Abstract
Primary objective
To study the mechanism of somatosensory-vestibular interactions, this study examined the effects of somatosensory inputs on body sway induced by galvanic vestibular stimulation (GVS) in healthy participants and persons with brain injury in the posterior insula, a region constituting a part of the parietoinsular vestibular cortex.
Research design
This study adopted an experimental, controlled, repeated measures design.
Methods and procedures
Participants were 11 healthy individuals, two persons with unilateral posterior insular injury and two age-matched controls. Bipolar GVS was applied to the mastoid processes while participants were sitting with their eyes closed, either lightly touching a stable surface with their index finger or not touching the surface with their index finger.
Main outcomes and results
In healthy participants, tilting was greater with right hemispheric stimulation than with left hemispheric stimulation. Moreover, with right hemispheric stimulation, tilting was greater with a right finger touch than with no touch. The person with right-brain injury showed tilting induced by GVS; however, finger touch had no modulatory effect. In contrast, finger touch enhanced tilting in the person with left-brain injury.
Conclusions
These preliminary results are discussed in light of a hypothesis of right hemispheric dominance of somatosensory-vestibular interactions in the posterior insula.
Keywords: Finger touch, GVS, PIVC, postural sway
Introduction
Touching something with a finger can stabilize a person who is about to lose his/her balance. The spatial acuity of the fingertip [1] is better defined than that of the vestibular system [2] and it is sensitive enough to detect small body sway. Tactile feedback from the finger is essential for reducing sway responses, yet no effects of fingertip-contact forces on postural sway previously have been reported [3]. The fact that postural sway induced by vestibular stimulation is reduced by finger touch [4] suggests that somatosensory inputs can modulate the vestibular processes that control postural balance. Bimodal neurons in the vestibular cortex converging vestibular and somatosensory inputs [5–7] may explain those somatosensory modulatory effects on vestibular responses. The vestibular cortex may combine multimodal reference frames to maintain the unity of the spatial experience [8]. However, the mechanisms of somatosensory modulatory effects on vestibular postural responses have not yet been clarified.
Vestibular, somatosensory and visual inputs provide complementary sources of information regarding body position and movement. It has been suggested that this type of multisensory integration is processed in the parietoinsular vestibular cortex (PIVC) [9, 10], a region located near the posterior end of the insular cortex in monkeys. Neuroimaging studies have suggested that the human PIVC is located in the posterior insula and the surrounding areas [11] and functional connections indicate that this area forms the core vestibular cortex [12]. Vestibular stimulation activates the PIVC [13] and damage to the PIVC is associated with postural deficits [14], spatial disorientation [15] and distortions in body ownership [16]. Furthermore, overlapping somatosensory and vestibular processing in the PIVC has been demonstrated [17]. PIVC responses to both vestibular and somatosensory inputs [6, 7, 10] suggest that somatosensory-vestibular interactions occur in the PIVC.
Vestibular modulation of the somatosensory system has previously been demonstrated. Caloric [18, 19] and galvanic [20] vestibular stimuli can improve tactile perception and somatosensory evoked potentials are known to be modulated by caloric vestibular stimulation [21]. It has been claimed that ‘[t]he vestibular system aids conscious tactile perception by introducing a bias in the neural system subserving body representation’ (p. 778) [22]. It was hypothesized that somatosensory-vestibular interactions are processed in the PIVC and consequently that a PIVC lesion can impair those interactions.
GVS can elicit vestibular sway reflexes safely with only minor adverse effects (moderate pain or itching under the electrodes) [23–25], making GVS suitable for studying the neural mechanisms of vestibular balance control. Binaural–bipolar stimuli have been shown to induce cathodal semi-circular canal and otoliths activity, evoking postural responses to the anodal side. GVS with the right anode and the left cathode induces neural activity mainly in the right hemisphere [26]. In this study, GVS was applied in the sitting position to reduce the effects of the leg muscles supporting balance control as in standing positions, as proprioceptive inputs from the legs providing sway perception [27] have been shown to disrupt the effects of finger touch on vestibular processes. Spontaneous postural sway in the standing position [28] can be diminished in the sitting position. The degree of tilting toward the anodal ear that is produced by bipolar GVS is reduced in a sitting position as compared to a standing position [29], enabling safe application of this methodology to persons with brain injury.
This study describes the somatosensory-vestibular interactions evoked by GVS with light finger touches. The modulation of the GVS-evoked tilt by finger touch was examined first in healthy participants in response to both right and left hemispheric stimulation. To investigate cortical involvement in somatosensory-vestibular interactions and potential interhemispheric differences, a person with right posterior insular lesion and a person with left posterior insular lesion also were examined using right hemispheric stimulation.
