Abstract
Individuals with Attention-Deficit/Hyperactivity Disorder (ADHD) have elevated smoking prevalence and reduced cessation rates compared to the general population. However, the effects of cigarette smoking on underlying brain activity in smokers with ADHD are not well characterized. Non-invasive Near-Infrared Spectroscopy (NIRS) was used to characterize how cigarette smoking affects prefrontal brain hemodynamics in smokers with and without ADHD. Prefrontal changes of oxy- and deoxyhemoglobin (HbO2 and HHb) were measured in six male adult smokers with ADHD and six age- and gender-matched control smokers. NIRS measurements were separated into four sequential time intervals, i.e., before smoking, during smoking, after smoking, and during a breath hold. Prefrontal HbO2 was lower during smoking in smokers with ADHD compared to control smokers. More specifically, smokers with ADHD showed decreased prefrontal HbO2 during smoking compared to breath hold, before and after smoking periods. In contrast, control smokers showed increased prefrontal HbO2 from before smoking to breath hold. Decreased prefrontal HbO2 in smokers with ADHD may reflect a smoking-induced change in prefrontal brain activity and microvasculature, which is not found in smokers without ADHD. The lower prefrontal HbO2 may be a biomarker for increased susceptibility to tobacco smoke in smokers with ADHD. Smoking in individuals with ADHD may increase vasoconstriction of cerebral arteries in the prefrontal cortex, which may contribute to a reduction in HbO2. The findings highlight the importance of smoking cessation, in particular in those smokers who use nicotine to self-medicate ADHD symptoms.
Keywords: Tobacco smoke, Attention-Deficit/Hyperactivity Disorder (ADHD), Brain activity, Prefrontal oxygenation, Near-Infrared Spectroscopy
1. Introduction
Cigarette smoking continues to be a major public health problem. The Centers for Disease Control and Prevention (CDC, 2009) estimated that cigarette smoking costs $193 billion per year in direct medical expenditures and costs associated with loss of productivity. Attention-Deficit/ Hyperactivity Disorder (ADHD) is characterized by inattention, impulsivity and hyperactivity (APA, 2000) and has been identified as a risk factor for smoking initiation and subsequent nicotine addiction (Gehricke et al., 2009; Gehricke et al., 2007; Kollins et al., 2005; Milberger et al., 1997). The smoking prevalence rates among young adults with this disorder have been at about 40% for the last 15 years (McClave et al., 2010; Molina and Pelham, 2001; Pomerleau et al., 1995) compared to 22% in the general population (CDC, 2011). In addition, smokers with ADHD are less successful in smoking cessation (Covey et al., 2008; Pomerleau et al., 1995).
The high prevalence and low cessation rates may be the result of individuals with ADHD self-medicating with nicotine (Wilens et al., 2007). As suggested previously (Gehricke et al., 2007), the elevated risk of dependence and the greater difficulty quitting in individuals with ADHD may result, in part, from nicotine’s effects on prefrontal cortical brain areas. Prefrontal cortex (PFC) areas are rich in nicotinic acetylcholine receptors (nAChRs) (Ghatan et al., 1998) and play a major role in the pathophysiology of ADHD (Arnsten, 2009). Research has shown that cigarette cravings during abstinence activate the PFC (Brody et al., 2007; Zubieta et al., 2005), whereas Varenicline (a partial agonist at alpha4beta2-nAChRs and antagonist at alpha7 nAChRs) reduced activity in some of these regions (Franklin et al., 2011). Similarly, acute cigarette smoking has been shown to reduce brain regional cerebral blood flow (rCBF) and brain hemodynamic reactivity (Ghatan et al., 1998; Terborg et al., 2002), vascular reactivity, and oxygen consumption (Siafaka et al., 2007).
