Abstract
It has become clear in recent years that multiple signal transduction pathways are employed upon GPCR activation. One of the major cellular effectors activated by GPCRs is extracellular signal-regulated kinase (ERK). Both G-protein and β-arrestin mediated signaling pathways can lead to ERK activation. However, depending on activation pathway, the subcellular destination of activated ERK1/2 may be different. G-protein -dependent ERK activation results in the translocation of active ERK to the nucleus, whereas ERK activated via an arrestin-dependent mechanism remains largely in the cytoplasm. The subcellular location of activated ERK1/2 determines the downstream signaling cascade. Many substrates of ERK1/2 are found in the nucleus: nuclear transcription factors that participate in gene transcription, cell proliferation and differentiation. ERK1/2 substrates are also found in cytosol and other cellular organelles: they may play roles in translation, mitosis, apoptosis and cross-talk with other signaling pathways. Therefore, determining specific subcellular locations of activated ERK1/2 mediated by GPCR ligands would be important in correlating signaling pathways with cellular physiological functions. While GPCR-stimulated selective ERK pathway activation has been studied in several receptor systems, exploitation of these different signaling cascades for therapeutics has not yet been seriously pursued. Many old drug candidates were identified from screens based on G-protein signaling assays, and their activity on β-arrestin signaling pathways being mostly unknown, especially regarding their subcellular ERK pathways. With today’s knowledge of complicated GPCR signaling pathways, drug discovery can no longer rely on single-pathway approaches. Since ERK activation is an important signaling pathway and associated with many physiological functions, targeting the ERK pathway, especially specific subcellular activation pathways should provide new avenues for GPCR drug discovery.
Keywords: GPCR, G-protein coupled receptor, ERK, extracellular signal-regulated kinase, nucleus, cytoplasm, subcellular localization, drug discovery.
INTRODUCTION
G protein-coupled receptors (GPCRs) are the largest group of cell surface receptors, nearly 800 members have been identified in the human genome [1]. The importance of GPCRs has been recognized by the pharmaceutical industry as the most successful drug targets. In fact, approximately 40% of all drugs target GPCRs [2]. Drugs targeted at these receptors treat a wie range of therapeutic indications including cardiovascular disease, asthma, diabetes, pain, obesity, Alzheimer's disease, Parkinson's disease, schizophrenia, learning and cognitive disorders.
GPCRs are structurally characterized by an extracellular N-terminus, seven membrane-spanning domains and an intracellular C-terminus. In response to extracellular stimuli, a GPCR changes its structural conformation and transduces this into intracellular signals. The signaling pathways activated by GPCRs include the cAMP / PKA (protein kinase A) pathway [3], the calcium / PKC (protein kinase C) pathway [4], the IP3/PLC (phospholipase C) pathway [5], the β-arrestin pathway [6], the PTK (protein tyrosine kinase) pathway [7], the ERK/MAPK (extracellular signal-regulated kinase) pathway [8], the PI-3 Kinase / AKT pathway [9], the Rho pathway [10], and G-protein gated ion channels including Ca channels and GIRKs [11]. The complexity of the signaling pathways challenges not only our basic understanding of GPCRs, but also drug discovery efforts targeting these receptors. GPCR drug discovery traditionally relied on G-protein-dependent signaling pathways such as cAMP and calcium assays for lead compound identification. Other signaling pathways were overlooked possibly due to a limited availability of assay tools. Many of the above signaling pathways are highly interconnected, but they can also be independently regulated. Notably, depending on how a GPCR is activated, ERK signaling pathways can be divided into at least two sub-pathways: nuclear and cytosolic pathways. The subcellular location of activated ERK determines the downstream signaling cascades and distinct cellular responses. Thus, targeting GPCR activated ERK signaling pathways represents a new promising direction for drug discovery.
GPCR ACTIVATED ERK SIGNALING PATHWAYS
GPCR signaling can be regulated at multiple levels. At the receptor level, GPCRs can form homomers, heteromers, and cluster with other cell surface receptors such as receptor tyrosine kinases [12-16]. At the ligand level, agonists, antagonists, inverse agonists, and allosteric ligands bind to receptors to induce or stabilize distinct conformations. At the intracellular level, receptors can recruit or interact with multiple, different cellular effectors, depending on distinct receptor conformations. Obviously, these receptor-receptor, receptor-ligand, receptor-signal effector interactions have made GPCR signaling mechanisms much more complex than previously thought. G-protein and β-arrestin signaling pathways have been extensively reviewed [17-19] and will not be discussed here. Receptor clustering and heteromerization are other active frontiers in current GPCR research that will not be discussed here. In this review, we will focus only on studies involving GPCR activated extracellular signal-regulated kinase (ERK) signaling pathways.