Materials and methods
Participants
Eleven right-handed individuals (three women and eight men) with an average age of 37.5 years (SD = 5.9, range = 29–49 years) participated in this study. In addition, a 62-year-old man and a 65-year-old man (both right-handed) served as age-controls for the persons with brain injury. No healthy participants showed any history of neurological, psychiatric or vestibular pathology. All experimental protocols were approved in advance by the Third Research Ethics Committee of RIKEN and were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to participation in the experiment.
Persons with brain injury
The selection of persons with brain injury was based on anatomical, sensorimotor and cognitive criteria. The anatomical criteria were a focal lesion in the unilateral posterior insula and a lesion within the insular cortex, determined on the basis of MRI images. The sensorimotor criteria were no paralysis or numbness in the whole body and walking without support. The cognitive criteria were the ability to understand and follow the experimental instructions with ease. The persons with brain injury met all of these criteria.
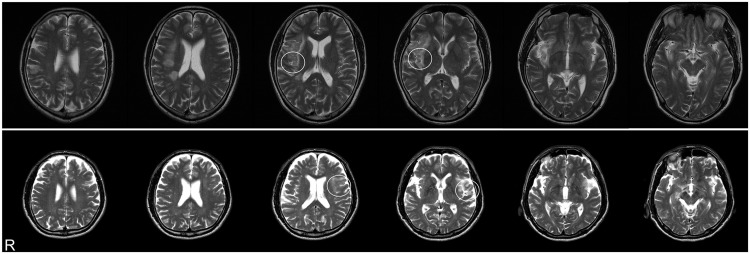
The person with right-brain injury was a 59-year-old man who had experienced a stroke to the region around the right posterior insular area 6 months previously (Figure 1, top). During the acute phase of his recovery, he had reported discomfort when stepping on an escalator. At the time of this experiment, he worked full time and drove a car without any difficulty. The person with left-brain injury was a 69-year-old man who had experienced a stroke in the left insular area (Figure 1, bottom) 3 months previously. He initially had demonstrated aphasic symptoms but had recovered from his aphasia by the time he participated in the study. He could easily respond to the handedness test [30] and could follow instructions in the experiment. Both persons with brain injury were right-handed and exhibited no tactile, motor or verbal deficits. They could stand still and walk with a normal posture and no spatial deficits (including neglect) were observed. Likewise, the persons with brain injury showed no visual deficits. The experiment was approved in advance by the Ethics Committee of Nippon Medical School and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from both persons with brain injury prior to participation in the experiment.
Figure 1.
Horizontal sections of MRI image showing lesion areas in persons with brain injury. The person with right-brain injury (top) and the person with left-brain injury (bottom) showed unilateral reductions in signal in the area of the posterior insula (white circles). R denotes right.
Materials
A force indicator (Digital force gauge FGP-5; NIDEC-SHIMPO CORPORATION, Kyoto, Japan) was used to measure the force of the fingertip during galvanic vestibular stimulation (GVS) under finger-touch conditions. Force was measured using the top of a hard-textured plastic cylinder (2 cm in diameter) that was parallel to the floor.
Procedures
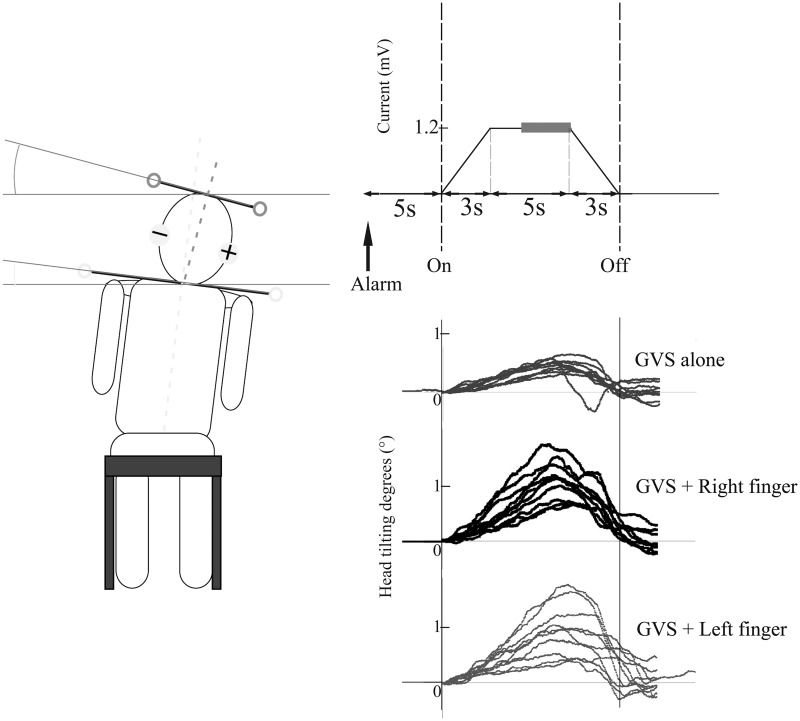
Participants were seated on a chair without arm or back support and with a cushion that had low rebound resilience. The participants faced forward with their arms at their sides and their feet touching the ground softly to prevent them from holding on (Figure 2, left). For the persons with brain injury, one of the authors stood by their side to provide support in the case of instability.
