Abstract
Chronic esophageal candidiasis is an infection that is mostly seen in immunocompromised conditions, among which is chronic mucocutaneous candidiasis (CMC). Recently an association between CMC and esophageal carcinoma has been reported. Here we present two patients with chronic esophageal candidiasis who developed esophageal squamous cell carcinoma and we discuss the etiologic role of Candida-induced nitrosamine production, the loss of STAT1 function and impaired tumor surveillance and T-lymphocyte function in the development of esophageal carcinoma.
Keywords: Esophageal candidiasis, Chronic mucocutaneous candidiasis, Esophageal cancer
1. Introduction
Esophageal candidiasis is a condition mostly seen in immunocompromised patients. It is usually caused by Candida albicans, but other species are occasionally found [1]. Patients at high risk are those infected with HIV, patients with hematologic malignancies or those receiving chemotherapy for solid tumors, and patients with congenital immunodeficiencies such as idiopathic CD4+ lymphopenia or chronic mucocutaneous candidiasis. Especially in the latter patient groups, infection is often prolonged and refractory to treatment.
Chronic mucocutaneous candidiasis (CMC) is a heterogenous group of clinical syndromes characterized by chronic or recurrent infections of the skin, nails and mucous membranes caused by Candida spp. Recently, several reports have suggested an association between CMC with oral squamous cell carcinomas [2–4] and esophageal carcinomas [5–8], especially in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). Esophageal carcinoma has also been described in CMC patients with concurrent IgA deficiency [6,7], which is associated with the development of solid and lymphoid tumors [9].
Here, we describe two patients with chronic esophageal candidiasis who developed esophageal carcinoma. One subject was diagnosed with autosomal dominant CMC due to mutations in the DNA sequence encoding the signal transducer and activator of transcription 1 (STAT1). The second patient had isolated refractory esophageal candidiasis for over 10 years, without other manifestations of CMC. We provide a review of the literature and we speculate about the mechanisms underlying the association between chronic esophageal candidiasis and the development of squamous cell carcinoma.
2. Cases
2.1. Case 1
The patient is a Dutch woman who presented with recurrent oral and vaginal Candida infections, and onychomycosis since birth. There was no consanguinity within her family. She experienced episodes of retrosternal pain and difficulty on swallowing solid food from the age of 12. At the age of 23, endoscopy of the upper digestive tract showed esophageal candidiasis and ketoconazole was prescribed. Immunological evaluation had shown normal lymphocyte counts, neutrophil function and immunoglobulin concentrations. Based on the clinical features, a diagnosis of chronic mucocutaneous candidiasis was made.
At 25 years of age, the patient presented with progressive dysphagia and retrosternal pain. There was no weight loss. She had smoked 20 cigarettes per day for the last 5 years, but used no alcohol. Endoscopy showed candidiasis of the proximal esophagus. Cytology samples revealed yeasts, as well as malignant epithelial cells. After two weeks of treatment with itraconazol 200 mg, endoscopy was repeated. Candidiasis had markedly improved but at 26 cm from the mouth an opaque white, irregular stenosis was found, histology of which showed poorly differentiated squamous cell carcinoma. Further staging did not reveal any evidence of locoregional spread or distant metastasis. An esophagectomy was performed, followed by reconstruction with colonic interposition. The reason for choosing a colonic interposition was that Candida species do not grow on colonic epithelium as they do on squamous cell mucosa (Dr. John E Bennett, personal communication). Although oral candidiasis persisted, repeated endoscopy showed no signs of candidiasis or malignancy in the neo-esophagus during 20 years of follow-up. In fact, below the proximal anastomosis of the neo-esophagus, candidal growth was never seen.
Her family history was notable for autosomal dominant CMC. She gave birth to three children, one of whom also suffered from CMC. The affected child was a son, who is now 17 years old. He experienced frequent Candida stomatitis since the age of 4 years, for which he was treated with fluconazol. At the age of 13 years he developed complaints of retrosternal pain. Esophagogastroduodensocopy showed normal mucosa without signs of malignancy or candidiasis. Multiple biopsies of the esophagus showed no abnormalities. The index patient's mother, who also had recurrent fungal infections of skin and mucous membranes, was diagnosed with esophageal cancer at the age of 32 years and had died one year later. Recently, a mutation in the DNA sequence encoding the signal transducer and activator of transcription (STAT1) was identified in both this patient and her affected son [10].
