ results from this study are consistent with previously observed values in disks. The main difference here was the addition of diskography-determined painful and nonpainful disks. We intentionally restricted the back pain patient cohort to a carefully selected population that was positive for disk pain. DHR and T1ρ are each able to separate individual IVDs into painful and nonpainful subgroups but combining these markers could better distinguish these cohorts. A possible application in the future of the MRI described here could be to prescreen back pain subjects prior to provocative diskography. For example, T1ρ MRI established a threshold (120 milliseconds here), and results above this are healthy. Although disks measuring low T1ρ (between 48 and 120 milliseconds here) would require disk height analysis to further distinguish whether the disk qualifies for diskography. Furthermore, although the current experiments were performed on a 3-T MRI scanner, the T1ρ MRI pulse sequence and T1 FLAIR can be performed on any MRI scanner field strength.
The MRI analyses are limited to evaluating disks that are not severely degenerated (i.e., less than grade V on the Pfirrmann classification system for lumbar disk degeneration based on T2-weighted MRI).7 This classification system assigns an integer grade (between I and V) to the disk based on structural morphology (e.g., homogeneity within the NP, distinction between the NP and AF, signal intensity, and disk height).
Additionally, disk herniations and end plate disruptions may produce spurious DHR measurements. DHR was derived from T1-weighted FLAIR MRI and appeared better suited than T2 for DHR measurements as performed previously due primarily to the clearer distinction of the IVD from surrounding tissues and the conspicuity of the annulus.25,26 A more detailed comparison of DHR using both MRI contrast mechanisms is underway. Diurnal variations in disk height have been observed and estimated to be in the order ∼1% and can vary with loading activities.27,28,29,30 There may be diurnal variations in T1ρ as well. These effects were not considered here because all MRI were done in a single session but may need to be accounted for in a more sophisticated predictive model of pain based on T1ρ and disk height among other markers.
In conclusion, there is a need for quantitative biomarkers for DDD and LBP. Significant differences are shown between painful and nonpainful disks from patients with LBP and between painful and healthy disks for both disk height and T1ρ measurements. These findings provide the basis for developing noninvasive imaging predictive models to guide diagnosis and treatment algorithms of LBP in the presence of DDD. Combining T1ρ MRI and disk height analysis shows a promising ability to distinguish nonpainful from potentially painful disks, thus minimizing the use of provocative diskography.
Acknowledgments
This work was performed at a NIH-NIBIB-supported Biomedical Technology Research Center (P41 EB015893) with additional funding from an AO Spine research grant (AOSBRC-07-05).
Footnotes
Disclosures Matthew Fenty, None Rachelle Crescenzi, None Bryan Fry, None Dawn Squillante, None Danielle Turk, None Philip M. Maurer, None Arijitt Borthakur, None
References
- 1.Adams M A, Roughley P J. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Rajasekaran S, Babu J N, Arun R, Armstrong B R, Shetty A P, Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 3.Haefeli M, Kalberer F, Saegesser D, Nerlich A G, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter J A. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro E M, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinckmann P, Grootenboer H. Change of disc height, radial disc bulge, and intradiscal pressure from discectomy. An in vitro investigation on human lumbar discs. Spine. 1991;16:641–646. doi: 10.1097/00007632-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Pfirrmann C W, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach J D, Johannessen W, Borthakur A. et al. In vivo quantification of human lumbar disc degeneration using T(1rho)-weighted magnetic resonance imaging. Eur Spine J. 2006;15 03:S338–S344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannessen W, Auerbach J D, Wheaton A J. et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenkrantz G, Li X, Han E T. et al. A feasibility study of in vivo T1rho imaging of the intervertebral disc. Magn Reson Imaging. 