Abstract
Transplantation of human fetal neural stem cells (hNSCs) previously demonstrated significant functional recovery after spinal cord contusion in rats. Other studies indicated that human mesenchymal stem cells (hMSCs) can home to areas of damage and cross the blood–brain barrier. The purpose of this article is to determine if combined administration of mesenchymal stem cells and neuronal stem cells improves functional outcomes in rats. The study design was a randomized controlled animal trial. Female adult Long-Evans hooded rats underwent laminectomy at T10 level. Moderate spinal cord contusion at T10 level was induced by the MASCIS Impactor. Four groups were identified. The MSC + NSC group received hMSCs intravenously (IV) immediately after spinal cord injury (acute) and returned 1 week later (subacute) for injection of hNSC directly at site of injury. The MSC-only group received hMSC IV acutely and cell media subacutely. The NSC-only group received cell media IV acutely and hNSC subacutely. The control group received cell media IV acutely and subacutely. Subjects were assessed for 6 weeks using Basso, Beattie, Bresnahan Locomotor Rating Score. Twenty-four subjects were utilized, six subjects in each group. Statistically significant functional improvement was seen in the MSC + NSC group and the NSC-only group versus controls (p = 0.027, 0.042, respectively). The MSC-only group did not demonstrate a significant improvement over control (p = 0.145). Comparing the MSC + NSC group and the NSC-only group, there was no significant difference (p = 0.357). Subacute transplantation of hNSCs into contused spinal cord of rats led to significant functional recovery when injected either with or without acute IV administration of hMSCs. Neither hMSCs nor addition of hMSC to hNSC resulted in significant improvement.
Keywords: spinal cord injury, neuronal stem cells, mesenchymal stem cells, stem cell, transplantation, functional analysis
Spinal cord injury (SCI) is a devastating clinical problem with significant neurological consequences leading to long-lasting functional disability and paralysis. Limited treatment options exist and most are ineffective in restoring neurological function. Stem cell transplantation is a promising technology that targets the fundamental pathological process of axonal degeneration, neuronal loss, and demyelination in SCI.1 The goal of stem cell transplantation is to replace lost neurons, reconnect interrupted axonal connections, and provide neuroprotective factors to allow for healing and recovery after SCI.
Neuronal stem cells (NSCs) are a type of progenitor stem cell derived from brain, spinal cord, and optic nerve tissue that is committed to a neural fate and has the potential for neural differentiation.2 Taking advantage of these characteristics, multiple studies have demonstrated the efficacy of NSC transplantation in SCI with migration of newly formed cells away from the SCI site and significant improvement in locomotor recovery.3 4 5 6 7 8 By aligning themselves and integrating along injured axonal pathways, NSCs can potentially reestablish disrupted axonal connections.6
Mesenchymal stem cells (MSCs) are another type of progenitor stem cell derived from bone marrow, an appealing source due to its easy accessibility and potential for autologous transplantation. MSCs are multipotent stem cells capable of differentiating into mesodermal tissues such as bone, cartilage, muscle, and fat.9 However, several studies have demonstrated that human MSCs can cross germ lines and transdifferentiate into neuronlike cells when expanded with neurotrophic growth factors.10 11 12 13 In addition, MSCs may produce a neuroprotective milieu within the injured spinal cord by releasing neurotrophic factors such as nerve growth factor and neurotrophin-3 and by activating neurotrophic receptors.14
Delivery of MSCs via direct injection into the injured spinal cord demonstrated improved locomotor function with histological evidence of MSC bundles guiding regenerating neurons through the spinal cord lesion.9 15 16 Interestingly, intravenous administration of MSCs has been shown to cross the blood–brain barrier, hone into the region of SCI, and aid remyelination to improve functional outcome.17 18 Previous research suggests increased permeability of the blood–spinal cord barrier occurs after SCI, allowing for MSC entry to the injury site.19 In addition, intravenous MSC delivery earlier after SCI has been shown to reduce cavitary formation and size, making timing of MSC administration important. Intravenous administration of stem cells is attractive clinically, given the ease of administration and avoidance of an invasive surgical procedure for direct injection into the site of injury.
