Abstract
While allergic bronchopulmonary aspergillosis and mycosis are well recognised, no cases have been described related to Alternaria spp. Alternaria is a common sensitising fungus in asthmatics and related to thunderstorm asthma. We report a case of an asthmatic who presented with worsening asthma control, mild eosinophilia on high dose inhaled corticosteroids (800 μg/day), a total IgE of 3800 KIU/L, an Alternaria-specific IgE of 21.3 KUa/L and positive skin prick test, negative specific IgE and skin prick test to Aspergillus fumigatus, Penicillium spp., Cladosporium spp., Trichophyton spp. and a normal CT scan of the thorax. He responded well to a short course of oral prednisolone and then oral itraconazole, given over 17 months but relapsed 1 month after stopping it.
Keywords: Aspergillosis, Itraconazole, Mycosis, IgE, Skin test, Alternaria
1. Introduction
Allergic bronchopulmonary mycosis (ABPM) is now a widely recognised entity. The most common form is allergic bronchopulmonary aspergillosis (ABPA), though numerous other fungi have been implicated in ABPM, including Cladosporium, Candida and Penicillium spp. [1]. Usually the diagnosis is made in an immunocompetent individual with either asthma or cystic fibrosis, and there is an underlying genetic predisposition [14]. There are no universally agreed criteria for diagnosis of ABPA or ABPM, but most proposed diagnostic guides for ABPA include the major criteria as shown in Table 1 [14]. It is proposed that the same would apply for ABPM not due to Aspergillus, in which case this would be replaced by the other fungus in the criteria.
Table 1.
Diagnostic criteria for ABPA.
| Major criteria for the diagnosis of ABPA |
| Asthma |
| Peripheral blood eosinophilia |
| Elevated total serum IgE (>1000 IU/ml)a |
| Positive immediate cutaneous reaction to Aspergillusa |
| Serum precipitating or IgG antibodies to A. fumigatus |
| Serum Aspergillus specific IgEa |
| Central bronchiectasis |
| Fleeting pulmonary infiltrates noted on chest radiograph or CT |
Essential.
Alternaria alternata is a ubiquitous saprophytic fungus found mostly in soil and plants, and has been described both indoors and outdoors as an allergen associated with asthma [32]. It has not been known to cause ABPM [1]. It has, however, been implicated in severe asthma with fungal sensitisation (SAFS) [25,26] and ‘thunderstorm asthma’. SAFS refers to a phenotype of severe asthma, involving evidence of fungal sensitisation, confirmed via skin-prick or fungus-specific serum IgE testing [1]. The diagnosis also requires the exclusion of ABPA [1]. Thunderstorm asthma is the term given to the long-described phenomenon of increased frequency of asthma exacerbations coinciding with thunderstorms, particularly at the end of or soon after summer [8]. It has been shown that thunderstorms are associated with a marked increase in air concentrations of fungal spores, with Alternaria mentioned by several studies as being significantly involved [8,9,27,12].
We describe here a case of ABPM and not SAFS or thunderstorm asthma, despite involving only sensitisation to Alternaria.
2. Case
A case of ABPM due to Alternaria from 2007 to 2011 is described, with reference to casenotes, radiological imaging and laboratory test results.
A 21 year old student with an 18 year history of asthma, hay fever, perennial rhinitis, and childhood eczema, presented with a 2 month history of worsening asthma, increasing bronchodilator use and a 4 day history of fever and sweats. Medication at that time (day 0) was cetirizine, nasal topical steroids and inhaled terbutaline. His new symptoms followed a house move; the prior owners had 2 dogs. Examination was unrewarding but a chest radiograph showed right lower zone consolidation (Fig. 1a).
Fig. 1.
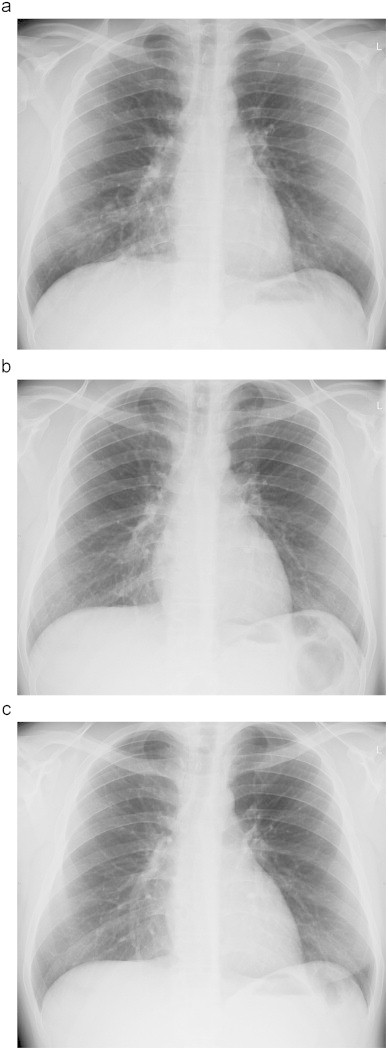
(a) Chest radiograph on day 0 showing right lower lobe infiltrate, (b) normal chest radiograph on day+28 and (c) chest radiograph on day+611.