2006;24:1001–1007. doi: 10.1016/j.mri.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen A M, Johannessen W, Yoder J H. et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Witschey W, Elliott M A, Borthakur A, Reddy R. Measurement of intervertebral disc pressure with T 1ρ MRI. Magn Reson Med. 2010;64:1721–1727. doi: 10.1002/mrm.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland J B, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannessen W, Auerbach J D, Wheaton A J. et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine. 2006;31:1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen A M, Johannessen W, Yoder J H. et al. Noninvasive quantification of human nucleus pulposus pressure with use of T1rho-weighted magnetic resonance imaging. J Bone Joint Surg Am. 2008;90:796–802. doi: 10.2106/JBJS.G.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tehranzadeh J. Discography 2000. Radiol Clin North Am. 1998;36:463–495. doi: 10.1016/s0033-8389(05)70038-5. [DOI] [PubMed] [Google Scholar]
- 17.Carragee E J, Alamin T F, Carragee J M. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine. 2006;31:505–509. doi: 10.1097/01.brs.0000201242.85984.76. [DOI] [PubMed] [Google Scholar]
- 18.Witschey W RT, Borthakur A, Elliott M A. et al. T1rho-prepared balanced gradient echo for rapid 3D T1rho MRI. J Magn Reson Imaging. 2008;28:744–754. doi: 10.1002/jmri.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy P A. Intervertebral disks on MR images: variation in signal intensity with the disk-to-magnetic field orientation. Radiology. 1996;200:143–147. doi: 10.1148/radiology.200.1.8657902. [DOI] [PubMed] [Google Scholar]
- 20.Farfan H F, Cossette J W, Robertson G H, Wells R V, Kraus H. The effects of torsion on the lumbar intervertebral joints: the role of torsion in the production of disc degeneration. J Bone Joint Surg Am. 1970;52:468–497. [PubMed] [Google Scholar]
- 21.Haefeli M, Kalberer F, Saegesser D, Nerlich A G, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31:1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 22.Berlemann U, Gries N C, Moore R J. The relationship between height, shape and histological changes in early degeneration of the lower lumbar discs. Eur Spine J. 1998;7:212–217. doi: 10.1007/s005860050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnstone B, Urban J P, Roberts S, Menage J. The fluid content of the human intervertebral disc. Comparison between fluid content and swelling pressure profiles of discs removed at surgery and those taken postmortem. Spine. 1992;17:412–416. doi: 10.1097/00007632-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Luoma K, Vehmas T, Riihimäki H, Raininko R. Disc height and signal intensity of the nucleus pulposus on magnetic resonance imaging as indicators of lumbar disc degeneration. Spine. 2001;26:680–686. doi: 10.1097/00007632-200103150-00026. [DOI] [PubMed] [Google Scholar]
- 25.Frobin W, Brinckmann P, Kramer M, Hartwig E. Height of lumbar discs measured from radiographs compared with degeneration and height classified from MR images. Eur Radiol. 2001;11:263–269. doi: 10.1007/s003300000556. [DOI] [PubMed] [Google Scholar]
- 26.Pfirrmann C W, Metzdorf A, Elfering A, Hodler J, Boos N. Effect of aging and degeneration on disc volume and shape: a quantitative study in asymptomatic volunteers. J Orthop Res. 2006;24:1086–1094. doi: 10.1002/jor.20113. [DOI] [PubMed] [Google Scholar]
- 27.Tyrrell A R, Reilly T, Troup J D. Circadian variation in stature and the effects of spinal loading. Spine. 1985;10:161–164. doi: 10.1097/00007632-198503000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Adams M A, Dolan P, Hutton W C. Diurnal variations in the stresses on the lumbar spine. Spine. 1987;12:130–137. doi: 10.1097/00007632-198703000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Roberts N, Hogg D, Whitehouse G H, Dangerfield P. Quantitative analysis of diurnal variation in volume and water content of lumbar intervertebral discs. Clin Anat. 1998;11:1–8. doi: 10.1002/(SICI)1098-2353(1998)11:1<1::AID-CA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Park C O. Diurnal variation in lumbar MRI. Correlation between signal intensity, disc height, and disc bulge. Yonsei Med J. 1997;38:8–18. doi: 10.3349/ymj.1997.38.1.8. [DOI] [PubMed] [Google Scholar]