Figure 2.
Schematic of the experimental design. Left panel displays a tilting response to the anodal direction in the sitting position. Participants were instructed to adjust their posture to their subjective vertical on their own accord with a notice alarm. After the notice alarm, 1.2 mA of direct current (trapezoidal pulse) was delivered to the mastoid processes via a right anode and a left cathode (right top panel). The displacement of the mean head positions during the last 3 seconds of the 5-second constant current period (grey bold line) from the time of GVS onset in each trial was analysed. Raw data from a representative healthy participant are shown (right bottom panel). Each condition consisted of 10 trials (lines) and lines show head tilting responses to the anodal direction during GVS.
Direct-current bipolar GVS was applied via electrodes that had been fitted to each participant’s mastoid process using a custom-made electric wave generator controlled by a personal computer and an isolator (SS-202j; NIHON KOHDEN, Tokyo, Japan). For healthy participants, two types of stimulation were applied: the anode on the right with the cathode on the left (right hemispheric stimulation) and the anode on the left with the cathode on the right (left hemispheric stimulation). The order of stimulation type was counterbalanced. For persons with brain injury, only stimulation with the anode on the right and the cathode on the left (right hemispheric stimulation) was applied because the right anodal stimulation induced significant somatosensory-vestibular modulation in healthy participants while no modulatory effects were seen with left anode stimulation. The individuals with brain injury closed their eyes and wore eye masks throughout the experiment. The GVS current intensity was 1.2 mA, a level at which all participants were aware of the stimulation, but the pain was tolerable. A trapezoidal pulse, 3-seconds to peak, 5-seconds constant current and 3-seconds fading out was used for the trial (Figure 2, top right). The inter-trial interval was 6 seconds. A notice alarm was emitted 5 seconds before GVS onset in order for the participants to adjust their posture to their subjective vertical position on their own accord.
The following three conditions were used: GVS alone (GVSa), GVS with right finger touch (+Rf) and GVS with left finger touch (+Lf). The orders of the conditions were GVSa, +Rf and +Lf for the persons with brain injury and for half of the healthy participants and +Lf, +Rf and GVSa for the other half of the healthy participants. Each condition consisted of 10 trials. During GVSa, the participants kept their arms at their sides. During the finger-touch conditions (+Rf and +Lf), participants used an index finger to touch the force indicator with a force of 0.5–2 N, while the other hand remained at their side. Participants were instructed to maintain roughly the same touch force. The top of the force indicator was located at the level of the iliac crest and 20 cm in front of each participant.
Head and shoulder motions were recorded from behind each participant using a video camera (29.97 frames s−1). Participants wore headphones to hold the electrodes in place and light-emitting diode (LED) bulbs were attached to both ends of a bar (25 cm) on the top of the headphones (Figure 2, left). LED bulbs also were attached to both ends of a bar (50 cm) placed on the participants’ shoulders. The line between the LED bulbs was defined as the position of the head or shoulders. Motion capture software (PV Studio 2D; OA Science Inc., Miyazaki, Japan) was used to capture head and shoulder movements. To calculate tilting angles, the head and shoulder positions at the GVS onset of each trial were used as a baseline for that trial. The mean head and shoulder positions during the last 3 seconds of the 5-second constant current GVS were used as the tilted positions in each trial (Figure 2, right top). Data from 10 trials of each condition were analysed. Only the tilting of the head was used in the analyses, as the amount of tilting of the shoulders generally was small. After each condition, participants were interviewed about their perceived body motions (direction, strength and body parts). Head tilting was also recorded from the top (∼50 cm above participant’s head) to monitor tilting in yaw and pitch axes for healthy participants; however, tilting in those two axes were too small to use in the analyses.
Statistical analyses
To examine hemispheric effects of finger touch on GVS-induced tilting responses in healthy participants, the ratio of tilting with finger touch to tilting without finger touch (+Rf/ GVSa, +Lf/ GVSa) was calculated and compared between those obtained using the right anode or the left anode using a 2 (anodal side: right or left) × 2 (conditions: +Rf/GVSa, and +Lf/GVSa) analysis of variance (ANOVA).
