Abstract
Non-invasive assays are increasingly being used in patients with suspected fungal infections. Limitations to these tests include limited sensitivity, specificity, and cross reactivity with other fungal pathogens. Herein we report a case of disseminated histoplasmosis producing a false positive serum and bronchoalveolar lavage (BAL) Aspergillus galactomannan assays. This test may have a role in the evaluation of patients with suspected histoplasmosis in settings where Histoplasma antigen testing is not widely available.
Keywords: Histoplasmosis, Aspergillosis, HIV/AIDS, Aspergillus galactomannan assay, Cross-reactivity
1. Introduction
Invasive fungal infections are an important cause of morbidity and mortality in patients with immunocompromised conditions, such as transplant recipients, HIV-infected patients, and recipients of corticosteroids. Histoplasmosis remains an important endemic mycosis, especially in patients with advanced HIV/AIDS. Often, more than one opportunistic infection may be present in those advanced HIV/AIDS patients, underscoring the importance of thorough clinical and laboratory evaluations [1,4]. In recent years, new diagnostic assays have become available to evaluate patients suspected of having invasive fungal infections, but with variable sensitivity and specificity. Histoplasma antigen and Aspergillus galactomannan (GM) testing are two widely used fungal diagnostic tests available in the United States.
The Histoplasma antigen enzyme-linked immunoassay (EIA) can be performed from samples in serum, urine, bronchoalveolar lavage (BAL) fluid or cerebrospinal fluid [2]. The Platelia® Aspergillus GM EIA has been commercially available in Europe since the mid-1990s and was approved by the US Food and Drug Administration in 2003. It is used to detect GM in serum or BAL fluid for diagnosis of invasive aspergillosis. GM antigen positivity has been incorporated into the microbiological diagnostic criteria proposed by European Organization for Research and Treatment of Cancer (EORTC) and Mycoses Study Group (MSG) for diagnosis of invasive aspergillosis [3,20]. Herein we describe a patient with advanced HIV infection and disseminated histoplasmosis who had a strongly positive Aspergillus GM test due to cross-reactivity between histoplasmosis infection and the Aspergillus GM assay and we briefly review literature for similar cases.
2. Clinical presentation
A 49-year-old man from the southeastern United States, diagnosed with HIV infection more than twenty years prior, presented with fever, dry cough, dyspnea, and left hip pain for four weeks. Two weeks prior to admission he noticed a skin rash that started in his left leg and spread sparsely to other parts of the body. He reported considerable amount of weight loss over the past 3 months but denied nausea, diarrhea or abdominal pain. He admitted that he had not been taking anti-retroviral therapy for the past year.
On examination, temperature was 102 °F, blood pressure was 92/54 mmHg, pulse was 130 beats per minute and respiratory rate was 18 breaths per minute. His oxygen saturation was 95% on room air, and 89% with minimal activity. Lungs were clear to auscultation. There were multiple punched out hemorrhagic ulcers over his left leg (Fig. 1). Similar skin lesions were scattered over face, scalp, behind the left ear (Fig. 2), upper back, chest and the both upper extremities. He had limited range of motion on left hip examination, especially on adduction, and required assistance with cane on walking. His oropharynx was clear and remainder of the physical examination was unremarkable.
Fig. 1.
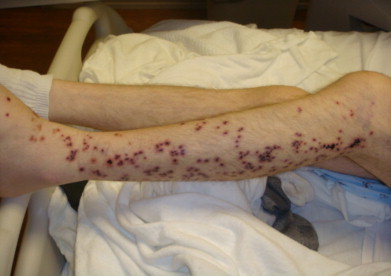
Multiple punched out hemorrhagic skin ulcers over the left leg.
Fig. 2.
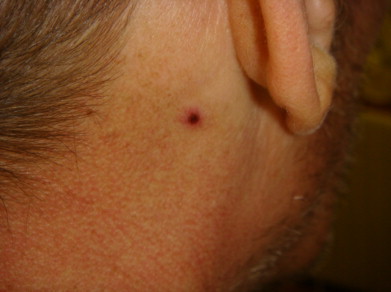
A hemorrhagic skin rash over the posterior part of the left ear.