It has become clear in recent years that multiple signaling transduction pathways are employed upon GPCR activation. In addition to classic heterotrimeric G-protein signaling pathways, G-protein-independent signaling pathways have attracted significant attention and have been exploited in drug discovery efforts. As new G-protein-independent assay tools [20-24] have become available (such as β-arrestin based assays), biased ligands that selectively activate one pathway over another, as well as show agonism in one pathway but antagonism in another pathway, have been discovered [25-27]. The findings of signaling pathway-dependent ligands challenged the early conventional views of GPCR activation and suggested the existence of multiple conformations induced by different ligands. Distinct GPCR conformations induced or stabilized by ligands can differentially recruit and interact with different cellular effectors to elicit specific signaling cascades. Therefore, exploiting biased ligands for therapeutic benefit represents a promising opportunity to develop safer and more efficacious drugs.
One of the major cellular effectors activated by GPCRs is extracellular signal-regulated kinase. Extracellular signal-regulated kinases, also called mitogen-activated protein kinases (MAPKs), include ERK1 (MAPK3) and ERK2 (MAPK1). ERK1 and ERK2 share 85% sequence identity and are expressed in a wide variety of tissues and cells. The ERK cascade is a central signaling pathway that regulates a wide variety of cellular processes including proliferation, differentiation, migration, survival, growth, growth arrest and apoptosis. Many stimuli including cytokines, growth factors, and GPCR ligands activate the ERK pathway. ERK activation by GPCRs can be mediated by the activation of Ras, Rap, PKC, tyrosine kinases (e.g., c-Src), transactivation of receptor tyrosine kinases, or via β-arrestins [28-30]. Unphosphorylated ERK is anchored in the cytoplasm by multiple components and forms a core signaling complex consisting of Raf, MEK, and ERK. Scaffold proteins such as KSR (kinase suppressor of Ras), β-arrestin, MEK partner-1, Sef and IQGAP1 also associate with the tethered three-kinase Raf/MEK/ERK architecture. Interactions with scaffolds ensure signaling fidelity, increase signaling efficiency, and target ERK1/2 to specific subcellular locations. Activation of ERK is achieved by sequential phosphorylation of a three-kinase signaling architecture. Activated ERK1/2 are released from the three-kinase complex, and then phosphorylate about 200 cellular substrates [31], thereby mediating diverse functions.
Both G-protein and β-arrestin mediated signaling pathways can lead to ERK activation [30, 32]. The activation of ERK cascades through G-protein α subunits including Gs, Gi, and Gq and G-protein βγ subunit signaling to Ras has been described [33-39]. Protein kinase A (PKA) and protein kinase C (PKC) are important components in G-protein-dependent signaling pathways. Pretreatment of cells with the PKA inhibitor H89 and the PKC inhibitor GF109203X can abolish G-protein-dependent activation of ERK1/2 [35, 40]. β-arrestin-dependent ERK activation has been illustrated with a mutant angiotensin type 1A receptor (AT1R) and a mutant angiotensin II peptide (SII-angiotensin) that acts as a biased AT1R agonist [28]. A mutant AT1R that lost G-protein signal transduction ability could still activate ERK1/2 when stimulated with angiotensin II. The biased AT1R agonist SII that is incapable of activating G-protein signaling could still induce ERK activation. These results indicate that both G-protein and β-arrestin mediated ERK activation pathways exist for a particular receptor, and that the two pathways are independent of each other [41]. In addition, both classically-defined agonists and antagonists can activate ERK1/2. For example, the β2-adrenergic agonist isoproterenol activates ERK1/2 using both pathways, whereas the antagonist ICI118551 activates ERK1/2 completely via the β-arrestin-dependent pathway [42]. Thus, the terms agonist and antagonist have to be pathway-defined.