To test whether GVSa evoked significant sway responses, one-sample t-tests were used for the 10 trials of each condition in persons with brain injury and the mean tilting responses of age-matched healthy participants. The tilting responses were compared between conditions (GVSa, +Rf, and +Lf) using a one-way ANOVA for each participant.
Finally, tilting responses in persons with brain injury were compared with those of healthy participants (n = 11) using the Crawford t-test [31].
Results
Hemispheric differences in finger-touch effects on vestibular processes in healthy participants
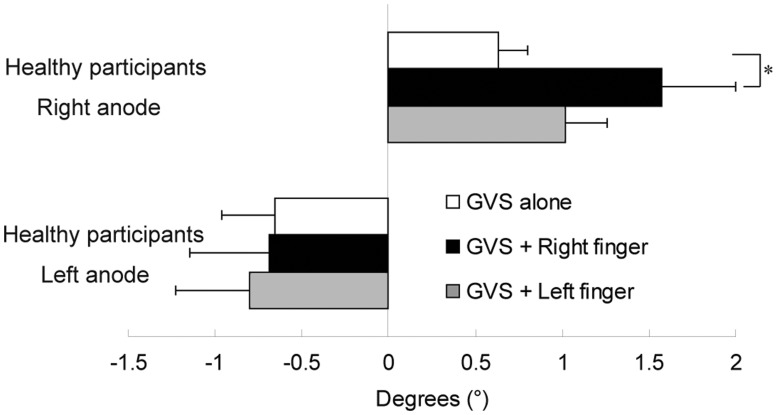
GVS induced tilt toward the anode in healthy participants (Figure 3). The mean degrees (°) of tilting in healthy participants were 0.63 (SD = 0.56), 1.57 (SD = 1.41) and 1.02 (SD = 0.81) to the right anodal side for GVSa, +Rf and +Lf, respectively. With the left anodal stimulations, tilting degrees were −0.66 (SD = 1.02), −0.69 (SD = 1.52) and −0.80 (SD = 1.40) for GVSa, +Rf and +Lf, respectively. The right anodal stimulations induced tilt toward the anode in all participants, whereas the left anodal stimulations induced responses to cathodal direction in one exceptional participant.
Figure 3.
Mean tilting responses induced by GVS alone and GVS with finger touch in healthy participants (n = 11). The right anode stimulations with finger touch (top) induced greater tilting responses than those of the left anode (bottom). In the right anode stimulation condition, GVS with right finger touch elicited greater responses than GVS without finger touch. Error bars represent standard error. Significant differences between conditions within each anode stimulation type are shown using asterisks. *p < 0.05.
The mean finger touch effects (tilting degrees with finger touch/without finger touch in each participant) with the right anode were 3.71 (SD = 3.71) and 2.58 (SD = 2.82) for the right and left finger, respectively. With the left anode, finger touch effects were 0.6 (SD = 0.36) and 1.94 (SD = 2.30) for the right and left finger, respectively. A 2 (anode right or left) × 2 (finger right or left) ANOVA revealed significantly greater effects of the right anode (F[1, 20] = 4.63, p < 0.05) than the left. No effects of right/left finger (F[1, 20] = 0.24, p = 0.63) nor interactions (F[1, 20] = 1.63, p = 0.22) were observed. Nevertheless, it was hypothesized that differences within the right anodal conditions would reveal effects of finger touch on vestibular processing. Analyses performed for the right anode stimulation did detect significant effects of finger touch (F[2, 20] = 3.55, p < 0.05) by an ANOVA and the post-hoc analysis (Ryan’s method) showed greater effects of the right finger touch than no finger touch (t = 2.65, p < 0.0166).
In all three conditions on both anodal sides, no correlations were observed between the tilting angle and age (right anode, GVSa: r = −0.01, p = 0.97; +Rf: r = −0.10, p = 0.78; +Lf: r = −0.45, p = 0.17; left anode, GVSa: r = 0.31, p = 0.36; +Rf: r = 0.40, p = 0.23; +Lf: r = 0.42, p = 0.19). In all healthy participants, the magnitude of finger-touch forces was generally kept constant (0.5–2 N).