His symptoms partially resolved with oral amoxicillin and clarithromycin, and initiation of inhaled budesonide 400 μg and then 800 μg daily. He responded rapidly and 4 weeks later (day+28) his chest radiograph was normal (Fig. 1b). However his total IgE was raised (3800 KIU/L), as was his mixed mould-specific IgE (24.6 KUa/L) and dog IgE (1.7 KUa/L), but his Aspergillus fumigatus IgE was 0.4 KUa/L (normal <0.4, Phadia) (Table 2). His pulmonary function tests were normal, with an FEV1/FVC ratio of 78%. His CT thorax (day+110) was normal, with no evidence of bronchiectasis. He did however describe occasional expectoration of brown plug-shaped mucus.
Table 2.
Serum total, Aspergillus-specific and Alternaria-specific IgE results.
| 22/6/07a (day+80) | 3/12/08a day+610) | 27/3/09b (day+726) | 30/11/09b (day+973) | 6/8/10c (day+1223) | 2/2/11d (day+1400) | Normal value | |
|---|---|---|---|---|---|---|---|
| Total IgE (KIU/L) | 3600 | 2900 | 2200 | 1700 | 1500 | 2200 | <100 |
| Aspergillus (KUa/L) | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | ||
| Alternaria (KUa/L) | 21.3 | 37.8 | 35 | 29 | 30.1 | 25.5 | <0.4 |
Before 1st course of itraconazole.
On 1st course.
After 1st course.
After 2nd course of itraconazole.
Subsequently (day+80) his Alternaria-specific IgE was found to be 21.3 KUa/L with a positive Alternaria skin prick test, negative specific IgE and skin prick test to Aspergillus fumigatus, Penicillium spp., Cladosporium spp., and Trichophyton spp. Total and fungi-specific serum IgE levels were measured using the Phadia (Uppsala, Sweden) CAP system as described in [25]. Percutaneous skin prick testing was carried out using equipment from Allergopharma (Reinbek, Germany). Fungal and non-fungal allergens, as well as a positive control (0.1% histamine) and a negative control (0.9% saline) were used, as described elsewhere [9]. Table 3 shows the results of skin prick testing. Over the next 9 months he had 3 probable chest infections, with a hazy right-sided chest radiograph abnormality and eosinophilia. On one occasion (day+380) he improved rapidly with prednisolone 30 mg for 3 days having failed oral cefalexin.
Table 3.
Results of skin prick testing in the patient.
| Allergen | Reaction |
|---|---|
| Positive control | 6 mm |
| Negative control | Nil |
| D. pteronyssinus | 10 mm |
| Cat | Nil |
| Dog | Nil |
| Grasses | 10 mm |
| Trees | Nil |
| Cockroach | Nil |
| Alternaria alternata (tenuis) | 9 mm |
| Aspergillus fumigatus | Nil |
| Trychophyton spp. | Nil |
| Cladosporium herbarum | Nil |
| Budgerigar | Nil |
A decision was made to start itraconazole 200 mg bd (day+610). This had good effect: his chest symptoms were better controlled, with less frequent exacerbations; his rhinitis became very mild, enabling cessation of the nasal steroid spray and his total IgE fell, at one point being 1500 KIU/L (day+1223) (Table 2). Variation in Alternaria-specific IgE did not correlate with itraconazole therapy or symptoms. Sputum culture both before (day+1, day+397) and during (day+642) itraconazole treatment was negative for pathogenic bacterial and fungal growth. The course lasted 17 months, with no adverse effects being experienced. Within one month of stopping (day+1200), there was a resurgence of nasal and chest symptoms. Increasing the dose of inhaled steroids from 200 μg to 400 μg daily had no effect, so itraconazole was restarted at the same dose (day+1255). However, five months later (day+1400), as there was no apparent benefit, it was stopped again. His chest symptoms are now controlled on a regular long acting beta agonist/inhaled corticosteroid combination (formoterol 12 μg/budesonide 400 μg daily).