Although many GPCRs can activate ERK1/2 through both pathways, the time course of ERK activation through the two pathways is different. Parathyroid hormone (PTH) activates ERK1/2 through its receptor PTH1R in two phases. There is an early rapid activation phase peaking at 5 min and a later sustained activation phase peaking at 30 to 60 min after stimulation [40]. Pretreatment of cells expressing PTH1R with the PKA inhibitor H89 and the PKC inhibitor GF109203X significantly diminished the early phase of ERK activation, but had little effect on the later phase (30 to 60 min) of ERK activation. The results indicate the early phase ERK activation is through a G-protein-dependent pathway, while the later phase ERK activation is through a G-protein-independent pathway. By using siRNA to knock down both β-arrestin-1 and β-arrestin-2 in cells, the time course of PTH-stimulated ERK activation was significantly changed. The rapid, transient ERK activation peaked at 5 min and then quickly diminished to basal levels. These results indicate the later phase of ERK activation is through a β-arrestin-dependent pathway. This biphasic response has been seen in other receptor systems as well, including non-GPCR systems [42].
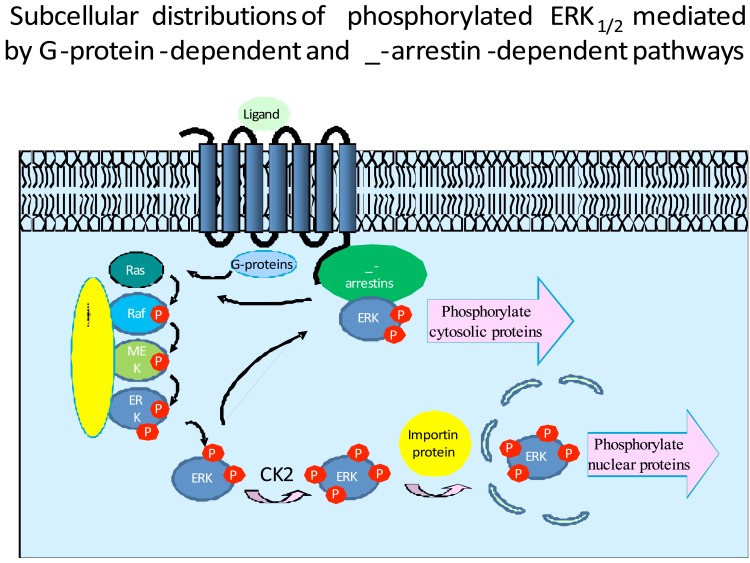
The subcellular destinations of activated ERK1/2 are different, depending on activation pathways (Fig. 1). In quiescent cells, unphosphorylated ERK is found mainly in the cytoplasm and is associated with anchoring proteins, such as MEK1 and vinculin. Upon activation, ERK1/2 are phosphorylated and released from the Raf/MEK/ERK signaling complex. The liberated, phosphorylated ERK1/2 then bind to other cellular proteins and are carried to new destinations. Subcellular distribution of activated ERK is regulated by interactions with various proteins. Interactions with these proteins also promote ERK cytoplasmic and nuclear retention [43]. For example, MEK1 is responsible for both cytoplasmic retention of ERK prior to its activation and nuclear export after its dephosphorylation [44, 45]. In general, the ERK1/2 phosphorylated via the G-protein-dependent pathway are further phosphorylated by Casein Kinase 2, and are able to bind to a nuclear anchor protein for nuclear translocation, where they can phosphorylate and activate various transcription factors [46, 47]. In contrast, the slower phosphorylation of ERK1/2 (10~60 min) mediated by the β-arrestin-dependent pathway, leaves ERK1/2 in the cytosol [45, 48, 49], where they can phosphorylate non-nuclear substrates [50, 51]. It has recently been demonstrated that receptor-associated β-arrestins preferentially bind to phosphorylated ERK2 [52], although co-immunoprecipitation of phosphorylated ERK and β-arrestins was reported a decade ago [53]. Free, non-receptor bound β-arrestins have weak affinity for inactive ERK2, but β-arrestin-1 has significantly more selectivity than β-arrestin-2. In the case of the PAR2 receptor, it has been estimated that as much as 80% of the active ERK pool is associated with the β-arrestin-bound receptor [54]. Thus, subcellular location of activated ERK is an important element in determining the biological consequences of ERK pathway activation.