Person with right-brain injury
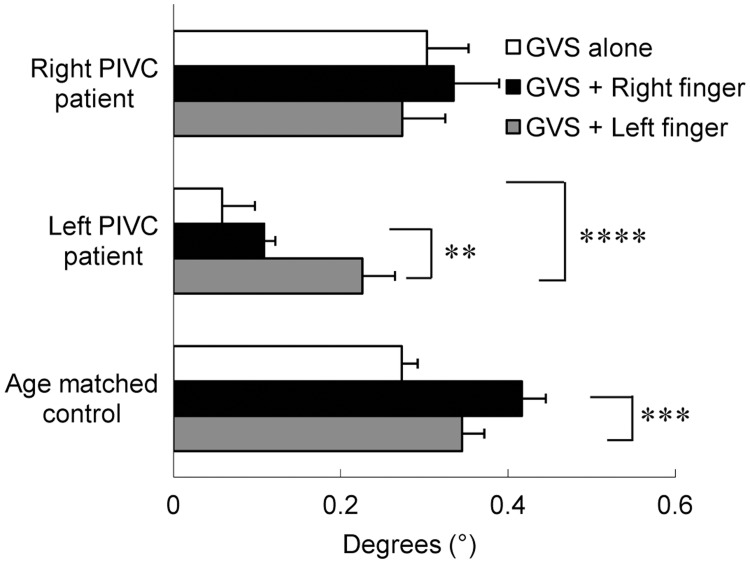
The mean degrees of tilting in the person with right-brain injury were 0.30 (SD = 0.15), 0.33 (SD = 0.17) and 0.27 (SD = 0.16) to the right anodal side for GVSa, +Rf and +Lf, respectively (Figure 4, top). In the person with right-brain injury, one-sample t-tests revealed significant tilt under all three conditions (GVSa: t = 5.71, p < 0.0005; +Rf: t = 4.97, p < 0.001; +Lf: t = 5.31, p < 0.0005), however, an ANOVA showed no significant differences between conditions (F[2, 18] = 0.27, p = 0.77). The person with right-brain injury reported a slight swinging sensation in his head and hip during +Rf, subtle tilting toward the cathode on his head during +Lf and very weak dizziness during GVSa. His ratings for his perceived body motion were +Rf > +Lf > GVSa.
Figure 4.
Tilting responses in persons with unilateral posterior insular lesion and age-matched control participants. The person with right-brain injury showed no differences in tilting responses between conditions. In the person with left-brain injury, finger touches enhanced tilting responses during GVS. In age-matched healthy participants, the right finger touch significantly modulated tilting responses. Error bars represent standard error. Only significant differences between conditions for each participant are shown using asterisks. **p < 0.01, ***p < 0.005, ****p < 0.001.
The mean finger-touch effects (+Rf/GVSa and +Lf/GVSa) were 1.11 (SD = 1.08) for the right finger and 0.90 (SD = 1.03) for the left finger. Crawford t-tests revealed no significant differences in finger-touch effects on GVS-induced tilting in the person with right-brain injury compared to healthy participants (+Rf: t = 0.67, p = 0.26; +Lf: t = 0.57, p = 0.29). In the persons with brain injury and age-matched participants, the magnitude of finger-touch forces were generally kept constant (0.5–2 N).
Person with left-brain injury
The tilting degrees for GVSa, +Rf and +Lf were 0.06 (SD = 0.12), 0.11 (SD = 0.04) and 0.23 (SD = 0.12), respectively (Figure 4, middle). Although GVS alone had no effect on tilting (t = 1.51, p = 0.16), GVS with finger-touch induced significant tilt ( + Rf: t = 7.93, p < 0.0001; +Lf: t = 5.75, p < 0.0005) in the person with left-brain injury. The ANOVA showed a main effect of condition (F[2, 18] = 10.71, p < 0.001) and post-hoc analyses revealed that +Lf induced greater tilt than GVSa (t = 4.51, p < 0.001) and +Rf (t = 3.17, p < 0.01). No significant differences were detected between +Rf and GVSa (t = 1.34, p = 0.20). The person with left-brain injury reported a sensation of slight tilting toward the cathode under all three conditions and his ratings for the degree of tilting were +Lf > +Rf > GVSa.
The mean finger-touch effects were 1.85 (SD = 0.35) for the right finger and 3.85 (SD = 1.01) for the left finger. No significant differences in GVS induced tilting responses were observed compared with healthy participants (+Rf: t = 0.48, p = 0.32, +Lf: t = 0.43, p = 0.34).
Age-matched control participants
The mean tilting in age-matched control participants was 0.27 (SD = 0.06), 0.42 (SD = 0.09) and 0.35 (SD = 0.08) to the right anodal side for GVSa, +Rf and +Lf, respectively (Figure 4, bottom). An ANOVA revealed a main effect of condition (F[2, 18] = 10.23, p < 0.005) and post-hoc analysis showed that +Rf induced greater tilting responses than GVSa (t = 4.53, p < 0.0003).