Due to the potential for MSCs to create a favorable environment for neuronal regeneration, this study investigated the ability of MSCs to enhance the local milieu and augment NSC transplantation in SCI. We hypothesized that acute transplantation of human MSCs (hMSCs) combined with subacute transplantation of human NSCs (hNSCs) after contusion SCI enhances functional recovery in the rat model.
Methods
Twenty-four adult female Long-Evans hooded rats were utilized in this study with approval from the Institutional Review Board. A power analysis was conducted prior to the initiation of the study to determine the minimal number of subjects required to detect a 3-point difference on an established locomotor scoring system. A minimum of six subjects per group was required to detect a significant difference between control and experimental groups.
Cultured hMSCs were previously harvested from adult bone marrow of a 24 year-old female donor. The marker expressions for this cell line were CD105+, CD73+, CD90+, human leukocyte antigen (HLA) DR, CD34−, and CD45−. In preparation for transplantation, the hMSCs were suspended and diluted with trypan blue by pipetting. The cell count was determined using hemacytometer. The cells were resuspended in 10 to 20 mL of sterile lactated Ringer's solution to obtain the desired cell concentration.
hNSCs were collected from a single 12-week-old fetal brain with family consent. Fetal brain tissue was freshly dissected and dissociated in TrypLE (Gibco/Invitrogen, Grand Island, NY) for 30 minutes at 37°C. Flow cytometry was utilized with the fetal brain tissue on a panel including: nestin; vimentin; neuronal nuclei; glial fibrillary acidic protein (GFAP); β-tubulin III; CD56; N-Cadherin (N-Cad); OB-Cadherin (OB-Cad); HLA-ABC; HLA-DR; CD34, and annexin. Only samples containing the highest proportion of stem cells and multipotent progenitors of neural types, and the least proportion of definitive cells and antigens of histocompatibility, were selected for further expansion. For the final culturing, only samples consisting of the following phenotype were selected: nestin + , and vimentin+ no less than 25%; HLA-DR+ and CD34+ no more than 5%; GFAP+ no more than 10%; β-tubulin+ no more than 20%; CD56+, N-Cad+, OB-Cad+, HLA-A,B,C+, and annexin+ no more than 15%; cell viability no less than 60%.20 For transplantation, neurospheres were seeded at 6 to 7 × 104 cells/cm2 in a T25 culture flask precoated with 0.01% poly-D-lysine (Sigma, St. Louis, MO) and 0.5 μg/cm2 laminin (Invitrogen, Grand Island, NY). Numbers of live cells were determined by a trypan blue exclusion assay. They were prelabeled with Cell Trace CFSE fluorescence dye from Invitrogen and then stored on ice until grafting. Two days before transplantation, neurospheres were enzymatically dissociated into single-cell suspensions and cultured in fresh medium.
A spinal cord contusion injury was performed in the spinal cords of adult Long-Evans rats (n = 24). A midline incision was made over the spinous processes of T6 through T12 vertebrae, and paravertebral muscles were separated from the vertebra. After the laminectomy and exposure of the underlying spinal cord at T10, the spinal cord was contused with the Multicenter Animal Spinal Cord Injury Study (MASCIS) Impactor weight-drop device, in which a 10 g weight impact rod was dropped from a height of 25 mm to produce a moderately contused SCI model.