Fig. (1).
Both G-protein and β-arrestin mediated signaling pathways can lead to ERK activation. However, the subcellular distributions of activated ERK1/2 are different. The phosphorylated ERK1/2 mediated by the β-arrestin signaling pathway largely remain in cytoplasm, while the phosphorylated ERK1/2 mediated by the G-protein signaling pathway translocate to the nucleus.
GPCR ligands can selectively determine subcellular destinations of activated ERK1/2. Activation of the μ-opioid receptor (MOR) can lead to both β-arrestin- and G protein-dependent activation of ERK1/2 [55, 56]. For example, MOR agonists morphine and etorphine activate ERK with similar time courses. Maximum phosphorylation of ERK was observed 10 min after the agonist treatment. Morphine-induced ERK activation could be blocked by a PKC inhibitor, whereas etorphine-induced ERK activation was not significantly affected [50]. These results suggest that morphine-induced ERK activation occurs via a G-protein-dependent pathway and etorphine-induced ERK activation via a β-arrestin-dependent pathway. Interestingly, morphine did not induce nuclear translocation of phosphorylated ERK even at 10 μM, the amount of phosphorylated ERK1/2 in the nucleus remained similar to the basal level. In addition, morphine-activated ERK1/2 remained in the cytoplasm and phosphorylated a cytosolic protein kinase 90RSK. On the other hand, for etorphine-activated ERK, phosphorylated ERK was found in nuclear fractions from cells treated with 10 nM etorphine. Translocated active ERK is known to phosphorylate nuclear transcription factor Elk-1 [57]. Using an EIK-1 driven luciferase reporter expression system, etorphine-stimulated luciferase activity was observed. Such pathway-dependent ERK phosphorylation by biased ligands has been observed in MOR over-expression HEK293 cells, in SHSY5Y cells expressing endogenous MOR, and in primary neuron cultures. Interestingly, the observations made here are counter to the prevailing dogma that ERK phosphorylation via β-arrestin leads to cytosolic signaling due to β-arrestin scaffolding effects on ERKs, and G-protein mediated ERK phosphorylation leads to nuclear translocation and gene transcription. Clearly, other factors are at play beyond the dogma, and functional selectivity of ligands can stimulate many different signaling pathways.
Importantly, subcellular location of activated ERK1/2 determines downstream ERK signaling cascades, and mediates overall cellular responses. Ligands that can selectively determine subcellular destinations of activated ERK may have distinct and valuable therapeutic properties. In the last two decades, GPCR drug discovery programs relied mainly on G-protein signaling pathways to assess a compound’s functional activity. Using G-protein based assays such as assessing changes in cAMP, IP3, and calcium levels, drug developers generated a large number of lead compounds. It is known that rank order of potency based on G-protein signaling assays and binding assays do not always correlate with potencies obtained in vivo studies (animal models and clinical data), although it is recognized that differences in pharmacokinetic properties may explain some of these differences. With the current knowledge of multiple signaling pathways, single signaling pathway approaches for identifying drug candidates are not adequately suited to detect the full repertoire of compounds that may have other signaling pathway activities. Drug developers have to examine multiple signaling pathways to link pathway activities to physiologic functions and pharmacological activity.
ERK PATHWAY AND SUBCELLULAR ERK LOCATION ASSOCIATION WITH DISEASES
While GPCR-stimulated selective ERK pathway activation has been studied in several receptor systems, as described above, exploitation of these different signaling cascades for therapeutics has not yet been seriously pursued. There is considerable study of the different pathways in relation to oncology, where activation by receptor tyrosine kinases has been implicated [58, 59]. There have also been efforts to identify compounds that inhibit activity along the ERK pathway, including ATP-site and allosteric inhibitors of the enzyme itself [60, 61].
Studies of the involvement of the ERK pathway in cardiovascular function are ongoing, demonstrating that activation of the ERK pathway following myocardial infarction promotes growth and survival of cardiomyocytes [62] as well as some remodeling. The ERK pathway is also involved in formation of neointima following vascular injury (particularly following balloon angioplasty). GPCR involvement in these processes, either in pathophysiology, or as potential targets for treatment, has not been well-studied to date.