2.2. Case 2
A 71-year-old Dutch man presented with a history of recurrent esophageal candidiasis for nine years. His medical history was unremarkable except for a Herpesvirus infection of the left eye at the age of 35, which necessitated corneal transplantation. He did not smoke or use alcohol. He used a proton pump inhibitor (omeprazole 40 mg) because of retrosternal pain. He did not use immunosuppressants. His CD4+ count was normal (1300×106/ml) as were his IgA, IgM and IgG immunoglobulin concentrations, complement activity (CH50, AP50) and Mannose Binding Lectin (MBL) concentration. Upper digestive endoscopy repeatedly showed extensive esophageal candidiasis. Cultures yielded Candida albicans, for which he was treated with multiple courses of fluconazole 200 mg daily for two weeks (MIC 1 mg/l). However, after each course, relapse occurred within a few weeks of cessation of antifungal therapy. Prolonged therapy with fluconazole 150 mg per day was effective during 10 months, after which he experienced difficulty in swallowing solid food and progressive retrosternal pain. A new upper digestive endoscopy again showed esophageal candidiasis (Fig. 1) and cultures now revealed Candida glabrata, resistant to fluconazole. He was treated with caspofungin (70 mg daily) in combination with amphotericin B (oral suspension, six times 400 mg) for two weeks. Follow-up endoscopy, however, showed persisting esophageal candidiasis and Candida albicans was isolated. During subsequent months, flucytosine (2000 mg intravenously every six hours) and high dose fluconazole (800 mg/d) were added to his antifungal regimen without success. Stimulation of antifungal immune response with recombinant G-CSF (300 μg subcutaneously three times per week for one month) and Interferon-gamma (IFNγ) (100 μg subcutaneously three times per week for one month) did not result in clinical and microbiological improvement.
Fig. 1.
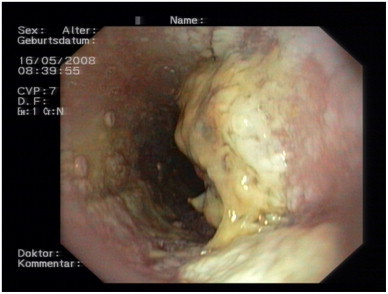
Upper digestive endoscopy showing extensive esophageal candidiasis.
CT scan of the thorax showed thickening of the esophageal wall and multiple slightly enlarged subcarinal and hilar lymph nodes. Biopsies of the esophageal wall showed no signs of malignancy despite repeated sampling (on ten occasions over 18 months time). Endobronchial Ultrasound Biopsy (EBUS) and two Transbronchial Needle Aspirations (TBNA) of mediastinal lymph nodes did not show signs of malignancy either. Despite nasogastric feeding, the patient lost 15 kg of body weight and his condition deteriorated. He refused further invasive diagnostic procedures or esophageal surgery. Respiratory failure occurred, leading to death. On autopsy a well-differentiated squamous cell carcinoma was found with invasive growth in mediastinal lymph nodes and bronchus (pT4). Candida glabrata was isolated from lung and esophageal tissues.
3. Discussion
We present two patients who developed esophageal carcinoma after prolonged esophageal candidiasis. One of these patients was diagnosed with autosomal-dominant chronic mucocutaneous candidiasis, the other patient had isolated esophageal candidiasis.
Multiple etiologic factors play a role in the development of squamous cell carcinoma of the esophagus, among which alcohol consumption and smoking are the most important [11]. The incidence of esophageal squamous cell carcinoma varies considerably among geographic regions, probably caused by a combination of common environmental risk factors and inherited predisposition. Ingestion of nitrosamines in food [12] and tobacco [13] have long since been implicated as risk factors for the development of esophageal cancer.