Groups 1 and 3 were administered 500 μL of control cell media intravenously (IV) within 3 hours of the SCI creation. Groups 2 and 4 received 4 × 106 hMSCs in 500 μL IV. All IV injections were performed at the major tail vein of the rats. Groups 3 and 4 returned for a secondary procedure 7 days after SCI to reexpose the surgical site, and a Hamilton syringe with a 30-gauge needle with 4 μL of cultured hNSCs (5 × 105 cells/4 μL) was used to transplant cells intrathecally into the subarachnoid space at the site of the SCI. Groups 1 and 2 also returned for a secondary procedure 7 days after SCI for intrathecal injection of control cell media into the subarachnoid space at the SCI site (Table 1). Immunosuppression was not performed in these experiments due to the concern that immunosuppression would inhibit wound and spinal cord healing, thereby allowing a variable that could limit functional recovery. Hori et al have also shown that fetal NSCs have low immunogenic nature and can survive in nonimmunoprivileged sites.21 Previous experiments in our laboratory demonstrated that stem cell transplantation of these human cell types led to minimal host immune responses within the blood–brain barrier with histological evidence of survival within the spinal cord tissue of the rat (Fig. 1).
Table 1. Four randomized and blinded groups with six animals per group.
| Group | Acute | Subacute |
|---|---|---|
| 1 | hMSC IV | hNSC direct injection |
| 2 | hMSC IV | Cell media direct injection |
| 3 | Cell media IV | hNSC direct injection |
| 4 | Cell media IV | Cell media direct injection |
Abbreviations: hMSC, human mesenchymal stem cells; hNSC, human fetal neural stem cells.
Fig. 1.
Human anti-nuclear antibody staining of large oligodendrocytic cells at the epicenter of the spinal cord injury after injection of stem cells.
Functional outcome was assessed using the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Score for rat hind limb motor function.22 Two trained observers, who were blinded to the experimental groups and scored independently in a noise-free environment, performed the BBB recordings. Animals were assessed during the course of a 4-minute exposure to an open-field arena consisting of a circular enclosure (90-cm diameter, 7-cm wall height). BBB scores were recorded weekly after creation of the SCI to assess functional recovery. Animals were monitored closely for 6 weeks following the subacute hNSC transplantation. Statistical significance was determined using repeated measures analysis of variance.
Results
Twenty-four subjects were utilized in this study, six in each of the four groups. Upon the completion of the study, a statistically significant functional improvement was seen in the combined hMSC + hNSC group and the hNSC-only group as compared with cell media group (p = 0.027 and 0.042, respectively; Figs. 1 and 2). At the end of the 6-week study period, the average BBB score for the combined hMSC + hNSC group was 10.5 as compared with 6.75 in the control group (Fig. 2). The average BBB score for the hNSC-only group was 11.08 at the end of 6 weeks (Fig. 3). When comparing the average BBB scores of the hMSC + hNSC group and the hNSC-only group, however, there was no significant difference (p = 0.357). In addition, the hMSC-only group did not demonstrate a significant improvement over the cell media group with an average BBB score of 9.17 at the end of 6 weeks (p = 0.145; Fig. 4).
Fig. 2.
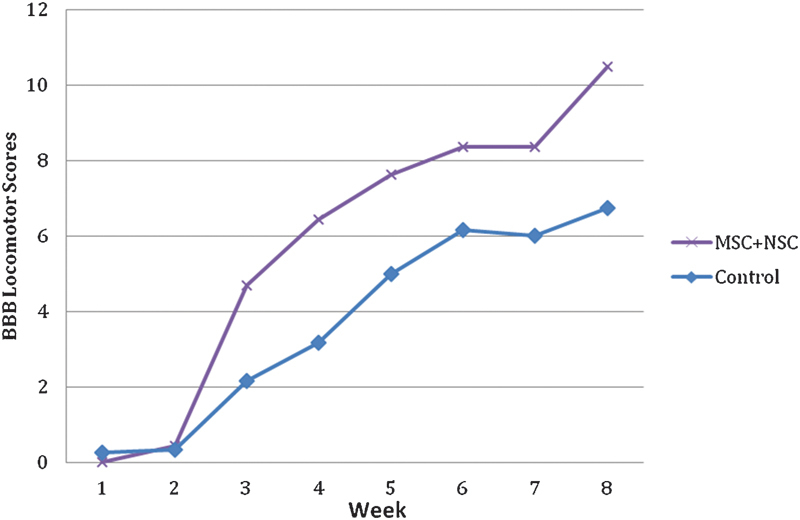
Basso, Beattie, Bresnahan (BBB) Locomotor Rating of the combined human mesenchymal stem cells and human fetal neural stem cells (hMSC + hNSC) group as compared with control (p = 0.027).