Laboratory studies revealed a white blood cell count of 900/mm3 (reference range 4000–11,000) with 92% neutrophils, hemoglobin level was 9 g/dL (12–15) and platelet count was 15,000/mm3 (150,000–400,000). Peripheral blood smear on admission showed no evidence of intracellular inclusions. Basic metabolic panel was within normal range. Liver function tests revealed alanine aminotransferase 200 U/L (10–44), aspartate aminotransferase 61 U/L (14–40), total bilirubin 1.1 mg/dL (0.4–1.4), albumin 2.3 g/dL (3.4–5.0), lactate dehydrogenase 266 U/L (120–240), with normal alkaline phosphatase level and international normalization ratio (INR). His CD4 count was 12 cells/μL (380–1500) and HIV RNA-PCR was 6.7 million copies/mL. Computed tomography of the chest and abdomen showed multiple pulmonary micro- and macro-nodules with right hilar lymphadenopathy and hepatosplenomegaly. Magnetic resonance imaging (MRI) of the pelvis noted an infiltrative mass over the proximal part of the left femur shaft (Fig. 3) without evidence of osteomyelitis or avascular necrosis of the femoral head.
Fig. 3.
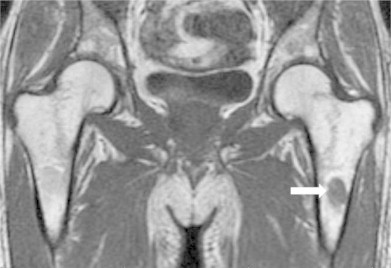
MRI of the pelvis showing oval-shaped infiltrated mass (white arrow) in the left proximal femoral shaft.
Blood cultures (bacterial, fungal and mycobacterial) were obtained and empiric intravenous vancomycin and piperacillin–tazobactam were initiated on admission. Biopsies were obtained from skin ulcers and left hip lesion. Anti-retroviral therapy with tenofovir, emtricitabine, ritonavir and atazanavir was started. Serum and urine Histoplasma antigen, and serum Aspergillus GM assays were performed on the day of admission. On day 4 of admission, the patient underwent bronchoscopy, BAL and transbronchial tissue biopsy with bacterial, fungal and mycobacterial cultures. BAL fluid for Aspergillus GM and Histoplasma antigen were sent as well.
On day 5 of hospitalization, peripheral blood smear showed intracellular inclusions suggestive of Histoplasma capsulatum (Fig. 4). Amphotericin B lipid complex (5 mg/kg daily) was started with significant improvement of symptoms, pancytopenia and transaminitis within 3 days. On day 6, intravenous vancomycin and piperacillin–tazobactam were discontinued after bacterial blood cultures returned negative and resolution of neutropenia. Fungal blood cultures were positive for H. capsulatum a week after collection (on day 7 of admission). H. capsulatum also grew from fungal cultures of skin ulcers and left femur bone tissue samples.
Fig. 4.
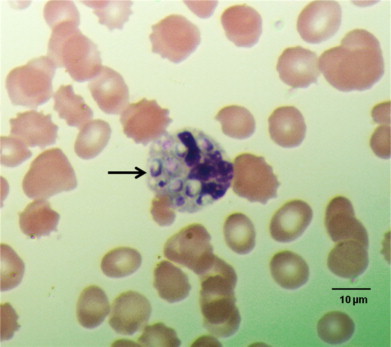
Peripheral blood smear illustrating the intracellular yeast (arrow) (Wright s stain, 1000 x magnification).