Candidiasis is often found in patients with esophageal carcinoma. A prospective study on the frequency of gastro-esophageal candidiasis in 465 patients who underwent endoscopy, showed that candidiasis was more frequent in patients with esophageal carcinoma (27%) than in patients with other forms of mucosal injury, such as oesophagitis (15%) [14]. Although candidiasis can develop secondary to malignancy, possibly due to impaired antifungal host defense due to mucosal damage, there is increasing evidence that Candida infection itself has carcinogenic properties and several reports have been published of increased incidence of oral and esophageal squamous cell carcinoma in patients with chronic candidiasis.
One important mechanism that has been proposed to underlie this phenomenon is the catalytic activity of Candida, which facilitates the production of carcinogenic nitrosamines such as nitroso-N-methylbenzylamine (NBMA) from their precursors [15]. A high nitrosation potential of Candida strains isolated from oral leukoplakia lesions was associated with more advanced precancerous changes, supporting the hypothesis that yeasts play a causal role in oral cancer by means of endogenous nitrosamine production [16]. These data are supported by experimental studies showing that Candida acts as a promoter of oral carcinogenesis in rats [17]. The long-term infection of esophageal epithelium by Candida, and the subsequent increased exposure to nitrosamine, may explain the development of esophageal carcinoma in our patient described in case 2, who did not have any other risk factors for this condition (smoking, alcohol or genetic predisposition).
Colonization of the esophagus by Candida is common even in healthy volunteers (7.5%) [18], while that of the colon is much less. Based on unpublished observations that candidiasis is less likely to occur on colonic epithelium, a colon interponate was chosen for reconstruction after esophagectomy in the first patient. A reason for this difference might be the differential capacity of the fungus to adhere to epithelial cells. In vitro experiments showed that adhesion, invasion and damage by C. albicans depend not only on fungal morphology and activity, but also on the epithelial cell type and the differentiation stage of the epithelial cells, indicating that epithelial cells differ in their susceptibility to the fungus [19].
The carcinogenic effect of nitrosamines on esophageal mucosa is mediated through the stimulation of nicotinic acetylcholine receptors (nAChRs) in target cells [20]. In vitro assays using immortalized human esophageal epithelial (Het-1A) cells showed that stimulation with nitrosamines upregulates STAT1 expression and protein binding activity [20]. STATs (signal transducer and activator of transcription) are a family of cytoplasmic proteins that play a role as signal messengers and transcription factors. STATs are activated by cytokines and they regulate the expression of several genes associated with cellular growth and differentiation. Furthermore, STAT1 activation is involved in apoptosis of esophageal squamous cell carcinoma cells and in the terminal differentiation of normal squamous cells [21]. In vitro stimulation of TE8 esophageal cancer cells showed that Epidermal Growth Factor (EGF) inhibits the growth of esophageal cancer cells that overexpress EGF receptor through a STAT1-dependent mechanism [22].
An association between chronic candidiasis and oral [2,3] or esophageal squamous cell carcinoma [5–7,24] has been described in CMC patients. Recently, a mutation in the gene encoding STAT1 was identified as the cause of autosomal dominant CMC in five families, including the patient described in case 1 [10]. Three out of sixteen CMC affected patients in these families also developed esophageal cancer (at the age of 41, 25 and 32) and one developed oral squamous cell carcinoma [8,10]. Interestingly, all CMC patients with oral or esophageal carcinoma had the Ala267Val mutation in the coiled-coil domain of STAT1. Functional studies in these patients showed defective T-lymphocyte immune responses, such as production of IFNγ IL-17 or IL-22. While IL-17 and IL-22 are crucial components of the mucosal antifungal defense, IFNγ plays a pivotal role in tumor surveillance. The latter has been illustrated in a study showing increased tumor induction in IFNγ receptor knock-out mice and in STAT1 knock-out mice (which are insensitive to IFNγ) when exposed to chemical carcinogens [25].
In a large cohort of patients with esophageal carcinoma (n=351), head and neck squamous cell carcinoma (n=375) and healthy controls (n=309), the Ala267Val mutation was not found [26]. However loss of STAT1 activation in cell lines established from esophageal carcinoma has been shown to be associated with a deleterious clinical course in patients [23].
Altogether, these data support the hypothesis that STAT1 plays an important role in the development of esophageal carcinoma induced by chronic Candida infection.
