Abstract
Basidiobolomycosis is a rare chronic subcutaneous infection caused by Basidiobolus ranarum. The disease usually occurs in children, less often in adolescent and rarely in adults. Males are more frequently affected than females. We report a case of subcutaneous zygomycosis of head and neck region caused by B. ranaram, in an immunocompetent adult female presenting with nontender firm swelling over the nape of neck and temporo-parietal region. The diagnosis was confirmed by histopathology, microbiology (culture) and DNA sequencing of molecular technique (sequencing). The patient was successfully treated with amphotericin B and potassium iodide
Keywords: Zygomycosis, Basidiobolus ranaram, Nape of neck, Amphotericin B and potassium iodine
1. Introduction
Basidiobolus ranaram is a saprophytic filamentous fungus belonging to the order Entomophthorales [1]. Unlike other Zygomycetes (Mucorales), B. ranarum causes subcutaneous zygomycosis in healthy individuals [2]. Zygomycosis caused by B. ranarum is a chronic inflammatory or granulomatous disease generally restricted to the subcutaneous tissue of the limbs, chest, back or buttocks, primarily occuring in children with male predominance [3]. Occasionally cases of gastrointestinal and systemic infections due to this agent are reported. B. ranaram occurs saprophytically in decaying plant material and has been frequently reported from the intestinal contents of several species of amphibians [4]. The disease is widely prevalent in tropical and subtropical regions of world [5]. In the past, clinical isolates of Basidiobolus were classified as B ranarum, B. meristosporus and B. haptosporus. But recent taxonomic studies based on antigenic analysis, isoenzyme banding and restriction enzyme analysis of rDNA indicate that all human pathogens belong to B. ranarum. Here, we report a rare case report of basidiobolus zygomycosis in an immunocompetent adult female involving nape and lateral aspect of neck and temporo-parietal region of head to demonstrate its atypical clinical presentation, discuss the differential diagnosis, highlight its characteristic diagnostic features and various treatment options available to treat this condition.
2. Case report
The 42 year old female reported to our outpatient clinic with swelling over right nape and lateral aspect of neck for seven months and swelling over right temporal region for 15 days. The swelling was insidious in onset and gradually progressive started over nape of right side of neck and progressed to involve the right side of face. It was associated with severe non radiating pain over the right side of face and neck which was relieved temporarily with analgesics. There was no history of fever, cough, weight loss, nasal obstruction, nasal discharge, dysphagia, respiratory distress, hoarseness, weight loss, diabetic mellitus, hypertension, bronchial asthma, or renal disease. She had consulted local doctors before and had undergone excision biopsy thrice before presenting to us. First excision biopsy was done at eight weeks after the appearance of the swelling which revealed aggregates of lymphocytes, eosinophils, few macrophages and plasma cells and no definite diagnosis could be made. Four weeks later, another swelling appeared the same region and excision of the swelling was done which was reported as a parasitic infestation, and was put on antihelminth treatment. In spite of this treatment the swelling slowly increased in size involving nape and lateral aspect of right neck and right temporoparietal region associated with deep boring pain. A third biopsy was repeated at 16 weeks. Histopathology showed granulomatous lesion suspicious of tuberculosis and the patient received anti-tubercular chemotherapy for four months but the patient discontinued as there was no improvement of symptoms and she reported to our institute.
Clinical systemic examination did not reveal any obvious abnormality. Examination of lesion showed diffuse, tender, firm to hard, nonfluctuant, non-pulsate, swelling over the nape of neck, right lateral region of neck, post-auricular and pre-auricular region, superior half of ear pinna, external auditory canal superiorly till temporo-parietal region leading to right periorbital puffiness (Fig. 1). A linear scar indicating previous surgical intervention measured 6×0.3 cm over the swelling along right lateral aspect of neck. There was no evident sinus, fistula, pus discharge, excoriation or ulceration over the swelling.
Fig. 1.
