Abstract
We report a case of an 80-year-old Brazilian man, farmer, with lesions on the dorsum of the hand. A direct mycological examination, cultivation and microculture slide observation was performed. The sequencing of ITS1-5.8S rDNA-ITS2 region was carried out and the etiological agent confirmed as Exophiala spinifera. The in vitro susceptibility of this isolate to antifungal agents alone and in combination was evaluated. This is the third case of phaeohyphomycosis caused by Exophiala spinifera in Brazil.
Keywords: Exophila spinifera, Phaeohyphomycosis, ITS rDNA, In vitro antifungal susceptibility, Checkerboard technique
1. Introduction
Exophiala spinifera is a dematiaceous fungus, with cosmopolitan distribution, but of rare occurrence, which can cause phaeohyphomycosis and chromoblastomycosis. Most of these infections are cutaneous and subcutaneous, but systemic infections are also reported [1–4].
The treatment is not well defined, and it is usually carried out empirically [5].
Microorganisms of the genus Exophiala are commonly found in the environment in plant materials, wood and soil [6]. The infection into cutaneous and subcutaneous tissues typically occurs by traumatic inoculation of fungal propagules, while systemic cases do not have well-defined portals of entry [7].
The first case of phaeohyphomycosis by Exophiala spinifera in Brazil was reported in 1984 by Lacaz et al. [8], where the mycosis was presented in a disseminated form. This case occurred in Midwestern Brazil. The second case occurred in the northeast of the country, where the lesions were in the form of subcutaneous abscesses involving skin, muscle and bone degeneration [9]. Here we report the third case of phaeohyphomycosis caused by Exophiala spinifera in Brazil, the first in the South of the country and the first Brazilian case with molecular identification of the etiologic agent.
2. Case
JAZ, male, farmer, 80 years, Brazilian, lives in rural area of Nova Santa Rita, Rio Grande do Sul, Brazil. Four years ago, small cutaneous lesions on the dorsum of his hand began to appear, which evolved slowly and drained secretion. He was admitted to a hospital in Porto Alegre, Rio Grande do Sul, Brazil, in August 2011, being treated twice with antibiotics for cutaneous cellulitis with little response. After 14 days, with the second antibiotic, the patient showed improvement in 30% of the lesions, clinical stabilization and was discharged. Soon after, he was sent to a dermatologist for evaluation. In the first consultation, the patient showed a severe edema of the arm and hand with pseudo skin fistulae, draining serous material upon compression (Fig. 1A). The clinical aspect of the lesion was of a mycetoma. The patient was sent to the Dermatology Service of Santa Casa de Porto Alegre, Rio Grande do Sul, Brazil, for the execution of direct microscopic examination and cultural test. Initially, the collected material was treated with 20% potassium hydroxide and the presence of fragments of moniliform dematiaceous hyphae was verified (Fig. 1B), while the presence of melanin was observed after Masson-Fontana staining (Fig. 1C), indicating a phaeohyphomycosis. Treatment was started with itraconazole at 100 mg, 12–12 h. After 1 month of treatment, the patient had already more than 60% improvement in lesion. The patient continued with itraconazole and is currently cured.
Fig. 1.
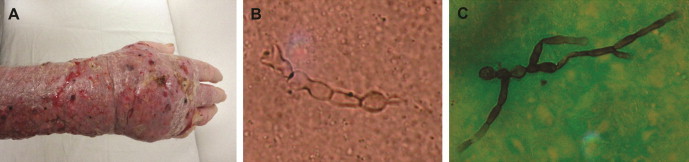
(A) Lesions caused by Exophiala spinifera (74110) in the patient's hand and arm. (B) Direct microscopic examination (KOH 20%) of material collected from the lesions revealed fragments of moniliform dematiaceous hyphae (40 X). (C) Direct microscopic examination using Masson-Fontana staining (40 X).
2.1. Mycology
Some of the material collected from the lesions was inoculated on Sabouraud Dextrose agar and incubated for 15 days at 25 °C (Fig. 2B). The growth of yeast-like, olivaceous black colonies with black pigmentation on the reverse side was verified. Microscopic study using slide culture techniques with Potato Dextrose Agar was conducted to induce sporulation. In this evaluation, straight, long, spine-like, brownish and septate conidiophores were observed. The conidia were obovoid and located at the apices of conidiogenous cells, as well as at intercalary places (Fig. 2A). Based on the morphological characteristics, the isolate was classified as an Exophiala species. This clinical isolate was inserted into the culture collection of pathogenic fungi laboratory of the Federal University of Rio Grande do Sul as 74110.