A study from Finland showed that 10% of all adult patients diagnosed with APECED had developed oral or esophageal squamous cell carcinoma at a mean age of 37 years [5]. APECED is an autosomal recessive disorder caused by mutations in the AIRE (autoimmune regulator) gene. AIRE encodes for a nuclear transcriptional protein that regulates immunological self-tolerance. It is characterized by chronic mucocutaneous candidiasis, hypoprathyroidism and Addison's disease. It has been suggested that in patients with APECED, T-lymphocyte immunological surveillance fails due to autoantibodies against IFNγ and IL-17, leading to chronic Candida infection as well as impaired clearance of precancerous cells. Esophageal carcinoma has also been described in CMC patients with concurrent IgA deficiency [6,7], and it remains to be investigated whether similar mechanisms are at work in these patients, as in those with STAT-1 defects.
In conclusion, these cases illustrate that patients with long-lasting esophageal Candida infection have an increased risk of developing oral and esophageal squamous cell carcinoma. The pathogenesis of development of esophageal malignancy in patients with chronic candidiasis remains to be fully elucidated, but it is probably multifactoral. Candida-related nitrosamine production seems to be an important risk factor for this increased incidence, while STAT1 defects may play an important role in failing to contain esophageal cell carcinoma in autosomal dominant CMC. Impairment of the function of T-lymphocytes is seen in various degrees in CMC patients, and this is hypothesized to play an important role. The high risk of malignancy in patients with chronic candidiasis warrants close follow-up in order to detect esophageal and oral carcinoma at an early stage. Aggressive treatment of oral and esophageal candidiasis is recommended, as well as repeating upper digestive endoscopy and biopsy of erosive lesions lasting more than 2 weeks [5]. Furthermore, patients should be advised to defer from use of alcohol and tobacco.
Conflict of interest statement
There are none.
References
- 1.Kliemann D.A., Pasqualotto A.C., Falavigna M., Giaretta T., Severo L.C. Candida esophagitis: species distribution and risk factors for infection. Revista do Instituto de Medicina Tropical de Sao Paulo. 2008;50(5):261–263. doi: 10.1590/s0036-46652008000500002. [DOI] [PubMed] [Google Scholar]
- 2.McGurk M., Holmes M. Chronic muco-cutaneous candidiasis and oral neoplasia. Journal of Laryngology and Otology. 1988;102(7):643–645. doi: 10.1017/s0022215100105985. [DOI] [PubMed] [Google Scholar]
- 3.Firth N.A., O'Grady J.F., Reade P.C. Oral squamous cell carcinoma in a young person with candidosis endocrinopathy syndrome: a case report. International Journal of Oral and Maxillofacial Surgery. 1997;26(1):42–44. doi: 10.1016/s0901-5027(97)80845-4. [DOI] [PubMed] [Google Scholar]
- 4.Bockle B.C., Wilhelm M., Muller H., Gotsch C., Sepp N.T. Oral mucous squamous cell carcinoma—an anticipated consequence of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) Journal of American Academy of Dermatology. 2010;62(5):864–868. doi: 10.1016/j.jaad.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Rautemaa R., Hietanen J., Niissalo S., Pirinen S., Perheentupa J. Oral and oesophageal squamous cell carcinoma—a complication or component of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, APS-I) Oral Oncology. 2007;43(6):607–613. doi: 10.1016/j.oraloncology.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Rosa D.D., Pasqualotto A.C., Denning D.W. Chronic mucocutaneous candidiasis and oesophageal cancer. Medical Mycology. 2008;46(1):85–91. doi: 10.1080/13693780701616023. [DOI] [PubMed] [Google Scholar]
- 7.Domingues-Ferreira M., Grumach A.S., Duarte A.J., De Moraes-Vasconcelos D. Esophageal cancer associated with chronic mucocutaneous candidiasis. Could chronic candidiasis lead to esophageal cancer? Medical Mycology. 2009;47(2):201–205. doi: 10.1080/13693780802342545. [DOI] [PubMed] [Google Scholar]