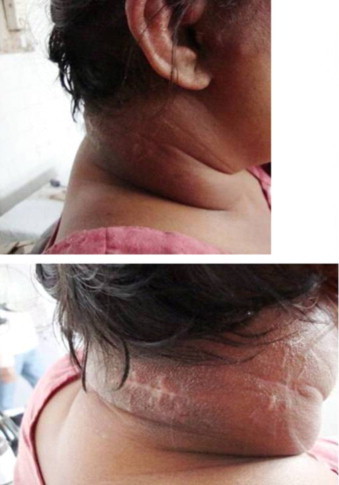
Preoperative photograph showing subcutaneous induration of the cervical and facial region showing linear scar over the neck.
Review of histopathology slides showed features of necrotizing vasculitis. Auto-immune disease was suspected and work up for the same including ANCA, rheumatoid factor, antinuclear antibody was normal. Routine blood investigations were normal.
Contrast enhanced computed tomography of neck and paranasal sinuses showed heterogenous soft tissue density diffusely involving muscles of the back of neck, posterior triangle of neck, post-aural, parotid, temporo-parietal region on the right side (Fig. 2). She was treated with intravenous antibiotics and analgesics for one week in view of tender, erythematous swelling depicting acute inflammation. The swelling persisted, however there was mild reduction in tenderness. With a suspicion of fungal etiology a request to cytologist was sent for fine needle aspiration cytology (FNAC) to look specifically for fungus. FNAC was done which showed numerous multinucleated giant cells and occasional epitheloid cell granuloma in the background of scattered polymorphs, eosinophils and macrophages. A few ill preserved structures, possibly fungal profiles, were noted. A 10% KOH and Blankphor wet mount of the tissue revealed broad, irregular, and aseptate hyphae as shown in (Figs. 3 and 4). Culture on Sabouraud dextrose agar showed a creamy white, membranous, radially folded colony. Examination of lacto-phenol cotton blue wet mount revealed aseptate hyphae with numerous smooth walled zygospores and characteristic conjugation beaks corresponding to the morphology of B. ranaram. Identity of the isolate was confirmed by DNA sequencing by molecular technique. The isolate has been deposited at the National Culture Collection of Pathogenic Fungi (NCCPF), PGIMER, Chandigarh.
Fig. 2.
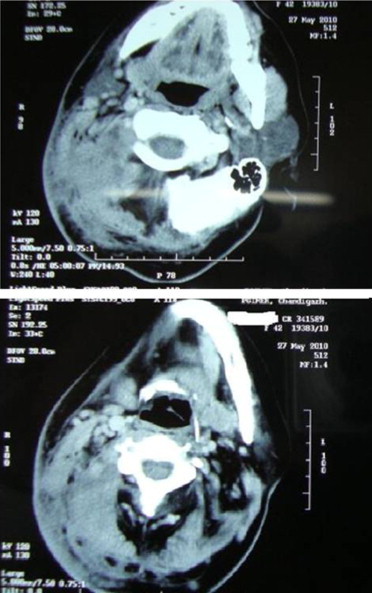
Contrast enhanced CTscan of neck showing heterogeneous soft tissue density involving diffusely right side of neck and right side of face.
Fig. 3.

Microscopy of the KOH mount shows broad ribbon like hyaline pauciseptate hyphae.
Fig. 4.
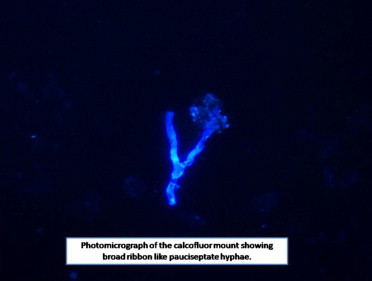
Photomicrograph of the calcofluor mount showing broad ribbon like pauciseptate hyphae.
She was treated with intravenous amphotericin B deoxycholate at the dose of 1 mg/kg body weight daily and cumulative dose of 2 gm was infused over a period of one and half month. In addition, oral potassium iodide given at a dose of five drops three times a day and gradually increased to 30 drops over a two-month period. The swelling completely disappeared on completion of the treatment. She is still under follow up and completely symptoms free (Figs. 5a and 6.)
