Head trauma and brain tumors are inextricably linked to epilepsy. Both traumatic brain injury and brain tumors are overrepresented in newly diagnosed patients with epilepsy, as well as in patients with drug-resistant focal epilepsy. Indeed, chronic seizures are the most cited problem associated with either of these conditions. However, managing complex cases of posttraumatic epilepsy or tumor-based epilepsy can be a challenge for many epilepsy specialists. Currently, there is no clear understanding of the basic science that underpins the mechanism for epileptogenesis in patients with tumors or trauma so at present one cannot identify appropriate targets that could help prevent seizures and epilepsy from occurring. Because of these challenges, complex lesional epilepsy was chosen as the theme for this year's 2012 American Epilepsy Society Annual Course.
The objectives for the course included understanding models for preventing epileptogenesis, creating best evidence algorithms that describe how to best manage patients with epilepsy related to brain tumors, including novel intraoperative monitoring techniques. Risk analyses were summarized to benefit treatment decisions regarding the prophylactic use of antiepileptic drugs in patients with CNS tumors or traumatic brain injury and to present the best management options with patients with metastatic brain tumors based on current knowledge.
Throughout two half-day sessions, post-traumatic epilepsy and tumoral epilepsy were explored in detail. Each session was framed by clinical cases involving adults and children. Through a variety of learning methodologies that included lectures, debates, and panel sessions, clinical management was highlighted, illuminating basic science and practice gaps. This discussion summarizes the salient take-home points of the session that are clinically useful for the practicing clinician.
Tumor-Related Epilepsy
Brain tumors are a common cause of lesional epilepsy. The frequency of epilepsy in patients who have brain tumors is estimated to be 40% (1). However, there is an inverse relationship between the degree of aggressiveness of a tumor and its relationship to epilepsy; the lower the grade of brain tumor pathology, the higher the likelihood for epilepsy. This is why tumors such as dysembryoplastic neuroepithelial tumors (DNETs), gangliogliomas, and other low grade gangliogliomas) are frequently associated with epilepsy in contrast to the lower incidence of epilepsy seen with more aggressive tumors (such as glioblastoma multiforme or metastatic brain tumors). Seizures that are a presenting symptom for tumors indicate a more favorable prognostic factor for survival from the tumor itself (2).
DNETs merit special attention. These lesions often have dual pathologies with major cortical dysplasias and mesial temporal sclerosis. The risk of intractability is 50%, almost twice as high as any other cause. Unfortunately, studies have shown that the longer the duration of epilepsy, the worse the chance of control of seizures for these patients.
Reflecting on the relationship between aggressiveness of tumor pathology and epilepsy, as many as 80% of patients with low-grade tumors present with seizures. The frontal lobe, temporal lobe, and insular regions are the most common sites of tumors that produce seizures. In contrast, 42% of patients with glioblastoma multiforme, have seizures as the presenting symptom; ultimately, 62% of these patients will develop some form of seizures.
Regarding management, both seizure control as well as anti-tumor therapy should be considered. In choosing antiepileptic drugs it is generally preferable to avoid enzyme-inducing agents to minimize potential interactions with chemotherapeutic agents.. There is no evidence supporting the use of prophylactic antiepileptic drugs to prevent seizures in patients with brain tumors with exception of the perioperative period. Valproic acid deserves special discussion: Valproic acid has been reported to improve survival in patients with high-grade brain tumors being treated with temozolomide (4). This is a fascinating consideration, given that the same drug is associated with increased teratogenicity in children of mothers who take this drug during pregnancy. Prospective studies on anticonvulsants with brain tumors are warranted, including their potential for favorable associations with chemotherapeutic agents and contribution to patient survival.
Antitumor therapy starts by obtaining a histological diagnosis—either by biopsy, surgery or, if possible, by gross total resection. Recent evidence supports early surgery for removal of low-grade gliomas over biopsy alone, irrespective of seizures, because of clear benefits on long-term survival (5). In addition, up to 50% of non-enhancing gliomas on imaging are actually anaplastic astrocytomas; therefore, it is difficult to rely on imaging alone for diagnosis and differentiation among tumor subtypes. On occasion, one may choose to use medical treatment only in patients with well controlled seizures and brain tumors that are benign in appearance or not easily accessible. Epilepsy surgery is particularly suited for patients with treatment-resistant seizures and benign or low-grade tumors. Resection should not only include tumor boundaries but possibly also entension beyond the lesion itself, possibly including hippocampal structures (if adjacent), given that the tumor margins are not always so distinct (6). The nature of the tumor will dictate subsequent treatment with radiation and chemotherapy; such treatment, where indicated, may also favorably impact seizure control. In fact, in one study, seizure reduction occurred in 60% of patients with tumor-related epilepsy who were receiving temozolomide versus 13% in the control group (7).
Figure 1 shows an algorithm for management of tumor-related epilepsy.
FIGURE 1.
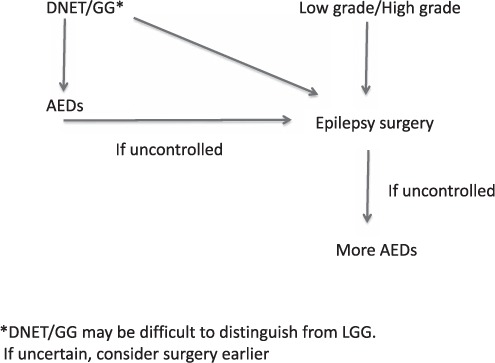
Management of tumor-based epilepsy.