Fig. 2.
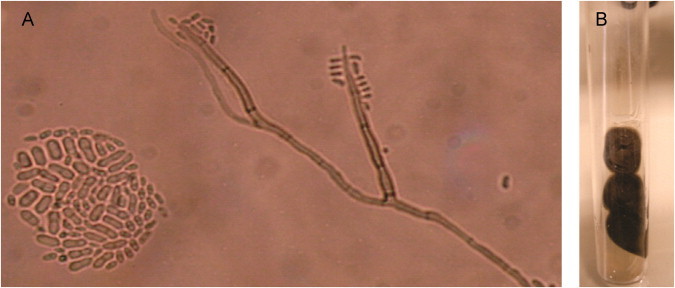
Exophiala spinifera (74110) (A) Slide culture techniques with Potato Dextrose agar, where straight, long, spine-like, brownish and septate conidiophores were observed. The obovoid conidia were located at the apices of conidiogenous cells, as well as at intercalary places. (B) Culture of the clinical isolate in Sabouraud Dextrose agar, where it had a yeast-like growth with olivaceous black colonies.
2.2. Molecular identification
For the molecular identification, the strain 74110 was grown aerobically in Sabouraud broth at 28 °C, with shaking at 180 rpm, until growth attained mid-log phase. Total genomic DNA was extracted and purified from 100 mL cultures using the UltraClean® Soil DNA Isolation Kit (Mobio, USA). Sequencing of the ITS1-5.8S rDNA-ITS2 region was performed using the Universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC -3′) [10]. The amplification conditions were: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 30 s, extension at 72 °C for 1 min, and final extension at 72 °C for 10 min. The PCR product was purified by the Ultraclean® PCR Clean-Up Kit (Mobio, USA), and sequenced at the Biotechnology Center of Federal University of Rio Grande do Sul (Cbiot/UFRGS), Brazil. The sequence was assembled and compared with sequences reported in GenBank using the basic local alignment search tool (BLAST) algorithm. Through this technique, the etiologic agent was confirmed as Exophiala spinifera, since it had 99% sequence identity with the type strain of this species (CBS 899.68).
2.3. In vitro antifungal susceptibility evaluation
The in vitro sensitivity to antifungal agents of Exophiala spinifera 74110 was determined by the broth microdilution methodology, according to the M38-A2 document of the Clinical and Laboratory Standards Institute [11]. The clinical isolate was subcultured onto potato dextrose agar (PDA; Difco) at 35 °C for 10 days. The spore suspension was produced by adding sterile saline solution (0.9%) and slightly scraping the surface of colonies with a sterile pipette. The homogeneous conidial suspension was transferred to sterile tube and the supernatants were adjusted on a spectrophotometer at 530 nm until to an optical density (OD) that ranged from 0.17 to 0.15 (68–71 T%). The inoculum was prepared by dilution in RPMI 1640 medium (Sigma, St. Louis, MO, USA) with L-glutamine, without sodium bicarbonate buffered to pH 7.0 with 165 mM morpholinepropanesulfonic acid (MOPS;Sigma) until a final concentration of 0.4×104–5×104 CFU/mL. After 5 days of incubation at 35 °C, the MIC was determined visually by comparison with the drug-free growth control well. Candida krusei ATCC 6258 was used as quality control. The antifungal agents assessed were amphotericin B, itraconazole, ketoconazole, terbinafine and voriconazole, at concentrations between 0.003 and 4 μg/mL for terbinafine and 0.03 and 16 μg/mL for amphotericin B, itraconazole, ketoconazole and voriconazole were included in these trials, besides a microorganism-free control (sterile), an antifungal-free control (growth control). The isolate was susceptible to itraconazole and ketoconazole, with minimum inhibitory concentration (MIC) of 0.25 μg/mL and 0.50 μg/mL, respectively, followed by terbinafine with an MIC 2 μg/mL, by amphotericin B with an MIC 4 μg/mL and by voriconazole with an MIC 8 μg/mL.
In parallel, an evaluation of antifungal agent combinations with the checkerboard technique [12] was carried out. The concentration ranges of the antifungal used are the same above mentioned. The interaction coefficient among drugs was quantitatively evaluated by means of the fractional inhibitory concentration index (FIC), which was calculated by the following formula: FIC=(MIC A in combination/MIC A)+(MIC B in combination/MIC B). The interaction was defined as synergistic if the FIC index was ≤0.5, no interaction if 0.5>FIC≤4.0, and antagonistic if FIC was >4.0. The associations of itraconazole and voriconazole (FCI=0.49), and of terbinafine and voriconazole (FCI=0.375) proved to be synergistic. The other associations were indifferent.