Fig. 3.
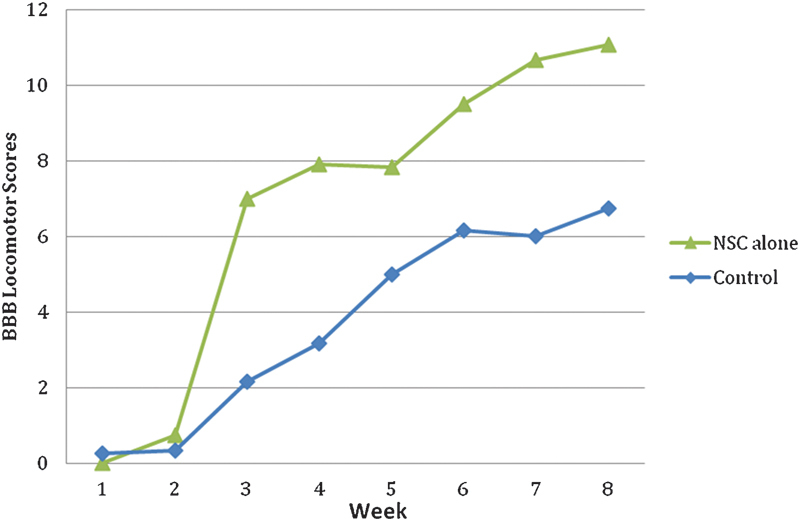
Basso, Beattie, Bresnahan (BBB) Locomotor Rating of the human fetal neural stem cell (NSC)-only group as compared with control (p = 0.042).
Fig. 4.
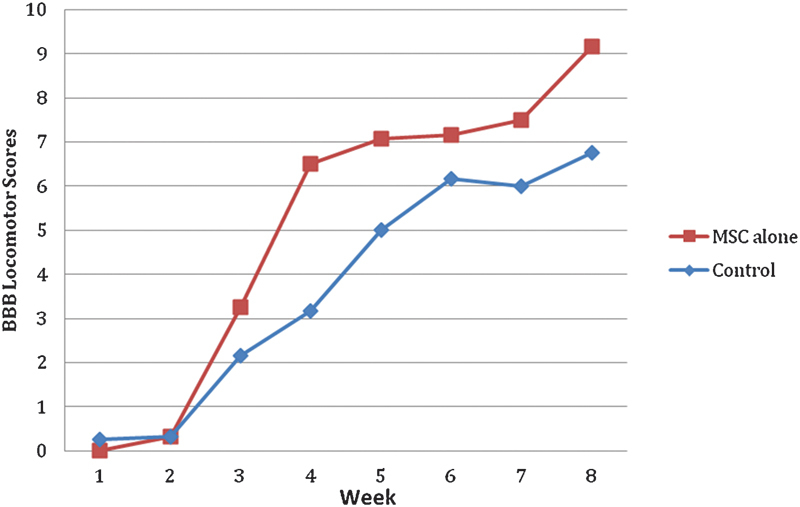
Basso, Beattie, Bresnahan (BBB) Locomotor Rating of the human mesenchymal stem cell (MSC)-only group as compared with control (p = 0.145).
Discussion
SCI is a complex clinical process with profound neurological consequences since the injured spinal cord has a limited ability to restore and regenerate lost neurons and axonal connections. The socioeconomic impact of SCI is also substantial since these typically young male patients require lifelong supportive care that affects families, caretakers, and medical personnel, representing a considerable social and financial burden. The National Spinal Cord Injury Statistical Center estimated the lifetime costs for a 25-year-old with quadriplegia to be 3.1 million dollars.23
The pathophysiological mechanism of SCI initially involves a primary process where energy is transferred to the spinal cord through a traumatic event with compression and deformation of the spinal cord.24 Free radicals, endogenous opioids, excessive excitatory neurotransmitter release have been implicated in the propagation of the SCI.24 25 From these processes, a caustic inflammatory environment exists after SCI that destabilizes cellular processes, leading to apoptosis and necrosis. Ultimately, the reactive glial scar develops within the spinal cord tracts, blocking axonal regeneration.