Fig. 5.
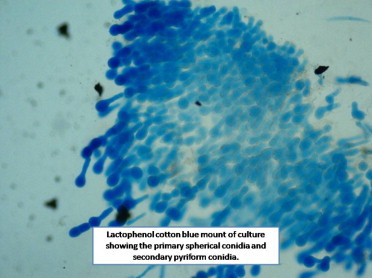
Lactophenol cotton blue mount of culture showing the primary spherical conidia and secondary pyriform conidia.
Fig. 6.
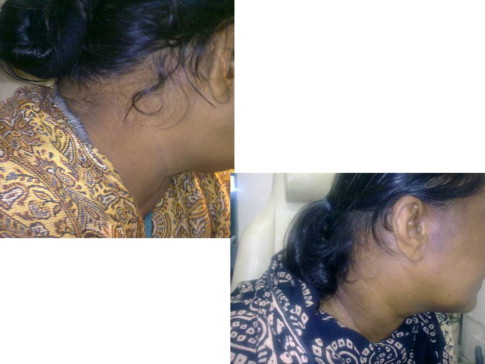
Postoperative photograph of patient complete resolution of lesion.
3. Discussion
The term zygomycosis embraces both deep and subcutaneous mycosis caused by fungi belonging to various genera of the class Zygomycetes, namely, mucormycosis and entomophthoromycosis. The term mucormycosis is used to refer to infections due to molds belonging to the order Mucorales. These organisms can cause rhino-cerebral, pulmonary, gastrointestinal, cutaneous, or disseminated infection in predisposed individuals. Infection with fungi from the order Entomopthorales typically occurs in immunocompetent patients residing in subtropical and tropical climates. Unlike Mucorales, Entomophthorales do not cause angioinvasive disease. Entomophthoromycoses has been subdivided into basidiobolomycosis and conidiobolomycosis.
Conidiobolomycosis is a chronic mycosis affecting the subcutaneous tissues. It originates in the nasal sinuses and spreads to the adjacent subcutaneous tissue of the face, causing disfigurement whereas basidiobolomycosis is a chronic subcutaneous infection of the trunk and limbs [6]. Basidiobolomycosis is encountered chiefly in the tropical regions in East and West Africa, Indonesia and India [7].
Basidiobolomycosis predominantly occurs in healthy individuals with apparently intact immunological status. Though infection with Basidiobolus can occur at any age, it is more common in children younger than 10 yr of age. Males are more frequently affected than females. No predisposing factors for infection are known. This fungus lives as a saprophyte in soil humus and on decomposing plant in moist and warm regions. It has also been isolated from feces of insects, amphibians and reptiles [8]. Infection is acquired through inhalation of spores, or their introduction into the nasal cavities by soiled hands or via minor trauma and insect bites [4,9]. Most cases affect men with agricultural or outdoor occupations.
B. ranarum was first described as an isolate from frogs in 1886 [10]. It was later cultured from the intestinal contents and ultimately the excreta of frogs. Infection by B. ranarum manifests itself as a slowly progressive subcutaneous tumefaction located on the trunk, buttocks or proximal portion of the limbs or as an invasion of the thoracic or abdominal cavities. The most common site of involvement is the buttocks and thighs [11]. The most common presentation is hardened nodule which expands and spreads locally and may eventually ulcerate. The nodular lesions contain inflammatory cellular material with many eosinophils, accounting for the associated erythema and warmth of the skin [4]. The condition is slowly progressive but seldom life threatening.
Our case is unique because of involvement of cervical and facial region which is rarely involved in basidiobolomycosis. The common site of involvement is the trunk and the gluteus region. Basidiobolomycosis is seen more commonly in young age groups while in our case the patient was adult female belonging to low socioeconomic group. She was a housewife and had less outdoor activity and had no history of insect bite.