The result of serum Aspergillus GM assay taken on admission was >3.0 (normal <0.5 Index), and the BAL Aspergillus GM assay showed a result of 4.72 (normal <0.5 Index). Serum Aspergillus GM assay was repeated 3 days after discontinuation of piperacillin–tazobactam antibiotic and it remained markedly elevated as >3.0 (normal <0.5 Index). However, BAL and transbronchial tissue biopsy cultures and microscopic examination were positive only for H. capsulatum (Fig. 5). Urine and BAL Histoplasma antigen tests were positive >19 ng/mL (above the limit of quantification). The patient's condition continued to improve with amphotericin B lipid complex therapy and after 2 weeks he was transitioned to oral itraconazole. At 1-month follow-up, he was doing well with resolution of skin lesions and respiratory symptoms. He had minimal left hip pain and he was able to walk unassisted. His CD4 count had increased to 115 cells/μL and HIV RNA-PCR was reduced to 2000 copies/mL.
Fig. 5.
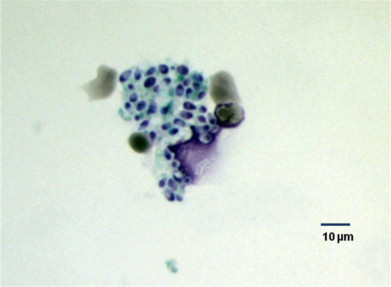
Histoplasma capsulatum yeasts on transbronchial tissue biopsy culture (Grocott's Methenamine Silver stain, 400 x magnification).
3. Discussion
Invasive fungal infections should be considered in immunocompromised hosts with proper clinical syndromes and the appropriate epidemiological exposures. Histoplasmosis is an endemic mycosis seen in the midwestern and southeastern United States (highly prevalent in the Mississippi and Ohio river valleys), and in many areas of Central and South America. In Europe, most patients of histoplasmosis have been reported from Italy, where the organism has been isolated from soil in the Po River valley. Other European countries, mainly United Kingdom, Spain, France, Germany and Belgium, have also published several cases of histoplasmosis [5–7]. Certain countries of Asia-Pacific region are endemic for histoplasmosis, such as Australia, India and Southeast Asia [8]. Histoplasmosis is also endemic in central and west areas of Africa as well as on the island of Madagascar [9]. Histoplasmosis can produce life-threatening manifestations in patients with HIV infection, especially with CD4 counts less than 100 cells/μL. Diagnosis is usually established by direct microscopy or cultures from respective specimens (blood, BAL, tissues, bone marrow, CSF) or by antigen detection (urine, blood, BAL or CSF). In patients with advanced HIV/AIDS, such as our patient, it is common to have more than one opportunistic infection, especially if the CD4 count is less than 50 cells/μL.
Invasive aspergillosis is an uncommon infection in patients with HIV infection. One of the studies analyzed 35,252 HIV-infected patients in the US showed the incidence of aspergillosis was as low as 3.5 cases per 1000 person-years [10]. An epidemiological survey conducted in France also demonstrated significant lower incidence (0.02–0.13% per annum) of aspergillosis in HIV patients compared to those in allogenic stem cell transplant recipients (12.8%), solid organ transplant patients (11.1%) and hematological malignancies (6.3–8%) [11]. However, invasive aspergillosis, although rare in HIV patients, is associated with poor survival. Risk factors for invasive aspergillosis in HIV patients include advanced HIV (CD4 count of less than 50 cells/μL), severe neutropenia (<500 cells/mm3) and recent corticosteroid use [12]. Co-infection with invasive aspergillosis in a patient with HIV/AIDS and disseminated histoplasmosis has been reported [4]. However, in our patient, there was no convincing clinical or radiological evidence for invasive aspergillosis.
Galactomannan is a polysaccharide cell wall component of Aspergillus species and the target of detection by the Platelia® Aspergillus GM enzyme-linked immunoassay (EIA). A Cochrane review reported the sensitivity of Aspergillus GM assay for invasive pulmonary aspergillosis as 70% (serum) and 85% (BAL) whereas its specificity was 89% (serum) and 95% (BAL) primarily in patients with hematological malignancies and stem cell transplantation [3]. Fewer data are available for solid organ transplant recipients. A study reported sensitivities of 25% in serum and 100% in BAL, with specificities of 97% in serum and 84% in BAL among patients with solid organ transplants [13]. No large studies are available for Aspergillus GM assay performance in HIV infected patients. Another drawback of this assay is its cross-reactivity with other fungal species other than Aspergillus in which GM is a component of their cell walls. These fungi include Histoplasma, Penicillium, Blastomyces, Paracoccidioides, Cryptococcus, Nigrospora, Paecilomyces, Trichothecium, Lichtheimia ramose, Fusarium and Geotrichum species [14–17]. Furthermore, false-positive Aspergillus GM tests have been reported in patients taking certain antibiotics (e.g. piperacillin–tazobactam and amoxicillin–clavulanate) [18]. In our patient, serum Aspergillus GM test was repeated 3 days after stopping the antibiotics as to avoid cross-reactivity between the Aspergillus GM assay and piperacillin–tazobactam. The repeat serum Aspergillus GM assay level was still elevated significantly above the level of quantification which effectively rules out possibility of cross-reactivity with piperacillin–tazobactam [19].