The ERK pathway is recognized as important in neural development, regulating progenitor proliferation or differentiation. Activation of ERK pathways in the hippocampus via multiple GPCR families has been described [63]. ERK2 knockout mice are embryonic lethal, whereas ERK1 knockout mice have deficits in neuronal development [64-66]. ERK1 knockouts appear normal, with deficits only elicited when challenged. Indeed, ERK1 knockouts are being used to study aspects of autism spectrum disorders. Conditional ERK2 knockouts show deficits in associative learning and fear conditioning. Given that there is often a strong hereditary component in neuropsychiatric diseases, disorders in ERK pathway have been studied from this perspective [66, 67]. Lithium and valproate are drugs used for the treatment of bipolar disorder and they enhance ERK activity.
Targeting different GPCRs to treat various CNS disorders is certainly well-studied; indeed drugs that act on GPCRs are among the most useful since agonists, antagonists and allosteric modulators can modulate GPCR function in different directions to obtain desired effects. However, further exploitation of the complexities of GPCR signaling is only beginning, and finding biased ligands that push signaling via one pathway over another is becoming possible with new biochemical screening tools. In particular, the GPCR-ERK pathway that can differentially signal to the cytosol and nucleus is worthy of further study.
CURRENT CELL-BASED ERK ACTIVATION ASSAYS
Since ERK activation represents a convergence point for signaling pathways in response to a variety of extracellular stimuli, measuring activation of ERK1/2 as a functional outcome has attracted widespread interest. Methods for assessing ERK activation are not only interesting for studying signaling mechanisms, but also for drug discovery efforts. G-protein coupled receptors, as a major drug target class, are known to mediate ERK activation. Regardless of signaling pathway, phosphorylated ERK1/2 is frequently a common endpoint of activation for a variety of GPCRs. Therefore, measuring downstream phosphorylated ERK could have some advantages in that one does not need to know the precise GPCR G-protein α-subunit coupling mechanism to establish assays. This could be especially useful for establishing orphan GPCR assays. Measuring phosphorylated ERK as an alternative read-out offers a physiologically relevant means for assessing GPCR drug candidates. However, one has to be cautious of ERK phosphorylation assay selectivity. A variety of endogenous receptors from GPCRs, cytokine receptors, to receptor tyrosine kinases in a given cell type can activate ERK signaling pathways in response to a wide range of extracellular stimuli.
ERK1/2 are activated via dual phosphorylation on specific tyrosine (Tyr204) and threonine (Thr202) residues by mitogen-activated or extracellular signal-regulated protein kinase kinase (MEK). Antibodies specific for dual phosphorylated ERKs (Phospho-Thr202 and Tyr204) have been developed for detecting phosphorylated ERKs (phospho-ERK). Current ERK activation assays with high throughput capability are almost all using antibodies detecting phospho-ERK1/2 with different readout technologies (see Table 1). The AlphaScreen SureFire ERK assay is a popular cell-based method of detecting phosphorylated ERK. Detection is achieved by immuno-sandwich capture of endogenous phosphorylated ERK1/2 in cell lysates. Antibody-coated Alpha-Screen beads with different excitation and emission wavelengths bind to phosphorylated ERK1/2 and produce a signal when in close proximity. Similarly, a HTRF ERK activation assay using two different specific anti-phosphorylated ERK monoclonal antibodies labeled with different dyes via a sandwich assay format can be used with cell lysates. Screening methods using fluorescence-conjugated or infrared-labeled secondary antibodies to detect phospho-ERK1/2 have also been developed [68, 69]. Other methods such as Western blot analysis and enzyme linked immunosorbent assays (ELISA) do not have HTS capability.
Table 1.