- 8.Koch D., Lilic D., Carmichael A.J. Autosomal dominant chronic mucocutaneous candidiasis and primary hypothyroidism complicated by oesophageal carcinoma. Clinical and Experimental Dermatology. 2009;34(8):e818–e820. doi: 10.1111/j.1365-2230.2009.03561.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham-Rundles C., Pudifin D.J., Armstrong D., Good R.A. Selective IgA deficiency and neoplasia. Vox Sanguinis. 1980;38(2):61–67. doi: 10.1111/j.1423-0410.1980.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 10.van de Veerdonk F.L., Plantinga T.S., Hoischen A., Smeekens S.P., Joosten L.A., Gilissen C. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. New England Journal of Medicine. 2011;365(1):54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 11.Engel L.S., Chow W.H., Vaughan T.L., Gammon M.D., Risch H.A., Stanford J.L. Population attributable risks of esophageal and gastric cancers. Journal of the National Cancer Institute. 2003;95(18):1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 12.Rogers A.E., Sanchez O., Feinsod F.M., Newberne P.M. Dietary enhancement of nitrosamine carcinogenesis. Cancer Research. 1974;34(1):96–99. [PubMed] [Google Scholar]
- 13.Boyland E., Roe F.J., Gorrod J.W., Mitchley B.C. The carcinogenicity of nitrosoanabasine, a possible constituent of tobacco smoke. British Journal of Cancer. 1964;18:265–270. doi: 10.1038/bjc.1964.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott B.B., Jenkins D. Gastro-oesophageal candidiasis. Gut. 1982;23(2):137–139. doi: 10.1136/gut.23.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krogh P. The role of yeasts in oral cancer by means of endogenous nitrosation. Acta Odontologica Scandinavica. 1990;48(1):85–88. doi: 10.3109/00016359009012738. [DOI] [PubMed] [Google Scholar]
- 16.Hsia C.C., Sun T.T., Wang Y.Y., Anderson L.M., Armstrong D., Good R.A. Enhancement of formation of the esophageal carcinogen benzylmethylnitrosamine from its precursors by Candida albicans. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(3):1878–1881. doi: 10.1073/pnas.78.3.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Grady J.F., Reade P.C. Candida albicans as a promoter of oral mucosal neoplasia. Carcinogenesis. 1992;13(5):783–786. doi: 10.1093/carcin/13.5.783. [DOI] [PubMed] [Google Scholar]
- 18.Bonavina L., Incarbone R., Reitano M., Tortorano A., Viviani M., Peracchia A. Candida colonization in patients with esophageal disease: a prospective clinical study. Diseases of the Esophagus. 2003;16(2):70–72. doi: 10.1046/j.1442-2050.2003.00297.x. [DOI] [PubMed] [Google Scholar]
- 19.Dalle F., Wächtler B., L'Ollivier C., Holland G., Bannert N., Wilson D. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cellular microbiology. 2010;12(2):248–271. doi: 10.1111/j.1462-5822.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 20.Arredondo J., Chernyavsky A.I., Grando S.A. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biology and Therapy. 2006;5(5):511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 21.Kaganoi J., Watanabe G., Okabe M., Nagatani S., Kawabe A., Shimada Y. STAT1 activation-induced apoptosis of esophageal squamous cell carcinoma cells in vivo. Annals of Surgical Oncology. 2007;14(4):1405–1415. doi: 10.1245/s10434-006-9274-7. [DOI] [PubMed] [Google Scholar]
- 22.Ichiba M., Miyazaki Y., Kitamura S., Kiyohara T., Shinomura Y., Matsuzawa Y. Epidermal growth factor inhibits the growth of TE8 esophageal cancer cells through the activation of STAT1. Journal of Gastroenterology. 2002;37(7):497–503. doi: 10.1007/s005350200077. [DOI] [PubMed] [Google Scholar]
- 23.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Journal of Clinical Endocrinology and Metabolism. 2006;91(8):2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dura P, ter Morsche RHM, Netea MG, van der Meer JWM, Peters WHM, Drenth JPH. Recently discovered mutation in the chronic mucocutaneous candidiasis STAT1 gene is not implicated in oesophageal and head and neck carcinoma, submitted for publication.
- 26.Watanabe G., Kaganoi J., Imamura M., Shimada Y., Itami A., Uchida S. Progression of esophageal carcinoma by loss of EGF-STAT1 pathway. Cancer Journal. 2001;7(2):132–139. [PubMed] [Google Scholar]