Given the elevated smoking prevalence rates and lower cessation rates, smokers with ADHD may show altered prefrontal hemodynamic characteristics in response to cigarette smoking. The primary aim of the study was to measure the acute prefrontal hemodynamic changes using Near-Infrared Spectroscopy (NIRS) method during smoking in smokers with and without ADHD. NIRS uses principles of optical spectroscopy to detect brain hemodynamic changes such as changes in oxyhemoglobin (HbO2) and deoxyhemoglobin (HHb) (Gratton et al., 1997; Gratton et al., 2005). A secondary aim of the study was to measure prefrontal hemodynamic changes after smoking in response to oxygen deprivation (i.e., a breath hold), which indicates cerebral vasomotor reactivity (VMR). VMR is age-dependent (Safanova et al., 2004) and a result of baroreceptors reacting to increased pressure of carbon dioxide, which induces vasodilatation in the resistance levels, increasing blood flow velocity in the cerebral arteries and reducing the resistance of vessels (Molinari et al., 2006). Cerebral blood flow is mediated by dopamine (Choi et al., 2006), which is depressed in individuals with ADHD (Volkow et al., 2007). Based on the prefrontal and dopaminergic dysfunctions associated with ADHD, we hypothesized that smokers with ADHD show altered PFC hemodynamic characteristics compared to control smokers.
2. Materials and Methods
2.1 Participants
Twelve young adult male smokers (6 ADHD and 6 Controls) were recruited as part of a larger study on the effects of smoking on brain circuitry. Similar to previous studies (Gehricke et al., 2009, Gehricke et al., 2011), each participant was assessed according to DSM-IV-TR criteria (APA, 2000) with the Structured Clinical Interview for DSM-IV, (SCID; First et al., 1996) and the QUEST method (Wigal et al., 2007). Participants were excluded if they were treated for any chronic illness such as heart disease, irregular heartbeat, hypertension, diabetes, skin allergies or skin diseases, even if currently controlled by medication. Participants did not take any medications to treat their ADHD symptoms and had no comorbid Axis I diagnosis. Smokers were defined as individuals who have been smoking at least 5 cigarettes per day to up to 20 cigarettes per day for at least two years. Cigarette smoking history and habits were assessed with the California Tobacco Survey (Davis, 2005). Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence (Fagerström, 1978). Each participant was asked to abstain from smoking overnight (i.e. at least 8 hours) prior to participation, which was validated via self-report and with expired carbon monoxide using a Portable CO Analyzer (National Draeger). The standard cut-off level for participation in the study was 8 ppm prior to the NIRS. The level of nicotine per cigarette was verified by content description on the cigarette package. None of the participants were using or dependent on illicit drugs. Abstinence from drugs of abuse prior to and during participation in the study was verified with commercially available urine drug screens (Integrated E-Z Split Key Cups 10 Panel; Drugformation.com). There were no significant differences in sample characteristics between smokers with ADHD and control smokers (see Table 1).
Table 1.
Sample characteristics
| Characteristics | ADHD (N =6) | Control (N = 6) |
|---|---|---|
| Ages, years (M ± SD) | 22.5 ± 0.84 | 24.8 ± 2.49 |
| Caucasian (%) | 83.33 | 50 |
| Education, years (M ± SD) | 12.0 ± 0.02 | 13.33 ± 1.63 |
| Employed (%) | 50 | 67 |
| Number of cigarettes/day (M ± SD) | 10.75 ± 3.92 | 10.92 ± 6.51 |
| Age started smoking regularly, years (M ± SD) | 17.17 ± 0.75 | 16.83 ± 2.93 |
| Number of quit attempts (M ± SD) | 2.67 ± 1.63 | 2.83 ± 2.04 |
| Nicotine per cigarette in mg (M ± SD) | 1.17 ± 0.14 | 1.00 ± 0.32 |
| Fagerström test for nicotine dependence (M ± SD) | 3.33 ± 1.03 | 4 ± 2.00 |
2.2. Experimental procedure
The study was approved by the Institutional Review Board of the University of California, Irvine. Subjects were instructed to abstain from smoking overnight prior to the NIRS session. On the morning of the NIRS measurements, each participant was tested for carbon monoxide, breath alcohol and illicit drug use via urine drug screen. The study protocol was performed on an outdoor patio. Participants sitting outside had their shoulders against a wall and faced a large sun-block umbrella arranged at a 45° angle, with the concave side in front of them to reduce outdoor light interference with our acquisition. The NIRS head band was placed on the subject’s forehead, held in place with an elastic band slightly attached to prevent vasoconstriction and covered with aluminum foil to reflect outdoor light. Participants also wore a breathing belt to detect variations in the breathing rate and smoking anomalies. The session started with approximately 2 minutes of relaxation (baseline). Measurements were taken over four time intervals: (1) Two minutes prior to lighting a cigarette; (2) during smoking (start-to-end) lasting approximately 4 minutes; (3) during an exhaled breath hold after smoking (average duration 24 seconds and standard deviation of 10.65 in smokers with ADHD and 31 seconds with a standard deviation of 13.57 in control smokers); and (4) an additional two minutes after smoking.