The cross-reactivity of Aspergillus GM assay has been observed in the management of a patient with histoplasmosis and some authors have suggested the Aspergillus GM assay may have utility as an adjunctive test for the diagnosis of histoplasmosis when other non-invasive tests are unavailable [20,21]. Table 1 enlists reported evidence of cross-reactivity of Platelia® Aspergillus GM assay in immunocompromised patients with culture-proven histoplasmosis. It is important to differentiate these two fungal infections because voriconazole has limited efficacy in the treatment of histoplasmosis and itraconazole has not been established as a preferred therapy for invasive pulmonary aspergillosis [26,27]. Because of the cross-reactivity of Aspergillus GM assay in patients with histoplasmosis, a false-positive result could easily obscure the correct diagnosis and lead to inappropriate therapy and suboptimal outcomes. Of note, patients with invasive aspergillosis who have high level of Aspergillus GM do not have cross-reactivity with Histoplasma antigen assay as it seems to be just “one-way” cross-reactivity [25].
Table 1.
Cross-reactivity of Platelia® Aspergillus Galactomannan (GM) antigen assay with culture-proven Histoplasma infections.
| Hosts | Type of Histoplasma Infection | Serum Platelia® Aspergillus GM (OD Index) | BAL Platelia® Aspergillus GM (OD Index) |
|---|---|---|---|
| Solid organ transplants (4 Renal transplants, 2 Liver transplants) [20,22] | Disseminated (2 patients), Pulmonary (4 patients) | 2.6–8.61 | 0.55–6.20 |
| Autologous stem cell transplant [23] | Disseminated, Pulmonary | 5.2 | NA |
| Rheumatoid Arthritis [24] | Disseminated, Pulmonary | 5.38 | NA |
| HIV (6 patients) [20] | Disseminated | 1.2–4.0 | NA |
| NA (6 patients) [25]a | Disseminated (4 patients), Pulmonary (2 patients) | 1.5–7.8 | NA |
| Advanced HIV/AIDS(Our index patient) | Disseminated (Blood, Cutaneous, Pulmonary, Bone, Bone Marrow, Liver) | >3.0 | 4.72 |
NA—Not Available; GM—Galactomannan; BAL—bronchoalveolar lavage fluid; OD—Optical Density; Platelia® Serum & BAL Aspergillus GM (OD Index)—Negative if less than 0.5.
Unable to elicit underlying host diseases on personal communication with the main corresponding study author.
4. Conclusion
Patients with HIV infection can have multiple co-infections and the treating physician may perform a diagnostic evaluation that includes non-invasive fungal diagnostic assays. The clinician should be aware of the possibility of cross-reactivity of Aspergillus GM assay in patients with histoplasmosis, but the potential to use this assay as a diagnostic tool for histoplasmosis requires further studies. It is important to recognize the limitations of these assays and understand that cultures and histology remain the gold standard for diagnosis of Aspergillus infection, especially in HIV-infected patients.
Conflicts of interest
There are none.
Acknowledgments
We thank Drs Victoria Johnson, Edward Hook and Peter Pappas for their extensive support and suggestions on writing this submitted manuscript. We also would like to extend our thanks to Drs Kierstin Lesile, Bassem Abazid, Catherine Coomer, Nakshatra Saxena, Steven Bleich, and Travis Steinke for their excellent management and care of the patient during hospitalization.