3. Discussion
Exophiala spinifera is an unusual causative agent of infection both in humans and in animals [5,13]. Until recently the identification of these agents was only based on macroscopic and microscopic aspects of the culture. Because of this, it is believed that the number of cases is underestimated by the morphological similarity between the species of the genus Exophiala [4,14].
There was a significant expansion in the knowledge about this genus with the advent of techniques of sequencing and the analysis of sequence data of the ribosomal DNA (rDNA) Internal Transcribed Spacer (ITS) regions [14,15]. Our case report is the fourth in the literature using molecular tools for identification of this fungus. The use of these methodologies is of utmost importance in the clinic, because among the different species there may be different patterns of susceptibility to antifungal agents [15].
Many drugs are used to treat infections caused by E. spinifera, such as itraconazole, pozaconazole, voriconazole, ketoconazole, fluconazole, terbinafine, amphotericin B, 5-flucytosine, and physical treatments such as cryosurgery, heat therapy and surgical excision [1–5,8,9,16–18]. However, there is currently no standard antifungal therapy for black fungi and little is known about the correlation between MICs obtained in tests in vitro and in vivo efficacy [18]. In our case, we can correlate the in vitro susceptibility and clinical response, in which the itraconazole showed a lower MIC and was effective against lesions caused by E. spinifera.
Itraconazole has proven to be the most efficient drug in the treatment of this fungus [4,16,17]. The literature reports that itraconazole requires low pH for its proper absorption. In the elderly, there may be a reduction in the production of gastric juice, which decreases the bioavailability of the drug, thus compromising its activity [19]. Our patient, despite his advanced age, showed excellent response to this antifungal.
Some studies have reported resistance of E. spinifera to itraconazole [2,3,5]. In this regard the use of combinations of antifungal agents in cases where there is no response to the antifungal used alone or in severe cases could be an alternative to increasing the efficacy of each drug and to obtain efficacy using lower doses [20]. In our study, combinations of antifungals were evaluated, in which we found that voriconazole, drug that was less active when used alone, showed synergism when combined with terbinafine and itraconazole, and in case of resistance, these combinations could have been used. At the clinic it has been observed that the association of the drugs is positive for the treatment of this etiologic agent [1].
Infections caused by this black fungi are more common in disseminated form in children, and cutaneous and subcutaneous forms in adults [9,4,18], pattern that is kept in our case.
Since the patient is a farmer and the fact that E. spinifera is present in the environment [6], it is probable that the patient has been infected at work, although he did not relate trauma injuries.
In Brazil, there were two other reported cases of infection caused by E. spinifera [8,9]. In both, the patients were children (5–12 years old) and amphotericin B was used, resulting in no clinical improvement with this drug. The first case was a disseminated phaeohyphomycosis, affecting various points throughout the extension outside of the body. The patient had a brain tumor, which was not biopsied, but was probably the source of infection. The patient was lost to follow-up. The second case reported a phaeohyphomycosis with abscesses that affected first the right side of the face. After one year, the lesions were also present on the fingers, elbow and left toe. Over time the abscesses suffered ulceration. By X-rays it was found degradation of the bone in the affected fingers. The patient died. The case reported by us is quite different from the other cases that occurred in Brazil, since the patient was an adult, with only localized cutaneous lesions and responsive to medication.
Based on our case, we can claim to be of extreme importance the molecular identification of the causative agents of phaeohyphomycosis for correct identification, as well as the evaluation of in vitro antifungal activity of the clinical isolate, as tools that can guide the clinical practice.
Conflict of interest
There are none.
Acknowledgments
The authors want to thank the CAPES (Coordenação de aperfeiçoamento de Pessoal de Nível Superior) and the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the financial support and scholarships.