Previous treatment strategies have focused on reducing the inflammatory reaction because final functional outcome will depend on the magnitude of this process. In the past, high-dose methylprednisolone was considered the standard of care of acute SCI treatment, based on the National Acute Spinal Cord Injury Study results.25 26 27 28 However, significant complications were also seen, including wound infection rates, sepsis, pneumonia, gastrointestinal hemorrhage, pulmonary embolus, and death.29 30 31
Cell-based therapy has targeted mechanisms that reduce secondary damage after SCI, attempting to minimize glial scar formation and preserve myelinated nerve tracts.1 Extensive research has been dedicated to technologies that may modulate the inflammatory reaction, replace lost neurons and oligodendrocytes, and remyelinate damaged spinal tracts, thereby allowing for functional recovery. Stem cells are an attractive option to achieve these goals due to their pluripotent nature. Multiple previous rodent experiments have shown the ability of pluripotent stem cells of either mesenchymal or neuronal lineage to survive transplantation and develop into neuronlike cells.32 33 34 35 These cells have been shown to reconstruct damaged neuronal structures, remyelinate axons, and restore motor function. Experiments of genetically engineered mice with severe dysmyelination of the central nervous system demonstrated patterns of significant myelination after transplantation of neural stem cells, providing further evidence that transplanted stem cells can create neuronal structures in vivo.36
This current study provides the first evidence in the scientific literature that combining MSC and NSC lines can result in significant functional recovery in SCI. We demonstrated that combined transplantation and subacute transplantation of hNSCs directly into the site of injury significantly improved functional outcomes 6 weeks after the SCI. These results demonstrate that hNSCs can augment healing and recovery in SCI even when given 7 days after the injury. This has important clinical implications because patients with SCI who have delayed surgical intervention may benefit from concurrent hNSC transplantation into the site of injury.
When examining the effect of hMSCs, no significant difference was detected between the combined and hNSC-only groups or between the hMSC-only and control groups. This likely signifies that the administration of hMSCs did not have an effect in the functional recovery of the rats. This may be due to the route of delivery because the hMSCs were administered intravenously and the cells may not have been able to cross the blood–spinal cord barrier to the SCI site. Another explanation is that the dose of hMSCs administered in this study may not have reached the critical threshold required to home to and cross the blood–spinal cord barrier. Based on our findings, we were unable to support the hypothesis that hMSCs enhance hNSC transplantation in SCI.
In summary, subacute transplantation of hNSCs provided significant functional improvement after contusion SCI in the rat model. Treatment with hMSCs did not provide statistically significant functional improvement on its own or enhance outcome when combined with hNSCs. Further study is required to determine the ideal route and dosage of hMSCs as a treatment modality in SCI.
Acknowledgment
The authors would like to thank Stemedica (San Diego, California) for providing the hNSCs and hMSCs for this study.