References
- 1.Mylonakis E., Barlam T.F., Flanigan T., Rich J.D. Pulmonary aspergillosis and invasive disease in AIDS: review of 342 cases. Chest. 1998;114(1):251–262. doi: 10.1378/chest.114.1.251. [DOI] [PubMed] [Google Scholar]
- 2.Wheat L.J. Improvements in diagnosis of histoplasmosis. Expert Opinion on Biological Therapy. 2006;6(11):1207–1221. doi: 10.1517/14712598.6.11.1207. [DOI] [PubMed] [Google Scholar]
- 3.Leeflang M.M., Debets-Ossenkopp Y.J., Visser C.E., Scholten R.J., Hooft L., Bijlmer H.A. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Systematic Review. 2008;8(Oct):4. doi: 10.1002/14651858.CD007394. [DOI] [PubMed] [Google Scholar]
- 4.Jones P.G., Cohen R.L., Batts D.H., Silva J., Jr. Disseminated histoplasmosis, invasive pulmonary aspergillosis, and other opportunistic infections in a homosexual patient with acquired immunodeficiency syndrome. Sexually Transmitted Disease. 1983;10:202–204. doi: 10.1097/00007435-198311000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Antinori S, Magni C, Nebuloni M, Parravicini C, Corbellino M, Wheat LJ, et al. Histoplasmosis among human immunodeficiency virus-infected people in Europe: report of 4 cases and review of the literature. Medicine (Baltimore). 2006 Jan;85(1):22–36. [DOI] [PubMed]
- 6.Wheat L.J. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49(4):274–282. doi: 10.1111/j.1439-0507.2006.01253.x. Jul. [DOI] [PubMed] [Google Scholar]
- 7.Ashbee HR, Evans EG, Viviani MA, Dupont B, Chryssanthou E, Surmont I, et al. ECMM working group on histoplasmosis. Histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Medical Mycology. 2008 Feb;46(1):57–65. [DOI] [PubMed]
- 8.Chakrabarti A., Slavin M.A. Endemic fungal infections in the Asia-Pacific region. Medical Mycology. 2011;49(4):337–344. doi: 10.3109/13693786.2010.551426. May. [DOI] [PubMed] [Google Scholar]
- 9.Gugnani H.C., Muotoe-Okafor F. African histoplasmosis: a review. Revista Iberoamericana De Micologia. 1997;14:155–159. [PubMed] [Google Scholar]
- 10.Holding K.J., Dworkin M.S., Wan P.C., Hanson D.L., Klevens R.M., Jones J.L. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Adult and adolescent spectrum of HIV disease project. Clinical Infectious Diseases. 2000;31(5):1253. doi: 10.1086/317452. [DOI] [PubMed] [Google Scholar]
- 11.Cornet M., Fleury L., Maslo C., Bernard J.F., Brücker G. Invasive aspergillosis surveillance network of the assistance Publique-Hôpitaux de Paris. epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. Journal of Hospital Infection. 2002;51(4):288. doi: 10.1053/jhin.2002.1258. [DOI] [PubMed] [Google Scholar]
- 12.Denning D.W., Follansbee S.E., Scolaro M., Norris S., Edelstein H., Stevens D.A. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. New England Journal of Medicine. 1991;324(10):654. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 13.Clancy CJ, Jaber RA, Leather HL, Staley B, Wheat LJ, Nguyen MH, et al. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid-organ transplant recipients. Journal of Clinical Microbiology. 2007 June; 45(6):1759–1765. Epub 2007 Apr 11. [DOI] [PMC free article] [PubMed]