3. Discussion
This patient suffered discrete periods of exacerbation of asthma symptoms, transient chest radiograph changes and remission in between exacerbations. The differential diagnosis would include asthma with intercurrent chest infections or non-infective exacerbations, and ABPM with sensitivity to Alternaria, or allergic bronchopulmonary alternariosis.
The case for the former would be that episodes resolved with antibiotics or oral steroids, fever and systemic upset during episodes, lack of bronchiectasis on CT scanning, and no sensitivity to Aspergillus or other fungi associated with ABPM on skin-prick testing or specific IgE RAST (Alternaria is not yet recognised as a cause for ABPM). The lack of response to the second course of itraconazole and control with usual inhaled asthma treatment could also support asthma with exacerbations.
The case for ABPM (Alternaria) would be fulfilment of the criteria for ABPA but with sensitivity to Alternaria. Specifically, the history of asthma, very high total IgE count, very high Alternaria-specific IgE, positive skin prick test to Alternaria and peripheral eosinophilia all point towards this diagnosis. In addition, the clinical response to oral itraconazole would support this as the diagnosis.
ABPM involving other fungi has been described in many studies (Table 4). There are, to our knowledge, no published cases of Alternaria in this context. If this is a case of allergic bronchopulmonary alternariosis, it is possible that many more people with recurrent asthma exacerbations also have undiagnosed ABPM, sensitive to fungi not currently thought to be important. This opens the way to use of a wider panel of fungal-specific serum IgE or skin-prick tests, perhaps after exclusion of commonly tested fungi, in the context of persistently high total serum IgE. Most fungal allergenic proteins are cross-reactive across one or more other species [7]. It is likely that this patient developed sensitivity to an Alternaria-specific protein, which must be an unusual circumstance in ABPA, but may be more common in asthma, depending on the predominant local fungal flora [36].
Table 4.
Fungi associated with ABPM in the literature.
| Fungal species |
|---|
| Bipolaris spp. [19] |
| Candida spp. [33,21,3] |
| Cladosporium spp. [24] |
| Curvularia spp. [22,19,35] |
| Drechslera spp. [22,17] |
| Fusarium spp. [5,31] |
| Geotrichum spp. [17] |
| Helminthosporium spp. [13] |
| Mucor-like spp. [16] |
| Paecilomyces spp. [2] |
| Penicillium spp. [30,17,34] |
| Pseudallescheria spp. [23,18] |
| Saccharomyces spp. [28] |
| Schizophyllum spp. [15,4] |
| Stemphylium spp. [6,17] |
| Torulopsis spp. [29] |
| Trichosporon spp. [11] |
Though this patient is controlled on inhaled steroids like some ABPA patients, so far only ABPA has been studied in detail. It is not known how many people have ABPM due to other fungi, some of whom may have severe or progressive disease. It is known that itraconazole is effective in ABPA [20] and in SAFS [10], but its role in ABPM due to other fungi is unclear. Therefore study in other mycoses is required to find out about the usual natural course and management options.
Funding source and potential conflicts of interest
This study was unfunded. Bhagteshwar Singh reports no potential conflicts of interest. Dr. Denning holds founder shares in F2G Ltd. a University of Manchester spin-out company and has received grant support from F2G as well as the Fungal Research Trust, The Medical Research Council, The Chronic Granulomatous Disease Research Trust, the National Institute of Allergy and Infectious Diseases, National Institute of Health Research, the European Union and AstraZeneca. He acts as an advisor/consultant to F2G and Myconostica (now part of Lab21 group) as well as other companies over the last 5 years including Pfizer, Schering Plough (now Merck), Nektar, Astellas and Gilead. He has been paid for talks on behalf of Merck, Astellas, Novartis, Merck, Dainippon and Pfizer.
Acknowledgements
The authors would like to thank Chris Harris and Joanne Sale for their invaluable support in the preparation of this case report.