Discussion
GVS evoked small but robust tilting responses in healthy participants and this tilting was modulated by a light finger touch while in a sitting position. Right anode bipolar vestibular stimulation induced greater tilt than left anode bipolar vestibular stimulation. In addition, right finger touch during GVS induced greater tilting responses than no finger touch. In the person with right posterior insular injury, somatosensory-vestibular interactions were not observed; however, GVS-evoked tilts were detected. In the person with left-brain injury, finger touch enhanced tilting responses as compared to no finger touch. These results suggest that a relevant somatosensory-vestibular interaction could occur in the right posterior insula.
Somatosensory-vestibular interactions in healthy participants
This study found enhanced postural sway responses due to a light finger touch during GVS. Both bottom-up and top-down interpretations have been put forth to explain somatosensory-vestibular interactions [18]. PIVC neurons respond to both vestibular and somatosensory inputs in monkeys [6, 7] and somatosensory and vestibular inputs to bimodal neurons may jointly enhance the somatosensory and/or vestibular processes that control posture. Thus, enhanced postural response caused by finger touch might induce excessive sway by such a mechanism. Vestibular stimulation has been suggested to modulate conscious body awareness [22]. Body representation that is modulated by vestibular inputs [32] may enhance somatosensory perception [19, 21] and/or attention. Enhanced and modulated somatosensory processes could conceivably trigger an exaggerated recalibration of postural control, thereby resulting in greater sway. In this study, modulation of vestibular processing using finger touch was greater with right hemispheric vestibular stimulation than with stimulation of the left hemisphere. These results are consistent with a right hemispheric dominance in vestibular processing, which previously has been reported in normal subjects [33–35]. Somatosensory, vestibular and spatial attention may be dominantly integrated in the right hemisphere.
The enhancement of sway responses by a light finger touch in this study is in contrast to the attenuation in sway previously observed when subjects were in an upright position [36]. A sitting position was used in this study to reduce the effects of the leg muscles that support balance control in standing positions [23]. In a sitting position, the leg muscles are not engaged in postural control and GVS evokes no responses in soleus electromyograms comparable to those elicited in standing postures [4]. Moreover, cutaneous information from the foot modulates vestibular responses both in standing [37] and sitting [38] positions. Somatosensory representation of the leg in the region of the posterior insula [9, 10] might be associated with postural control and somatosensory-vestibular interactions. The lack of leg muscle engagement in postural responses in the sitting position used in the present study might, therefore, reduce the stabilizing effects of light finger touch.
Right/left posterior insular injury and somatosensory-vestibular interactions
Hemispheric effects of posterior insula lesions on the modulation of vestibular-evoked postural responses by finger touch were observed. The person with right-brain injury showed similar responses to those of a healthy control participant in the GVS alone condition; however, modulations by finger touch were not observed. Hence, the modulation of vestibular postural responses by finger touch could be associated with the right posterior insula. In contrast to the person with right-brain injury, the person with left-brain injury did show modulations of tilt during GVS by finger touch. Taken together, the results suggest a right hemispheric dominance in the posterior insula for somatosensory-vestibular interactions, a result that is consistent with previous findings in persons with brain injury [39–41]. Persons with right brain lesion show deficits in subjective visual vertical and these deficits ameliorate with right anodal GVS [42]. The right PIVC could therefore be responsible for multimodal interactions/integrations concerning one’s own body [16] and could be involved in somatosensory-vestibular interactions.
Tilting responses were not detectable with GVS alone in the person with left-brain injury, although the responses to GVS were significantly increased with a light finger touch, suggesting that modulation occurs in the intact right hemisphere. These results suggest that GVS alone could be used as a control condition for GVS with finger touch.
Study limitations
In this small sample, no significant differences in finger touch effects on vestibular processing were observed between healthy participants and persons with posterior insular injury. Variations in tilting degrees in healthy participants should be taken into consideration when considering these results and the use of a larger number of both healthy subjects and persons with brain injury is necessary in future studies to fully explore the effects of finger touch on vestibular processing. Although the variations in tilting degrees were large in healthy participants, all healthy participants, including age-matched controls, showed somatosensory modulatory effects on vestibular responses with the right anodal stimulation. The person with left insular injury also showed the modulation; however, the hand was contralateral. In contrast, the person with right insular injury showed no modulatory effects. These results suggested dissociations in somatosensory modulatory effects on vestibular responses between healthy participants and persons with unilateral insular injury.
The younger age and longer duration after lesion of the person with right insular injury suggested more recovery than the person with left insular injury and the person with right injury showed no somatosensory modulatory effects on vestibular responses while the person with left injury showed effects similar to those of healthy participants. In addition, this study examined a wide age range of healthy participants and, while no effects of age on tilting angle were observed, small tilting responses in persons with brain injury and age-matched participants suggest advanced age might undermine vestibular responses.