2.3 Near-infrared spectroscopy
A single channel Oximeter (model 9620)8, ISS Inc., Champaign, IL) was used, which operates at a modulation frequency of 110 MHz and a cross-correlation frequency of 6 kHz, with 8 light sources, 4 emitting light at 690 nm, and 4 at 830 nm. Light intensity was modulated at a frequency of 9.76 Hz, providing an acquisition frequency of 10 data points per second. Light sources were arranged at multiple distances ranging from 2.40 cm to 3.99 cm from the photon-multiplier tube (PMT) detector, in a linear way and encased in a silicon head band for optimal contact with the subject’s forehead. Before measuring, the sensor was calibrated on a silicon block with optical properties comparable to those of the human brain. Similar to previous research (Safonova et al., 2004), measurements were performed on the left forehead of each subject.
2.4 Optical data analysis
The Oximeter provided a calculated measure of light intensity modulation, demodulation, from which values of the absorption μa and reduced scattering μs` coefficients and phase delay were calculated. From these values the physiological quantities of HbO2 and HHb were reconstructed as a function of wavelength (690 nm and 830 nm) and followed the frequency-domain multiple-distanced method (Gratton et al., 1997). Time series data were low pass filtered and the grand average of the initial baseline period was subtracted to reference zero change at baseline. Specifically, we applied a finite impulse response, FIR, filter with a zero phase delay. The filter kernel is defined by an algorithm implemented in the BoxyRead software. The implementation has a left-right symmetry and proceeds according to the recursion coefficient. We used a low band pass coefficient of order 0.8, which effectively suppresses all frequencies below 1s. In addition, we removed signal frequencies due to systemic changes. Specifically the heart and breathing frequencies were removed according to the procedure explained in Gratton and Corballis (1995).
2.5 Statistical analysis
HbO2 and HHb were analyzed with a 2 group (ADHD versus Controls) and 4 time points (before smoking, during smoking, during breath hold, and after smoking) linear mixed model analysis of variance (ANOVA; SPSS 17.0). Interaction effects were decomposed with t-tests. All statistical tests were conducted at alpha level of 0.05.