References
- 1.Agarwal R. Severe asthma with fungal sensitization. Current Allergy and Asthma Reports. 2011;11(5):405–413. doi: 10.1007/s11882-011-0217-4. [DOI] [PubMed] [Google Scholar]
- 2.Akhunova A.M. Infectious-allergic bronchopulmonary paecilomycosis. Terapevticheskii Arkhiv. 1991;63(10):19–24. [PubMed] [Google Scholar]
- 3.Al-Mobeireek A.F., El-Rab M.O.G.A.D., Al-Hedaithy S.S., Alasali K., Al-Majed S., Joharjy I. Allergic bronchopulmonary mycosis in patients with asthma: period prevalence at a university hospital in Saudi Arabia. Respiratory Medicine. 2001;95(5):341–347. doi: 10.1053/rmed.2001.1047. [DOI] [PubMed] [Google Scholar]
- 4.Amemiya Y., Shirai R., Tokimatsu I., Oka H., Iwata A., Otani S. Allergic bronchopulmonary mycosis induced by Schizophyllum commune—case report and review of the literature. Nihon Kokyuki Gakkai Zasshi. 2009;47(8):692–697. [PubMed] [Google Scholar]
- 5.Backman K.S., Roberts M., Patterson R. Allergic bronchopulmonary mycosis caused by Fusarium vasinfectum. American Journal of Respiratory and Critical Care Medicine. 1995;152:1379–1381. doi: 10.1164/ajrccm.152.4.7551398. [DOI] [PubMed] [Google Scholar]
- 6.Benatar S.R., Allan B., Hewitson R.P., Don P. Allergic bronchopulmonary stemphyliosis. Thorax. 1980;35(7):515–518. doi: 10.1136/thx.35.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowyer P., Fraczek M., Denning D.W. A comparative genomic analysis of fungal allergens reveals the presence of multiple cross reactive proteins. BMC Genomics. 2006;7:251. doi: 10.1186/1471-2164-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dales R.E., Cakmak S., Judek S., Dann T., Coates F., Brook J.R. The role of fungal spores in thunderstorm asthma. Chest. 2003;123(3):745–750. doi: 10.1378/chest.123.3.745. [DOI] [PubMed] [Google Scholar]
- 9.Denning D.W., O’Driscoll B.R., Hogaboam C.M., Bowyer P., Niven R.M. The link between fungi and severe asthma: a summary of the evidence. European Respiratory Journal. 2006;27(3):615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 10.Denning D.W., O’Driscoll B.R., Powell G., Chew F., Atherton G.T., Vyas A. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. American Journal of Respiratory and Critical Care Medicine. 2009;179(1):11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 11.Gondor M., Michaels M.G., Finder J.D. Non-aspergillus allergic bronchopulmonary mycosis in a pediatric patient with cystic fibrosis. Pediatrics. 1998;102(6):1480–1482. doi: 10.1542/peds.102.6.1480. [DOI] [PubMed] [Google Scholar]
- 12.Halonen M., Stern D.A., Wright A.L., Taussig L.M., Martinez F.D. Alternaria as a major allergen for asthma in children raised in a desert environment. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):1356–1361. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 13.Hendrick D.J., Ellithorpe D.B., Lyon F., Hattier P., Salvaggio J.E. Allergic bronchopulmonary helminthosporiosis. American Review of Respiratory Disease. 1982;126(5):935–938. doi: 10.1164/arrd.1982.126.5.935. [DOI] [PubMed] [Google Scholar]
- 14.Hogan C., Denning D.W. Allergic bronchopulmonary aspergillosis and related allergic syndromes. Seminars in Respiratory and Critical Care Medicine. 2011;32:682–692. doi: 10.1055/s-0031-1295716. [DOI] [PubMed] [Google Scholar]
- 15.Kamei K., Unno H., Nagao K., Kuriyama T., Nishimura K., Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clinical Infectious Diseases. 1994;18(3):305–309. doi: 10.1093/clinids/18.3.305. [DOI] [PubMed] [Google Scholar]
- 16.Kino T., Yamada Y., Honda K., Fujimura N., Matsui Y., Izumi T. Diagnosis and treatment of a case of allergic bronchopulmonary mycosis caused by Mucor-like fungus. Nihon Kyobu Shikkan Gakkai Zasshi. 1983;21(9):896–903. [PubMed] [Google Scholar]
- 17.Lahoute C., Tonnel A.B., Fournier E., Ramon P., Voisin C. Bronchopulmonary pathology with hypereosinophilia of fungal origin (excluding allergic bronchopulmonary aspergillosis) Le Poumon et le Coeur. 1983;39(2):87–93. [PubMed] [Google Scholar]