- 14.Giacchino M., Chiapello N., Bezzio S., Fagioli F., Saracco P., Alfarano A. Aspergillus galactomannan enzyme-linked immunosorbent assay cross-reactivity caused by invasive Geotrichum capitatum. Journal of Clinical Microbiology. 2006;44(9):3432–3434. doi: 10.1128/JCM.00856-06. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings J.R., Jamison G.R., Boudreaux J.W., Howles M.J., Walsh T.J., Hayden R.T. Cross-reactivity of non-Aspergillus fungal species in the Aspergillus galactomannan enzyme immunoassay. Diagnostic Microbiology and Infectious Disease. 2007;59:113–115. doi: 10.1016/j.diagmicrobio.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Xavier M.O., Pasqualotto A.C., Cardoso I.C., Severo L.C. Cross-Reactivity of Paracoccidioides brasiliensis, Histoplasma capsulatum, and Cryptococcus species in the commercial platelia Aspergillus enzyme immunoassay. Clinical and Vaccine Immunology. 2009;16(1):132. doi: 10.1128/CVI.00310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrás R., Roselló P., Chilet M., Bravo D., de Lomas J.G., Navarro D. Positive result of the Aspergillus galactomannan antigen assay using bronchoalveolar lavage fluid from a patient with an invasive infection due to Lichtheimia ramosa. Journal of Clinical Microbiology. 2010 Aug;48(8):3035–3036. [DOI] [PMC free article] [PubMed]
- 18.Boonsarngsuk V., Niyompattama A., Teosirimongkol C., Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scandinavian Journal of Infectious Diseases. 2010;42(6-7):461. doi: 10.3109/00365541003602064. [DOI] [PubMed] [Google Scholar]
- 19.Singh N., Obman A., Husain S., Aspinall S., Mietzner S., Stout J.E. Reactivity of platelia Aspergillus galactomannan antigen with piperacillin–tazobactam: clinical implications based on achievable concentrations in serum. Antimicrobial Agents and Chemotherapy. 2004;48(6):1989–1992. doi: 10.1128/AAC.48.6.1989-1992.2004. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranque S., Pelletier R., Michel-Nguyen A., Dromer F. Platelia Aspergillus assay for diagnosis of disseminated histoplasmosis. European Journal of Clinical Microbiology and Infectious Diseases. 2007;26:941–943. doi: 10.1007/s10096-007-0380-7. [DOI] [PubMed] [Google Scholar]
- 21.Rivière S, Denis B, Bougnoux ME, Lanternier F, Lecuit M. Lortholary O. Serum Aspergillus galactomannan for the management of disseminated histoplasmosis in AIDS. American Journal of Tropical Medicine and Hygiene. 2012 Aug;87(2):303-305. [DOI] [PMC free article] [PubMed]
- 22.Vergidis P., Walker R.C., Kaul D.R., Kauffman C.A., Freifeld A.G., Slagle D.C. False-positive Aspergillus galactomannan assay in solid organ transplant recipients with histoplasmosis. Transplant Infectious Disease. 2012;14:213–217. doi: 10.1111/j.1399-3062.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones O., Cleveland K.O., Gelfand M.S. A case of disseminated histoplasmosis following autologous stem cell transplantation for Hodgkin's lymphoma: an initial misdiagnosis with a false-positive serum galactomannan assay. Transplant Infectious Disease. 2009;11:281–283. doi: 10.1111/j.1399-3062.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 24.Narreddy S., Chandrasekar P. False-positive Aspergillus galactomannan (GM) assay in histoplasmosis. Journal of Infection. 2008;56:80–81. doi: 10.1016/j.jinf.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Wheat L.J., Hackett E., Durkin M., Connolly P., Petraitiene R., Walsh T.J. Histoplasmosis-associated cross-reactivity in the BioRad platelia Aspergillus enzyme immunoassay. Clinical and Vaccine Immunology. 2007;14(5):638. doi: 10.1128/CVI.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheat L.J., Freifeld A.G., Kleiman M.B., Baddley J.W., McKinsey D.S., Loyd J.E. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2007;45(7):807–825. doi: 10.1086/521259. Oct 1. [DOI] [PubMed] [Google Scholar]
- 27.Walsh T.J., Anaissie E.J., Denning D.W., Herbrecht R., Kontoyiannis D.P., Marr K.A. Treatment of Aspergillosis: clinical practice guidelines of the infectious diseases society of America. Clinical Infectious Diseases. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]