3. Results
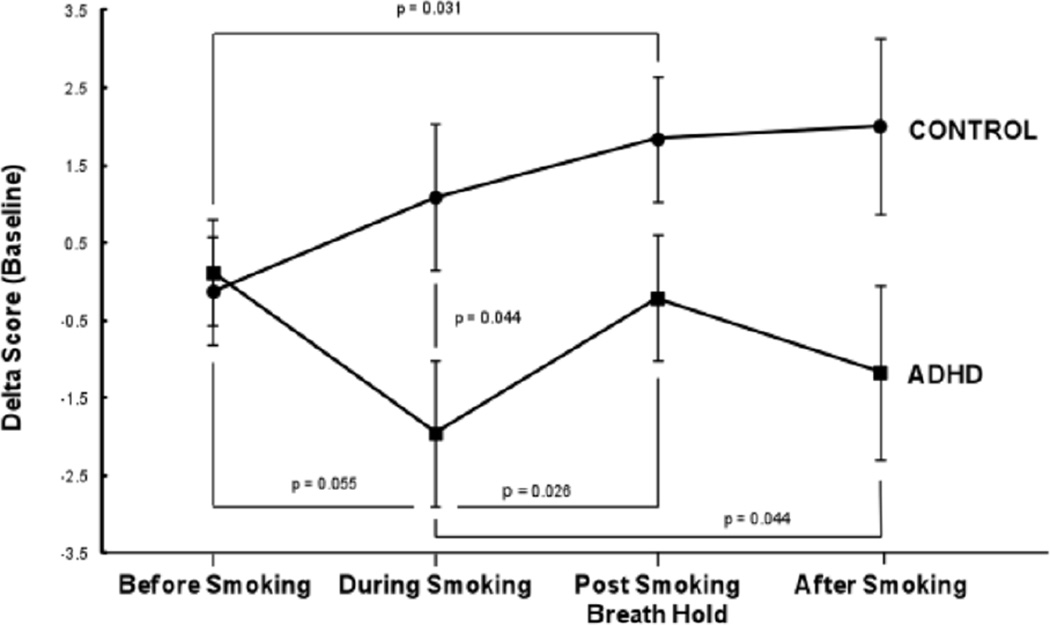
A significant group by time point interaction effect was found for HbO2 (F(3,8) = 3.07, p = 0.043). As depicted in Fig. 1, smokers with ADHD showed a significant decrease in HbO2 during smoking compared to control smokers (t(10) = 3.18, p = 0.010). In addition, smokers with ADHD showed a borderline significant reduction in HbO2 from before to during smoking (t(5) = 2.49, p = 0.055), a significant reduction from during smoking to breath hold (t(5) = 3.12, p = 0.026) and from during smoking to after smoking (t(5) = 2.67, p = 0.044). In contrast, control smokers showed a significant increase in HbO2 during breath hold compared to before smoking (t(5) = 2.99, p = 0.031). No other significant differences were found for HbO2 and HHb.
Figure 1.
Prefrontal oxygenation variations. Means and standard errors of oxyhemoglobin changes in smokers with ADHD and control smokers before smoking, during smoking, during breath hold, and after smoking.
4. Discussion
The findings corroborate the hypothesis of altered smoking-induced PFC HbO2 in smokers with ADHD compared to control smokers (Gehricke et al., 2007; McClernon, 2009). More specifically, smokers with ADHD showed reduced PFC HbO2 in response to cigarette smoking. Such reduction in prefrontal HbO2 may reflect structural changes at the level of the microvasculature due to the toxic effects of tobacco smoking on the vessels and the associated vasomotor reactivity, the ability to change the vessel diameter, and microcirculatory function (Siafaka et al., 2007; Terborg et al., 2002). Although the VMR in response to the breath hold did not differ significantly between groups, smokers with ADHD showed a shorter breath holding duration compared to control smokers, which has been associated with smoking abstinence intolerance and may be a predictor of smoking lapse during quitting (Kahler et al., 2013). Nicotine may trigger a process of oxygen exchange, possibly due to a mechanism of increased vascular dilation or larger vascular recruitment in smokers with ADHD (Safonova et al., 2004). In contrast, control smokers showed an increase from before smoking to post smoking breath hold in HbO2. In a previous study, Pucci et al. (2009) showed a significant slow increase in total hemoglobin concentration due to cigarette smoking. Our results corroborate the findings by Pucci et al. (2009) for HbO2 in control smokers compared to smokers with ADHD.
The nature of the smoking response in smokers with ADHD may suggest that a minor hypoxic event occurs during smoking, in which more oxygen is used than is replaced. Smoking-induced hypoxia may occur because oxygen is not available or perhaps because the neurovascular regulation is impaired due to partial collapse of arteriolar walls or excessive stiffness due to stressed constriction-dilation. Similar effects are reported in clinical populations such as patients with obstructive sleep apnea (Safonova et al., 2004).