- 18.Lake F.R., Tribe A.E., McAleer R., Froudist J., Thompson P.J. Mixed allergic bronchopulmonary fungal disease due to Pseudallescheria boydii and Aspergillus. Thorax. 1990;45(6):489–491. doi: 10.1136/thx.45.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lake F.R., Froudist J.H., McAleer R., Gillon R.L., Tribe A.E., Thompson P.J. Allergic bronchopulmonary fungal disease caused by Bipolaris and Curvularia. Australian and New Zealand Journal of Medicine. 1991;21(6):871–874. doi: 10.1111/j.1445-5994.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 20.Lazarus A.A., Thilagar B., McKay S.A. Allergic bronchopulmonary aspergillosis. Disease-a-Month. 2008;54(8):547–564. doi: 10.1016/j.disamonth.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee T.M., Greenberger P.A., Oh S., Patterson R., Roberts M., Liotta J.L. Allergic bronchopulmonary candidiasis: case report and suggested diagnostic criteria. Journal of Allergy and Clinical Immunology. 1987;80(6):816–820. doi: 10.1016/s0091-6749(87)80271-3. [DOI] [PubMed] [Google Scholar]
- 22.McAleer R., Kroenert D.B., Elder J.L., Froudist J.H. Allergic bronchopulmonary disease caused by Curvularia lunata and Drechslera hawaiiensis. Thorax. 1981;36:338–344. doi: 10.1136/thx.36.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M.A., Greenberger P.A., Amerian R., Toogood J.H., Noskin G.A., Roberts M. Allergic bronchopulmonary mycosis caused by Pseudallescheria boydii. American Review of Respiratory Disease. 1998;148(3):810–812. doi: 10.1164/ajrccm/148.3.810. [DOI] [PubMed] [Google Scholar]
- 24.Neas L.M., Dockery D.W., Burge H., Koutrakis P., Speizer F.E. Fungus spores, air pollutants, and other determinants of peak expiratory flow rate in children. American Journal of Epidemiology. 1996;143:797–807. doi: 10.1093/oxfordjournals.aje.a008818. [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulmonary Medicine 2005;5:4 [PubMed PMID:15720706; PubMed Central PMCID:PMC550663]. [DOI] [PMC free article] [PubMed]
- 26.O’Driscoll B.R., Powell G., Chew F., Niven R.M., Miles J.F., Vyas A. Comparison of skin prick tests with specific serum immunoglobulin E in the diagnosis of fungal sensitization in patients with severe asthma. Clinical & Experimental Allergy. 2009;39:1677–1683. doi: 10.1111/j.1365-2222.2009.03339.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Hollaren M.T., Yunginger J.W., Offord K.P., Somers M.J., O’Connell E.J., Ballard D.J. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. New England Journal of Medicine. 1991;324(6):359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa H., Fujimura M., Tofuku Y. Allergic bronchopulmonary fungal disease caused by Saccharomyces cerivisiae. Journal of Asthma. 2004;41(2):223–228. doi: 10.1081/jas-120026080. [DOI] [PubMed] [Google Scholar]
- 29.Patterson R., Samuels B.S., Phair J.J., Roberts M. Bronchopulmonary torulopsosis. International Archives of Allergy and Immunology. 1982;69(1):30–33. doi: 10.1159/000233142. [DOI] [PubMed] [Google Scholar]
- 30.Sahn S.A., Lakshminarayan S. Allergic bronchopulmonary penicilliosis. Chest. 1973;63(2):286–288. doi: 10.1378/chest.63.2.286. [DOI] [PubMed] [Google Scholar]
- 31.Saini S.K., Boas S.R., Jerath A., Roberts M., Greenberger P.A. Allergic bronchopulmonary mycosis to Fusarium vasinfectum in a child. Annals of Allergy, Asthma and Immunology. 1998;80(5):377–380. doi: 10.1016/S1081-1206(10)62986-9. [DOI] [PubMed] [Google Scholar]
- 32.Salo P.M., Arbes S.J., Sever M., Jaramillo R., Cohn R.D., London S.J. Exposure to Alternaria alternata in US home is associated with asthma symptoms. Journal of Allergy and Clinical Immunology. 2006;118(4):892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandhu R.S., Mehta S.K., Khan Z.U., Singh M.M. Role of Aspergillus and Candida species in allergic bronchopulmonary mycoses. A comparative study. Scandinavian Journal of Respiratory Diseases. 1979;60(5):235–242. [PubMed] [Google Scholar]
- 34.Sarkar A., Mukherjee A., Ghoshal A.G., Kundu S., Mitra S. Occurrence of allergic bronchopulmonary mycosis in patients with asthma: an Eastern India experience. Lung India. 2010;27(4):212–216. doi: 10.4103/0970-2113.71949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travis W.D., Kwon-Chung K.J., Kleiner D.E., Geber A., Lawson W., Pass H.I. Unusual aspects of allergic bronchopulmonary fungal disease: report of two cases due to Culvularia organisms associated with allergic fungal sinusitis. Human Pathology. 1991;22(12):1240–1248. doi: 10.1016/0046-8177(91)90106-y. [DOI] [PubMed] [Google Scholar]
- 36.Knutsen A.P., Bush R.K., Demain J.G., Denning D.W., Dixit A., Fairs A. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol. 2012;129(2):280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]