Cell-based ERK Activation HTS Assays
| Name | Assay Principle | Detection Technology | Provider |
|---|---|---|---|
| In-cell Western | Using ERK1/2 primary antibodies and infrared-labeled secondary antibodies sandwich assay to detect infrared signal in fixed cells | Infrared fluorescence | LI-COR Biosciences |
| MSD ERK Activation Assay | Using ERK1/2 antibody conjugated with an electrochemiluminescent compound and capture antibody for phosphorylated ERK1/2 coated in microplate wells for detection | Electrochemiluminesence | Meso-Scale Discovery |
| AlphaScreen SureFire™Cellular ERK Assay | Using antibody-coated AlphaScreen beads to generate a fluorescent signal when in close proximity due to binding of phospho-ERK1/2 (Thr202/Tyr204) | Fluorescence emission | TGR BioSciences & Perkin Elmer |
| Cayman ERK/MAPK (Thr202/Tyr204) Cell-based Assay | A sandwich assay using anti-Thr202/Tyr204 primary antibody with a DyLight conjugated secondary antibody | Immunofluorescence staining | Cayman |
| Phospho-ERK (Cellul'erk) Assay | A sandwich assay format using 2 different specific monoclonal antibodies: the anti-phospho-ERK antibody labeled with d2 and the anti-ERK antibody labeled with Eu3+-cryptate. | HTRF | Cisbio |
| LanthaScreen® Activity Assay | Terbium (Tb)-labeled antibody binds the dually phosphorylated (Thr185, Tyr187) ERK2-GFP fusion protein expressed in cells. This association allows energy transfer to occur between the excited state Tb fluorophore and GFP, leading to an increase in TR-FRET signal | TR-FRET | Life Technologies |
The subcellular location of activated ERK1/2 determines the downstream signaling cascades [70]. Many substrates of ERK1/2 are found in the nucleus; they are nuclear transcription factors and participate in gene transcription, cell proliferation and differentiation. ERK1/2 substrates are also found in the cytosol and other cellular organelles; they may play roles in translation, mitosis, apoptosis and cross-talk with other signaling pathways. Therefore, determining specific subcellular location of activated ERK1/2 mediated by GPCR ligands would be important in correlating signaling pathways to cellular physiological functions. The antibody-based assays measure the phosphorylation status of ERK1/2 in cell lysates or fixed cells, and lack the ability to distinguish G-protein-dependent or arrestin-dependent ERK activation. Other pull-down assay methods to examine the ability of ERK to recruit cellular effectors or to interact with substrates have quantitation and throughput limitations. Cell-based assay methods that can specifically assess dynamic distributions of activated ERK1/2 and distinguish G-protein-dependent and β-arrestin-dependent ERK activation pathways would be highly desirable.
Cellular signal transduction processes depend on protein-protein interactions. Utilizing activated ERK1/2 interacting with its cellular effectors or adaptor proteins to develop cell-based assays could allow readouts of GPCR activation of distinct ERK cellular signal transduction cascades. It would be desirable to develop such tools with HTS capability to identify ERK pathway-selective or biased ligands for drug development. Whether all G-protein-dependent ERK activation results in active ERK nuclear translocation and all β-arrestin-dependent ERK activation results in cytosolic retention remains to be explored. Although it is a challenge to predict therapeutic effects to a specific signaling pathway, evaluating existing drugs and drug candidates on ERK pathway activity should shed light on this question. Many old drug candidates were identified from screens based on classical G-protein signaling assays, and their activity on β-arrestin signaling pathways are mostly unknown, especially regarding any subcellular ERK pathway selectivity. In addition, β-arrestin pathway biased ligands were potentially missed in those screens. With today’s knowledge of complex GPCR signaling pathways, drug discovery can no longer rely on single-pathway approaches. Perhaps it may be worth taking a second look at failed drug candidates on their activities in different signaling pathways. Since ERK activation is an important signaling pathway that is associated with a myriad of physiological functions, targeting ERK pathways, especially differential subcellular activation pathways should provide some new directions in GPCR drug discovery.
SUMMARY AND CONCLUSIONS
GPCRs are recognized as being highly “druggable” – assays are available to identify lead molecules and optimize them into drug-like compounds that reach clinical studies. Additionally, the function of GPCRs can be stimulated, inhibited or modulated in an allosteric manner. Hence, GPCRs are the target of many pharmaceutical interventions for a broad range of diseases. The biological significance of this receptor class and its therapeutic potential were illustrated by the 2012 Nobel prize in chemistry, awarded to Brian Kobilka and Robert Lefkowitz for their work that was "crucial for understanding how G protein–coupled receptors function." Some ligands that target G-proteins have been recently found to have functional selectivity – that is, an agonist of one signal transduction pathway can be an antagonist at another. Hence the choice of signaling pathway is important in the identification of new compounds to treat diseases. The β-arrestin pathway was pioneered in the Lefkowitz lab, and led to the discovery of G-protein-independent signaling to stimulate ERKs. The ability of ERKs to signal in the cytoplasm as well as in the nucleus makes this pathway particularly interesting and novel for drug discovery. Assays that allow independent measurement of these two pathways would facilitate efforts aimed at discovering new, functionally selective drugs.