Laterality of somatosensory input
The right-handed healthy participants showed greater responses with the right than the left finger condition, although this modulatory effect was observed only in the right anode condition. The person with right-brain injury showed no differences between the right and left finger conditions, whereas the person with left-brain injury showed greater tilting responses with his left finger touch than with his right finger touch. Although the hemispheric difference in somatosensory inputs for somatosensory-vestibular interactions could not be determined from these results, further experiments using left-handed individuals may provide answers to this question.
Conclusion
In summary, somatosensory-vestibular modulation was observed with the condition of finger touch during right hemispheric GVS in healthy participants and double dissociations in responding were found in persons with unilateral damage to the posterior insula. The person with right posterior insular injury showed GVS-evoked sway responses, although no somatosensory-vestibular modulation by finger touch was detected. In contrast, finger touch did modulate GVS-evoked tilting in the person with left posterior insular injury. These preliminary results suggest that the right posterior insula is likely to be involved in somatosensory-vestibular interactions.
Acknowledgements
We thank S. Hihara for helping with data collection from healthy participants and N. Ishibashi for assisting with data collection from persons with brain injury.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work was supported by JPSP, the Funding Program for World-leading Innovative R&D on Science and Technology, and MEXT Japan.
References
- 1.Stevens JC, Choo KK. Spatial acuity of the body surface over the life span. Somatosensory & Motor Research. 1996;13:153–166. doi: 10.3109/08990229609051403. [DOI] [PubMed] [Google Scholar]
- 2.Lackner JR, DiZio P, Jeka J, Horak F, Krebs D, Rabin E. Precision contact of the fingertip reduces postural sway of individuals with bilateral vestibular loss. Experimental Brain Research. 1999;126:459–466. doi: 10.1007/s002210050753. [DOI] [PubMed] [Google Scholar]
- 3.Rabin E, DiZio P, Ventura J, Lackner JR. Influences of arm proprioception and degrees of freedom on postural control with light touch feedback. Journal of Neurophysiology. 2008;99:595–604. doi: 10.1152/jn.00504.2007. [DOI] [PubMed] [Google Scholar]
- 4.Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Experimental Brain Research. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- 5.Avillac M, Ben Hamed S, Duhamel JR. Multisensory integration in the ventral intraparietal area of the macaque monkey. Journal of Neuroscience. 2007;27:1922–1932. doi: 10.1523/JNEUROSCI.2646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grusser OJ, Pause M, Schreiter U. Vestibular neurones in the parieto-insular cortex of monkeys (Macaca fascicularis): Visual and neck receptor responses. Journal of Physiology. 1990;430:559–583. doi: 10.1113/jphysiol.1990.sp018307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grusser OJ, Pause M, Schreiter U. Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis) Journal of Physiology. 1990;430:537–557. doi: 10.1113/jphysiol.1990.sp018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez C, Schreyer HM, Preuss N, Mast FW. Vestibular stimulation modifies the body schema. Neuropsychologia. 2012;50:1830–1837. doi: 10.1016/j.neuropsychologia.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner U, Iannetti GD, Zambreanu L, Stoeter P, Treede RD, Tracey I. Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: A high-resolution fMRI study. Journal of Neurophysiology. 2012;104:2863–2872. doi: 10.1152/jn.00253.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cerebral Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- 11. Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 2012;212:159--179. [DOI] [PubMed]
- 12.Zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60:162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Stephan T, Deutschlander A, Nolte A, Schneider E, Wiesmann M, Brandt T, Dieterich M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005;26:721–732. doi: 10.1016/j.neuroimage.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- 15.Karnath HO, Dieterich M. Spatial neglect – a vestibular disorder? Brain. 2006;129:293–305. doi: 10.1093/brain/awh698. [DOI] [PubMed] [Google Scholar]
- 16.Lopez C, Halje P, Blanke O. Body ownership and embodiment: Vestibular and multisensory mechanisms. Clinical Neurophysiology. 2008;38:149–161. doi: 10.1016/j.neucli.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Fasold O, Heinau J, Trenner MU, Villringer A, Wenzel R. Proprioceptive head posture-related processing in human polysensory cortical areas. Neuroimage. 2008;40:1232–1242. doi: 10.1016/j.neuroimage.2007.12.060. [DOI] [PubMed] [Google Scholar]