A recent model of brain circulation and metabolism (Banaji et al., 2008) explains how oxygen transport and consumption induce changes in the vascular compartment due to physiological variations and cell metabolism. As near-infrared light travels in the brain it encounters products of mitochondrial metabolism as well as oxygen transport and consumption in the microvasculature of the brain tissue. Thus, the PFC in smokers with ADHD may have undergone an adaptive metabolic response to smoking. A similar response has been observed in a study of nitric oxide-dependent mechanisms, showing impairment of cerebral arterioles, and morphological and functional alterations in the vascular endothelium, as a consequence of nicotine infusion (Fang et al., 2003). These effects reflect the impairment of the nitric oxide synthase-dependent mechanism that is responsible for the dilation and constriction of the arterioles due to nicotine.
However, the present study has several limitations, which include a small sample size and NIRS measurement, which was restricted to the left prefrontal cortex. Higher spatial resolution and more detail on inter-regional oxygen regulation can be achieved by adding other measurement locations. However, the NIRS cannot distinguish between specific prefrontal brain areas. In addition, the results of the study have to be interpreted with caution because the subject sample had low dependency scores and a large range of cigarettes per day. Further research is necessary to address these limitations, exploring control conditions and populations of non-smokers with imitated smoking, to provide a more comprehensive model of the effects of smoking on the brain. On a final note, it remains to be investigated whether the group differences reflect changes in brain activity or microvasculature.
5. Conclusions
The decreased prefrontal HbO2 in response to smoking in smokers with ADHD was not found in control smokers. Smoking in individuals with ADHD may either reduce brain activity or increase vasoconstriction of cerebral arteries in the prefrontal cortex, which may contribute to a reduction in HbO2. The decrease in prefrontal HbO2 may be a biomarker for increased susceptibility to tobacco smoke in smokers with ADHD. Such reduction in the prefrontal brain hemodynamics in smokers with ADHD during smoking may reflect a minor hypoxic event, which could increase the risk for cancer. The findings highlight the importance of smoking cessation in particular in those smokers who use nicotine to self-medicate attention deficits and emotional dysfunctions (Gehricke et al., 2007). In addition, the present study documents the utility of NIRS in measuring brain hemodynamic changes in response to cigarette smoking. The NIRS is a noninvasive and complimentary method to other techniques of measuring brain activity such as functional Magnetic Resonance Imaging and Positron Emission Topography.
Highlights.
We measured brain oxygenation during cigarette smoking
We compared individuals with and without Attention-Deficit/Hyperactivity Disorder (ADHD)
Prefrontal oxygenation levels during smoking were reduced in smokers with ADHD
Individuals with ADHD may show increased vasoconstriction while smoking
Acknowledgements
We thank Jessel Villegas, Minjee Kwon, Christine Ly, Jenny Nguyen, and Kimberley Leung for their help in implementing the research protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have no financial interests to disclose concerning the conduct or reporting of this study. This study was supported by the National Institute on Drug Abuse grant DA025131 to Jean Gehricke. Enrico Gratton acknowledges support from NIH-P41-GM103540.