ACKNOWLEDGEMENTS
We thank Dr. Paul Wright for reviewing the manuscript and providing helpful comments.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Bjarnadóttir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schiöth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–73. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Filmore D. It's a GPCR world. Mod Drug Discov. 2004;7:24–8. [Google Scholar]
- 3.Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol Ther. 2005;106:405–21. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Chen-Izu Y, Xiao RP, Izu LT, et al. G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca(2+) channels. Biophys J. 2000;79:2547–56. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. β-Arrestin: a protein that regulates β-adrenergic receptor function. Science. 2000;248:1547–50. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 7.Dikic I, Blaukat A. Protein tyrosine kinase-mediated pathways in G protein-coupled receptor signaling. Cell Biochem Biophys. 1999;30:369–87. doi: 10.1007/BF02738120. [DOI] [PubMed] [Google Scholar]
- 8.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta/gamma subunits. Nature. 1994;369:418–20. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 9.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase gamma. J Biol Chem. 1998;273:19080–5. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 10.Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003;278:41253–58. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- 11.Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–43. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 12.Park PS, Filipek S, Wells JW, Palczewski K. Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry. 2004;43:15643–56. doi: 10.1021/bi047907k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan M. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signaling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32:443–50. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–7. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–97. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce KL, Premont RT, Lefkowitz RJ. 7 Transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–50. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 19.DeFea K. β-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br J Pharmacol. 2008;153:S298–309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 21.Barnea G, Strapps W, Herrada G, et al. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA. 2008;105:64–9. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eishingdrelo H, Cai J, Weissensee P, Sharma P, Tocci MJ, Wright PS. A cell-based protein-protein interaction method using a permuted luciferase reporter. Curr Chem Genomics. 2011;5:122–8. doi: 10.2174/1875397301105010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer MM, Wehrman TS, Blau HM. A novel enzyme complementation-based assay for monitoring G-protein-coupled receptor internalization. FASEB J. 2007;21:3827–34. doi: 10.1096/fj.07-8777com. [DOI] [PubMed] [Google Scholar]
- 24.Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J Biomol Screen. 2005;10:463–75. doi: 10.1177/1087057105275344. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopa S, Keshava I, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–86. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy S, Lefkowitz RJ. Distinct conformational changes in b-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–93. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gbahou F, Rouleau A, Morisset S, et al. Protean agonism at histamine H3 receptors in vitro and in vivo. Proc Natl Acad Sci USA. 2003;100:11086–91. doi: 10.1073/pnas.1932276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei H, Ahn S, Shenoy SK, et al. Independent {beta}-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–7. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leroy D, Missotten M, Waltzinger C, Martin T, Scheer A. G protein-coupled receptor-mediated ERK1/2 phosphorylation: towards a generic sensor of GPCR activation. J Recept Signal Transduct Res. 2007;27:83–97. doi: 10.1080/10799890601112244. [DOI] [PubMed] [Google Scholar]
- 30.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–25. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 31.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 32.Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26:3122–42. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 33.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein gamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282:24099–108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan JD, Carey KD, Stork PJS, Iyengar R. Modulation of Rap activity by direct interaction of G alpha(o) with Rapl GTPase-activating protein. J Biol Chem. 1999;274:21507–10. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki N, Ohba Y, Kiyokawa E, et al. Activation of the ERK/MAPK pathway by an isoform of Rap1GAP associated with G alpha(i) Nature. 1999;400:891–4. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 36.Antonelli V, Bernasconi F, Wong YH, Vallar L. Activation of B-Raf and regulation of the mitogen activated protein kinase pathway by the G(o) alpha chain. Mol Biol Cell. 2000;11:1129–42. doi: 10.1091/mbc.11.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inglese J, Koch WJ, Touhara K, Lefkowitz RJ. G-beta-gamma interactions with pH domains and Ras- MAPK signaling pathways. Trends Biochem Sci. 1995;20:151–6. doi: 10.1016/s0968-0004(00)88992-6. [DOI] [PubMed] [Google Scholar]