The diagnosis of basidiobolomycosis requires a high degree of clinical suspicion by the clinician and the mycologist. The differential diagnosis of such a lesion includes tuberculosis (M. tuberculosis and M. ulcerans), localized filarial elephantiasis, onchocerciasis, Burkitt's lymphoma, scleroderma, Wegener granulomatosis [12]. Histopathology may be suggestive of basidiobolomycosis but culture remains the gold standard to diagnose this rare clinical entity [13]. Histopathology shows inflammatory granulomatous reaction with a predominant mononuclear cell infiltrate consisting of lymphocytes, histiocytosis and multinucleate giant cells. Sometimes broad thin walled hyphae with irregular branching may be seen in the center of lesion. Splendore-Hoeppli phenomenon (presence of eosinophilic material around the hyphae) may be seen. Typically there is no evidence of invasion of blood vessels, necrosis and tissue infarction [12]. In our case the diagnosis of basidiobolomycosis was suspected in fourth biopsy. A high index of suspicion is must for diagnosing such rare lesions.
Basidiobolus can be cultured on the routine media used in the diagnostic mycology like Sabouraud dextrose agar (SDA), potato dextrose agar or cornmeal agar [14]. The colonies are typically flat and furrowed, are yellowish to grayish on the surface with pale reverse and have wavy texture and produce musty odor. Microscopically the colonies produce large vegetative hyphae which become increasingly septate as they mature [15]. The role of KOH smear cannot be underestimated and is valuable aid in diagnosis. Examination of the Blankophor (fluorescent dye) wet-mount preparation of tissue biopsy under fluorescent microscope increases the sensitivity of diagnosis. However isolation of the causative agent by culture and molecular confirmation has epidemiological significance and also helps in the definitive diagnosis and determine the susceptibility to the antifungal agents.
Various pharmaceutical agents have been used to successfully treat basidiobolomycosis including most commonly potassium iodide (KI), trimethoprim-sulfamethoxazole, amphotericin B, oral azoles [16–18]. Treatment of basidiobolomycosis is not always successful, and no single drug has proved effective in the treatment of all cases of basidiobolomycosis [18]. Hence, till date, no single therapy is recommended for the treatment of basidiobolomycosis. Potassium iodide appears to be effective when given for at least three months (1.5–2 g/day), but patients often do not comply due to adverse effects. In-vitro antifungal susceptibility studies have shown amphotericin B not to be an effective agent against Basidiobolus isolates [19]. Among the azoles, ketoconazoles have shown variable efficacy and may require months of continuous treatment for cure [19]. Recently, Goyal et al. has reported a case of basidiobolomycosis involving nose and facial region in a 32 year male who was successfully treated with itraconazole plus terbinafine [20]. In another report (Ramesh et al.) of similar presentation of basidiobolomycosis involving a 5 year male child was treated with potassium iodide alone [21]. We could successfully treat our patient with combination of intravenous amphotericin B (50 mg/day, cumulative dose of 2 gm) and KI (1.5 g/day) for about two months. Hence, we recommend this combination therapy of amphotericin and potassium iodide for this very rare infection.
Conflict of interest
There is no financial or personal interests that might be potentially viewed to influence the work presented.