References
- 1.Padhye A.A., Kaplan W., Neuman M.A., Case P., Radcliffe G.N. Subcutaneous phaeohyphomycosis caused by Exophiala spinifera. Sabouraudia. 1984;22 493–00. [PubMed] [Google Scholar]
- 2.Padhye A.A., Hampton A.A., Hampton M.T., Hutton N.W., Prevost-Smith E., Davis M.S. Chromoblastomycosis caused by Exophiala spinifera. Clinical Infectious Diseases. 1996;22:331–335. doi: 10.1093/clinids/22.2.331. [DOI] [PubMed] [Google Scholar]
- 3.Negroni R., Helou S.H., Petri N., Robles A.M., Arechavala A., Bianchi M.H. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clinical Infectious Diseases. 2004;38:e15–e20. doi: 10.1086/380840. [DOI] [PubMed] [Google Scholar]
- 4.Harris J.E., Sutton D.A., Rubin A., Wickes B., De Hoog G.S., Kovarik C. Exophiala spinifera as a cause of cutaneous phaeohyphomycosis: case study and review of the literature. Medical Mycology. 2009;47:87–93. doi: 10.1080/13693780802412611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal A., Pandhi D., Bhattacharya S.N., Das S., Aggarwal S., Mishra K. Pheohyphomycosis caused by Exophiala spinifera: a rare occurrence. International Journal of Dermatology. 2008;47:44–47. doi: 10.1111/j.1365-4632.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- 6.Conti-Dìaz I., MacKinnon J.E., Civila E. Isolation and identification of black yeasts from the external environment in Uruguay. Pan American Journal of Public Health. 1977;356:109–114. [Google Scholar]
- 7.Brandt M.E., Warnock D.W. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. Journal of Chemotherapy. 2003;15:36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 8.Lacaz C.S., Porto E., Andrade J.G., Queiroz Telles F. Feohifomicose disseminada por Exophiala spinifera. Anais Brasileiros de Dermatologia. 1984;59:238–243. [Google Scholar]
- 9.Campos-Takaki G.M., Lobo Jardim M. Report of chronic subcutaneous abscesses caused by Exophiala spinifera. Mycopathologia. 1994;127:73–76. doi: 10.1007/BF01103061. [DOI] [PubMed] [Google Scholar]
- 10.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press Incorporation; New York: 1990. pp. 315–322. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute (CLSI) 2nd ed. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA: 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved Standard M38-A2. [Google Scholar]
- 12.Cuenca-Estrella M. Combinations of antifungal agents in therapy–what value are they? Antimicrobial Agents and Chemotherapy. 2004;54:854–869. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- 13.Kettlewell P., Mcginnis M.R., Wilkinson G.T. Phaeohyphomycosis caused by Exophiala spinifera in two cats. Journal of Medical and Veterinary Mycology. 1989;27:257–264. [PubMed] [Google Scholar]
- 14.De Hoog G.S., Vicente V., Caligiorne R.B., Kantarcioglu S., Tintelnot K., Gerris van den Ende A.H.G. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. Journal of Clinical Microbiology. 2003;41:4767–4778. doi: 10.1128/JCM.41.10.4767-4778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale R.G., De Hoog G.S. Molecular diversity, new species and antifungal susceptibilities in the Exophiala spinifera clade. Medical Mycology. 2002;40:545–556. doi: 10.1080/mmy.40.6.545.556. [DOI] [PubMed] [Google Scholar]
- 16.Rajendran C., Khaitan B.K., Mittal R., Ramam M., Bhardwajw M., Data K.K. Phaeohyphomycosis caused by Exophiala spinifera in lndia. Medical Mycology. 2003;41:437–441. doi: 10.1080/1369378031000153820. [DOI] [PubMed] [Google Scholar]
- 17.Takahara M., Imafuku S., Matsuda T., Uenotsuchi T., Matsumoto T., Padhye A.A. Concurrent double infections of the skin: Phaeohyphomycosis and nocardiosis in a patient with idiopathic thrombocytopenic púrpura. Journal of the American Academy of Dermatology. 2005;53:S277–S280. doi: 10.1016/j.jaad.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Badali H., Chander J., Bayat M., Seyedmousavi S., Sidhu S., Rani H. Multiple subcutaneous cysts due to Exophiala spinifera in an immunocompetent patient. Medical Mycology. 2012;50:207–213. doi: 10.3109/13693786.2011.603367. [DOI] [PubMed] [Google Scholar]
- 19.Hof H. Mycoses in the elderly. European Journal of Clinical Microbiology and Infectious Diseases. 2010;29:5–13. doi: 10.1007/s10096-009-0822-5. [DOI] [PubMed] [Google Scholar]
- 20.Yu J., Ruoyu L., Zhang M., Liu L., Wan Z. In vitro interactions of terbinafine with itraconazole and amphotericin B against fungi causing chromoblastomycosis in China. Medical Mycology. 2008;46:745–747. doi: 10.1080/13693780802163438. [DOI] [PubMed] [Google Scholar]