- 38.Hawes BE, Vanbiesen T, Koch WJ, Luttrell LM, Lefkowitz RJ. Distinct pathways of G(i)- and G(q)- mediated mitogen-activated protein kinase activation. J Biol Chem. 1995;270:17148–53. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 39.Van Biesen T, Hawes BE, Raymond JR, Luttrell LM, Koch WJ, Lefkowitz RJ. G(o)-protein alpha subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J Biol Chem. 1996;271:1266–9. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- 40.Gesty-Palmer D, Chen M, Reiter E, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–64. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 41.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 42.Azzi M, Charest PG, Angers S, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell. 2001;93:71–9. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogen-activated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–8. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- 45.Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–56. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuderland D, Konson A, Seger R. Identification and characterization of a general nuclear translocation signal in signaling proteins. Mol Cell. 2008;31:1–12. doi: 10.1016/j.molcel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Plotnikov A, Chuderland D, Karamansha Y, Livnah O, Seger R. Nuclear extracellular signal-regulated kinase 1 and 2 translocation is mediated by casein kinase 2 and accelerated by autophosphorylation. Mol Cell Biol. 2011;3:3515–30. doi: 10.1128/MCB.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Luttrell LM. G protein-coupled receptor signaling in neuroendocrine systems: ‘Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J Mol Endocrinol. 2003;30:117–26. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- 49.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–36. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 50.Zheng H, Loh HH, Law P. β-Arrestin-dependent µ-opioid receptor-activated extracellular signal-regulated kinases (ERK1/2 ) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–90. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wortzel I, Seger R. The ERK cascade distinct functions within various subcellular organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coffa S, Breitman M, Hanson, et al. The effect of arrestin conformation on the recruitment of C-Raf1, MEK1, and ERK1/2 activation. PLoS One. 2011;6:e28723. doi: 10.1371/journal.pone.0028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luttrell LM, Roudabush FL, Choy EW, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–54. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-arrestin-dependent endocytosis of proteinase activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belcheva MM, Clark AL, Haas PD, et al. µ and κ opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–9. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–65. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mebratu Y, Tesfaigzi Y. How ERK1/2 Activation Controls Cell Proliferation and Cell Death Is Subcellular Localization the Answer? Cell Cycle. 2009;8:1168–75. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santarpia L, Lippman SL, El-Naggar AK. Targeting the Mitogen-Activated Protein Kinase RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin Ther Targets. 2012;16:103–19. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–64. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yap JL, Worlikar S, MacKerell Jr AD, Shapiro P, Fletcher P. Small Molecule Inhibitors of the ERK Signalling Pathway: Towards Novel Anti-cancer Therapeutics. Chem Med Chem. 2011;6:38–48. doi: 10.1002/cmdc.201000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muslin AJ. MAPK Signaling in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Targets. Clin Sci (Lond) 2008;115:203–18. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berkeley J, Levey AI. Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol Pharmacol. 2003;63:128–35. doi: 10.1124/mol.63.1.128. [DOI] [PubMed] [Google Scholar]
- 64.Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–7. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel SR, Creson TK, Hao Y, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–61. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levitt P, Campbell DB J. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. Clin Invest. 2009;119:747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalkman HO. Potential opposite roles of the extracellular signal-regulated kinase (ERK) pathway in autism spectrum and bipolar disorders. Neurosci Biobehav Rev. 2012;36:2206–13. doi: 10.1016/j.neubiorev.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Osmond RIW, Sheehan A, Borowicz R, et al. GPCR Screening via ERK 1/2: A novel platform for screening G protein-coupled receptors. J Biomol Screen. 2005;10:730–7. doi: 10.1177/1087057105277968. [DOI] [PubMed] [Google Scholar]
- 69.Wong SK. A 384-well cell-based phospho-ERK assay for dopamine D2 and D3 receptors. Anal Biochem. 2004;333:265–72. doi: 10.1016/j.ab.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Luttrell DK, Luttrell LM. Signaling in time and space: G protein-coupled receptors and mitogen-activated protein kinases. Assay Drug Dev Technol. 2003;1:327–38. doi: 10.1089/15406580360545143. [DOI] [PubMed] [Google Scholar]