Footnotes
Disclosures D. Y. Park, None R. E. Mayle, None R. L. Smith, None I. Corcoran-Schwartz, None A. I. Kharazi, Stock/stock options: Stemedica I. Cheng, None
References
- 1.Ronsyn M W, Berneman Z N, Van Tendeloo V F, Jorens P G, Ponsaerts P. Can cell therapy heal a spinal cord injury? Spinal Cord. 2008;46:532–539. doi: 10.1038/sc.2008.13. [DOI] [PubMed] [Google Scholar]
- 2.Parr A M, Kulbatski I, Zahir T. et al. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155:760–770. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 3.McDonald J W, Liu X Z, Qu Y. et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 4.Stokes B T, Reier P J. Fetal grafts alter chronic behavioral outcome after contusion damage to the adult rat spinal cord. Exp Neurol. 1992;116:1–12. doi: 10.1016/0014-4886(92)90171-l. [DOI] [PubMed] [Google Scholar]
- 5.Teng Y D, Lavik E B, Qu X. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 7.Parr A M, Kulbatski I, Tator C H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- 8.Pallini R Vitiani L R Bez A et al. Homologous transplantation of neural stem cells to the injured spinal cord of mice Neurosurgery 2005571014–1025., discussion 1014–1025 [DOI] [PubMed] [Google Scholar]
- 9.Hofstetter C P, Schwarz E J, Hess D. et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodbury D, Schwarz E J, Prockop D J, Black I B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Ramos J, Song S, Cardozo-Pelaez F. et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 12.Deng W, Obrocka M, Fischer I, Prockop D J. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282:148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- 13.Zeng R, Wang L W, Hu Z B. et al. Differentiation of human bone marrow mesenchymal stem cells into neuron-like cells in vitro. Spine. 2011;36:997–1005. doi: 10.1097/BRS.0b013e3181eab764. [DOI] [PubMed] [Google Scholar]
- 14.Pisati F, Bossolasco P, Meregalli M. et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16:41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- 15.Park W B, Kim S Y, Lee S H, Kim H W, Park J S, Hyun J K. The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 2010;11:119. doi: 10.1186/1471-2202-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlichenko N, Sokolova I, Vijde S. et al. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama Y, Radtke C, Honmou O, Kocsis J D. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osaka M, Honmou O, Murakami T. et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Popovich P G, Horner P J, Mullin B B, Stokes B T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 20.Poltavtseva R A, Marey M V, Aleksandrova M A, Revishchin A V, Korochkin L I, Sukhikh G T. Evaluation of progenitor cell cultures from human embryos for neurotransplantation. Brain Res Dev Brain Res. 2002;134:149–154. doi: 10.1016/s0165-3806(02)00274-2. [DOI] [PubMed] [Google Scholar]
- 21.Hori J, Ng T F, Shatos M, Klassen H, Streilein J W, Young M J. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- 22.Basso D M, Beattie M S, Bresnahan J C. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 23.National Spinal Cord Injury Statistical Center, Birmingham, Alabama. Spinal cord injury facts and figures at a glance. Available at: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202012%20Feb%20Final.pdf
- 24.Gupta R, Bathen M E, Smith J S, Levi A D, Bhatia N N, Steward O. Advances in the management of spinal cord injury. J Am Acad Orthop Surg. 2010;18:210–222. doi: 10.5435/00124635-201004000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bracken M B, Holford T R. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg. 1993;79:500–507. doi: 10.3171/jns.1993.79.4.0500. [DOI] [PubMed] [Google Scholar]
- 26.Bracken M B, Collins W F, Freeman D F. et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 27.Bracken M B, Shepard M J, Collins W F. et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 28.Bracken M B, Shepard M J, Holford T R. et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 29.Gerndt S J, Rodriguez J L, Pawlik J W. et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. 1997;42:279–284. doi: 10.1097/00005373-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001;26:426–430. doi: 10.1097/00007632-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine. 2009;34:2121–2124. doi: 10.1097/BRS.0b013e3181b613c7. [DOI] [PubMed] [Google Scholar]
- 32.Park H W, Cho J S, Park C K. et al. Directed induction of functional motor neuron-like cells from genetically engineered human mesenchymal stem cells. PLoS ONE. 2012;7:e35244. doi: 10.1371/journal.pone.0035244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto Y, Abematsu M, Falk A. et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- 34.Kakinohana O, Juhasova J, Juhas S. et al. Survival and differentiation of human embryonic stem cell-derived neural precursors grafted spinally in spinal ischemia-injured rats or in naïve immunosuppressed minipigs: a qualitative and quantitative study. Cell Transplant. 2012 doi: 10.3727/096368912X653200. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda A, Tsuji O, Shibata S. et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983–1994. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- 36.Uchida N, Chen K, Dohse M. et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]