References
- APA. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (text revision) 4th ed. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Banaji M, Mallet A, Elwell CE, Nicholls P, Cooper CE. A model of brain circulation and metabolism: NIRS signal changes during physiological challenges. PLoS Comput Biol. 2008;4:e1000212. doi: 10.1371/journal.pcbi.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Great American Workout. Morbidity and Mortality Weekly Report. 2009;58:1227. [Google Scholar]
- CDC. Vital signs: current cigarette smoking among adults aged ≥18 years --- United States 2005–2010. Morbidity and Mortality Weekly Report. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking cessation and inattention or hyperactivity/impulsivity: a post hoc analysis. Nicotine Tob Res. 2008;10:1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. California Tobacco Survey [electronic version] 2005:1–16. [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. Am J Physiol Heart Circ Physiol. 2003;284:H528–H534. doi: 10.1152/ajpheart.00752.2002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, et al. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Whalen CK, Steinhoff K, Wigal TL. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychol Addict Behav. 2009;23:644–655. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Wigal TL, Chan V, Doan A. ADHD medication reduces cotinine levels and withdrawal in smokers with ADHD. Pharmacol Biochem Behav. 2011;98:485–491. doi: 10.1016/j.pbb.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob Res. 2007;9(Suppl 4):S523–S536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gratton G, Corballis PM. Removing the heart from the brain: compensation for the pulse artifact in the photon migration signal. Psychophysiology. 1995;32:292–299. doi: 10.1111/j.1469-8986.1995.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Gratton E, Fantini S, Franceschini MA, Gratton G, Fabiani M. Measurements of scattering and absorption changes in muscle and brain. Philos Trans R Soc Lond B Biol Sci. 1997;352:727–735. doi: 10.1098/rstb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton E, Toronov V, Wolf U, Wolf M, Webb A. Measurement of brain activity by near-infrared light. J Biomed Opt. 2005;10:11008. doi: 10.1117/1.1854673. [DOI] [PubMed] [Google Scholar]
- Kahler CW, McHugh RK, Metrik J, Spillane NS, Rohsenow DJ. Breath holding duration and self-reported smoking abstinence intolerance as predictors of smoking lapse behavior in a laboratory analog task. Nicotine Tob Res. 2013;15:1151–1154. doi: 10.1093/ntr/nts231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100:2464–2472. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ. Neuroimaging of Nicotine Dependence: Key Findings and Application to the Study of Smoking-Mental Illness Comorbidity. J Dual Diagn. 2009;5:168–178. doi: 10.1080/15504260902869204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Molina BS, Pelham WE. Substance use, substance abuse, and LD among adolescents with a childhood history of ADHD. J Learn Disabil. 2001;34:333–342. doi: 10.1177/002221940103400408. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari F, Liboni W, Grippi G, Negri E. Relationship between oxygen supply and cerebral blood flow assessed by transcranial Doppler and near-infrared spectroscopy in healthy subjects during breath-holding. J Neuroeng Rehabil. 2006;3:16. doi: 10.1186/1743-0003-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Pucci O, Stepanov S, Toronov V. Transcranial Near-Infrared Spectroscopy of smoking brains. Journal of Innovative Optical Health Sciences. 2009;2:227–234. [Google Scholar]
- Safonova LP, Michalos A, Wolf U, Wolf M, Hueber DM, Choi JH, et al. Age-correlated changes in cerebral hemodynamics assessed by near-infrared spectroscopy. Arch Gerontol Geriatr. 2004;39:207–225. doi: 10.1016/j.archger.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Siafaka A, Angelopoulos E, Kritikos K, Poriazi M, Basios N, Gerovasili V, et al. Acute effects of smoking on skeletal muscle microcirculation monitored by near-infrared spectroscopy. Chest. 2007;131:1479–1485. doi: 10.1378/chest.06-2017. [DOI] [PubMed] [Google Scholar]
- Terborg C, Bramer S, Weiller C, Rother J. Short-term effect of cigarette smoking on CO(2)-induced vasomotor reactivity in man: a study with near-infrared spectroscopy and tanscranial Doppler sonography. J Neurol Sci. 2002;205:15–20. doi: 10.1016/s0022-510x(02)00308-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Wigal T, Wigal SB, Steinhofff K, Kollins S, Newcorn JH, Steinberg-Epstein R, et al. Establishing a clinical diagnosis of ADHD in adults: the QUEST method. Advances in ADHD. 2007:17–24. [Google Scholar]
- Wilens TE, Adamson J, Sgambati S, Whitley J, Santry A, Monuteaux MC, et al. Do individuals with ADHD self-medicate with cigarettes and substances of abuse? Results from a controlled family study of ADHD. Am J Addict. 2007;16(Suppl 1):14–21. doi: 10.1080/10550490601082742. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]