- 18.Ferre ER, Bottini G, Haggard P. Vestibular modulation of somatosensory perception. European Journal of Neuroscience. 2011;34:1337–1344. doi: 10.1111/j.1460-9568.2011.07859.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferre ER, Sedda A, Gandola M, Bottini G. How the vestibular system modulates tactile perception in normal subjects: A behavioural and physiological study. Experimental Brain Research. 2010;208:29–38. doi: 10.1007/s00221-010-2450-9. [DOI] [PubMed] [Google Scholar]
- 20.Kerkhoff G, Hildebrandt H, Reinhart S, Kardinal M, Dimova V, Utz KS. A long-lasting improvement of tactile extinction after galvanic vestibular stimulation: Two Sham-stimulation controlled case studies. Neuropsychologia. 2011;49:186–195. doi: 10.1016/j.neuropsychologia.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Ferre ER, Bottini G, Haggard P. Vestibular inputs modulate somatosensory cortical processing. Brain Structure & Function. 2012 doi: 10.1007/s00429-012-0404-7. 217:859--864. [DOI] [PubMed] [Google Scholar]
- 22.Bottini G, Cappa SF, Sterzi R, Vignolo LA. Intramodal somaesthetic recognition disorders following right and left hemisphere damage. Brain. 1995;118:395–399. doi: 10.1093/brain/118.2.395. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. Journal of Applied Physiology. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 24.Utz KS, Korluss K, Schmidt L, Rosenthal A, Oppenlander K, Keller I, Kerkhoff G. Minor adverse effects of galvanic vestibular stimulation in persons with stroke and healthy individuals. Brain Injury. 2011;25:1058–1069. doi: 10.3109/02699052.2011.607789. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson D, Zubko O, Sakel M. Safety of repeated sessions of galvanic vestibular stimulation following stroke: A single-case study. Brain Injury. 2009;23:841–845. doi: 10.1080/02699050903232541. [DOI] [PubMed] [Google Scholar]
- 26.Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, Zilles K, Dieterich M. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: An fMRI study with clinical implications. Neuroimage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. Journal of Physiology. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day BL, Guerraz M. Feedforward versus feedback modulation of human vestibular-evoked balance responses by visual self-motion information. Journal of Physiology. 2007;582:153–161. doi: 10.1113/jphysiol.2007.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: A vestibularly driven balance protection mechanism. Journal of Physiology. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 31.Crawford JR, Howell DC. Comparing an individual's test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- 32.Lopez C, Schreyer HM, Preuss N, Mast FW. Vestibular stimulation modifies the body schema. Neuropsychologia. 2012;50:1830–1837. doi: 10.1016/j.neuropsychologia.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) Journal of Neurophysiology. 2001;85:886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- 34.Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003;13:994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- 35.Klingner CM, Volk GF, Flatz C, Brodoehl S, Dieterich M, Witte OW, Guntinas-Lichius O. Components of vestibular cortical function. Behavioural Brain Research. 2013;236C:194–199. doi: 10.1016/j.bbr.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 36.Jeka JJ, Lackner JR. Fingertip contact influences human postural control. Experimental Brain Research. 1994;100:495–502. doi: 10.1007/BF02738408. [DOI] [PubMed] [Google Scholar]
- 37.Muise SB, Lam CK, Bent LR. Reduced input from foot sole skin through cooling differentially modulates the short latency and medium latency vestibular reflex responses to galvanic vestibular stimulation. Experimental Brain Research. 2012;218:63–71. doi: 10.1007/s00221-012-3002-2. [DOI] [PubMed] [Google Scholar]
- 38.Thomas KE, Bent LR. Subthreshold vestibular reflex effects in seated humans can contribute to soleus activation when combined with cutaneous inputs. Motor Control. 2013;17:62–74. doi: 10.1123/mcj.17.1.62. [DOI] [PubMed] [Google Scholar]
- 39.Alessandrini M, Napolitano B, Bruno E, Belcastro L, Ottaviani F, Schillaci O. Cerebral plasticity in acute vestibular deficit. European Archives of Oto-Rhino-Laryngolog. 2009;266:1547–1551. doi: 10.1007/s00405-009-0953-4. [DOI] [PubMed] [Google Scholar]
- 40.Bense S, Bartenstein P, Lochmann M, Schlindwein P, Brandt T, Dieterich M. Metabolic changes in vestibular and visual cortices in acute vestibular neuritis. Annals of Neurology. 2004;56:624–630. doi: 10.1002/ana.20244. [DOI] [PubMed] [Google Scholar]
- 41.Saj A, Honore J, Bernard-Demanze L, Deveze A, Magnan J, Borel L. Where is straight ahead to a patient with unilateral vestibular loss? Cortex. 2013;49:1219–1228. doi: 10.1016/j.cortex.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Saj A, Honore J, Rousseaux M. Perception of the vertical in patients with right hemispheric lesion: Effect of galvanic vestibular stimulation. Neuropsychologia. 2006;44:1509–1512. doi: 10.1016/j.neuropsychologia.2005.11.018. [DOI] [PubMed] [Google Scholar]