References
- 1.Bandeira V., Lascet I.G. Zigomicose. In: Talharis, Neves R.G., editors. Dermatologia tropical. Medsi; SãoPaulo: 1995. pp. 191–202. [Google Scholar]
- 2.Williams AO. Pathology of phycomycoses due to Entomopthora and Basidiobolus species. Archives of Pathology. 1969;86:113–120. [PubMed] [Google Scholar]
- 3.Joe L.K., Njo-Imjo T.E. Basidiobolus ranarum as a cause of subcutaneous mycosis in Indonesia. Archives of Dermatology. 1956;74:378–383. doi: 10.1001/archderm.1956.01550100046008. [DOI] [PubMed] [Google Scholar]
- 4.Cameroon H.M. Entomophthoromycosis. In: Mahgoub E.S., editor. Tropical mycoses. Janssen Research Council; Beerse (Belgium): 1990. pp. 186–198. [Google Scholar]
- 5.Sugar A.M. Agents of mucormycosis and related species. In: Mandell G.L., Douglas R.G., Bennett J.E., editors. Principlesand practice of infectious diseases. 3rd ed. Churchill Livingstone, Inc.; NewYork, N.Y: 1990. pp. 1962–1972. p. [Google Scholar]
- 6.Echetebu K.O., Ononogbu I.C. Extracellular lipase and proteinase of Basidiobolus haptosporus: possible role in subcutaneous mycosis. Pathologia. 1982;80:171–177. doi: 10.1007/BF00437580. [DOI] [PubMed] [Google Scholar]
- 7.CClark B.M. Epidemiology of phycomycosis. In: Wolstenhome G.E.W., Porter R., editors. Systemic mycoses. Little, Brown & Co.; Boston, Mass: 1968. pp. 179–192. p. [Google Scholar]
- 8.Thaxter R. The Entomophthoreae of the United States. Memoirs Read Before the Boston Society of Natural History. 1988;4:133–201. [Google Scholar]
- 9.Vismer H.F., DeBeer H.A., Dreyer L. Subcutaneous phycomycosis caused by Basidiobolus haptosporus (Dreschler, 1947) South African Medical Journal. 1980;58:644–647. [PubMed] [Google Scholar]
- 10.Eidam E. Basidiobolus, eine neue Gattung der Entomophthoraceen. Beitrage zur Biologie der Pflanzen. 1886;4:181–251. [Google Scholar]
- 11.Goodman N.L., Rinaldi M.G. 1991. Agents of zygomycosis. p. 674–92. [Google Scholar]
- 12.Sugar A.M. Agents of mucormycosis and related species. In: Mandell G.L., Bennett J.E., Dolin R., editors. 4th ed. vol. 2. 1995. pp. 2311–2321. (Mandell, Douglas and Bennett's principles and practice of infectious diseases). [Google Scholar]
- 13.Chandler F.W., Watts J.C. ASCP Press, Chicago, Ill. Churchill Livingstone, Inc.; New York, N.Y: 1987. Pathologic diagnosis of fungal Infections. 85–95. [Google Scholar]
- 14.Beneke E.S., Rogers A.L. Star Publishing Co.; Belmont, Ca: 1996. Medical mycology and human mycoses. 182–206. [Google Scholar]
- 15.Kwon-Chung K.J., Bennett J.E. Lea & Febiger; Philadelphia: 1992. Medical mycology. 449–63. [Google Scholar]
- 16.Bittencourt A.L., Arruda S.M., de Andrade J.A.F., Carvalho. E.M. Basidiobolomycosis: a case report. Pediatric Dermatology. 1991;8:325–328. doi: 10.1111/j.1525-1470.1991.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamalam A., Thambiah A.S. Muscle invasion by Basidiobolus haptosporus. Sabouraudia and Journal of Medical and Veterinary Mycology. 1984;22:273–277. [PubMed] [Google Scholar]
- 18.Restrepo A. Treatment of tropical mycoses. Journal American Academy of Dermatology. 1994;31:S91–S102. doi: 10.1016/s0190-9622(08)81277-7. [DOI] [PubMed] [Google Scholar]
- 19.Guaro J, Aguilar C, Pujol I. In-vitro antifungal susceptibilities of Baisidiobolus and Conidiobolus spp. strains. The Journal of Antimicrobial Chemotherapy. 1999;44:557–560. doi: 10.1093/jac/44.4.557. [DOI] [PubMed] [Google Scholar]
- 20.Arun Goyal, Neelima Gupta, Shukla Das, Sarika Jain. Basidiobolomycosis of the nose and face: a case report and a mini-review of unusual cases of basidiobolomycosis. Mycopathologia. 2010;170(3):165–168. doi: 10.1007/s11046-010-9310-9. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh A., Deka R.C., Vijayaraghavan M., Ray R., Kabra S.K., Rakesh K. Entomophthoromycosis of the nose and paranasal sinus. Indian Journal of Pediatrics. 2000;67(4):307–310. doi: 10.1007/BF02758182. [DOI] [PubMed] [Google Scholar]