Post-Traumatic Epilepsy
There is clearly an increased risk of epilepsy associated with traumatic brain injury (8–30). The increased risk, however, is limited to those who are diagnosed with moderate or severe civilian traumatic brain injury, which comprises almost 5% of all cases of head trauma. In people who are under the age of 35, traumatic brain injury increases the risk for both generalized tonic–clonic seizures and complex partial seizures. Risk factors for the development of post-traumatic epilepsy include age 65 years or older, the presence of a brain contusion, sub-dural hematoma, linear depressed skull fracture, and loss of consciousness for more than 24 hours. It is also known that the risk for epilepsy after civilian traumatic brain injury is greatest in the first two years after traumatic brain injury in most studies; one study suggests that an increased risk for the development of seizures after severe traumatic brain injury can persist for up to 10 years.
Sadly, with all of the focus on traumatic brain injury, antiepileptogenesis remains a concept yet to translate to clinical management and treatment. Although several new biomarkers of epileptogenic risk are promising (e.g. imaging of hippocampal changes, fMRI, and interictal EEG spike features), there are clearly yet to be defined genetic biomarkers. Promising potential new tools include pathological high-frequency oscillations, alpha methyltryptophan, and positron emission tomography imaging (PET), as well as other PET ligand markers.
Prophylactic antiepileptic drugs can reduce early acute symptomatic seizures after severe traumatic brain injury, but they do not prevent the development of late post-traumatic epilepsy. Antiepileptic drugs are really only useful in severe traumatic brain injury to prevent seizures within the first week after severe trauma (29–31).
Diagnostic tools, such as EEG and MRI, are very important in the diagnosis of posttraumatic epilepsy. Different techniques may be better at identifying different lesion types. These studies help provide data to identify those at risk for developing posttraumatic epilepsy. It may also be important to identify candidates for therapeutic intervention, when anti-epileptogenic agents are found.
Antiepileptic drugs are the mainstay of therapy for post-traumatic epilepsy. No particular agent or combination of agents is considered to be singularly more beneficial than others, and studies have yet to confirm comparative differences for the use of various agents in posttraumatic epilepsy.
Surgical intervention for drug-resistant posttraumatic epilepsy is a viable option for some patients. However, there are caveats: The best candidates are those patients who have a single lesion in contrast to those who have apparent nonlesional or multifocal epilepsy. Those with a multifocal epileptiform EEG tend to have a worse prognosis and may not be the best candidates for surgical intervention. There is a need for multimodal assessment tools to better identify when surgery can be offered in these complex situations.
It is also essential to confirm the diagnosis of epilepsy in patients who have traumatic brain injury and present with spells. Traumatic brain injury and spells do not always imply epilepsy. Some individuals with a history of head trauma will have psychogenic nonepileptic seizures. Indeed those patients who have mild or minimal traumatic brain injury tend to have a stronger association with psychogenic nonepileptic spells and are less likely to have epilepsy. Posttraumatic stress disorder is often the cause associated with psychogenic nonepileptic spells. This is based on military population studies, but may be generalizable to civilian populations.
Figure 2 outlines an algorithm for the management of posttraumatic epilepsy. If patients have traumatic brain injury, they will fall into 1 of 3 categories: mild, moderate, or severe head trauma. If in the severe category, consideration should be given for prophylaxis with antiepileptic drugs in the first 7 days—not to prevent epilepsy but to prevent seizures within that week. This has been found helpful and is a guideline for management by the American Academy of Neurology (31). If spells continue, then one may need to confirm the diagnosis of epilepsy. In patients with mild and moderate traumatic brain injury, particularly if the history is atypical and seizures continue despite antiepileptic drug therapy, confirming the diagnosis of seizures and epilepsy with video EEG monitoring may be necessary. If epilepsy is found then further antiepileptic drug therapy is warranted. Failure of drug therapy should lead to assessment for seizure surgery, recognizing that this still offers the chance for curative treatment and improved quality of life. If a patient fails seizure surgery or is not a candidate, then other options would include additional antiepileptic drugs, neuromodulation with devices such as vagus nerve stimulation and newer stimulation techniques, the use of ketogenic or modified Atkins diet and, clearly, research trials.
FIGURE 2.
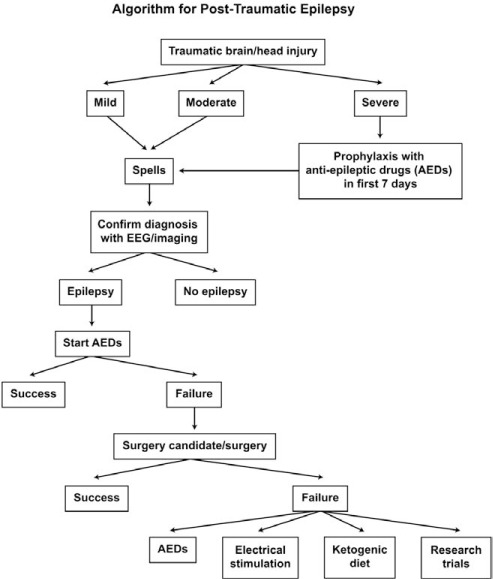
Management of posttraumatic epilepsy.
In the end, it is essential to remember that epilepsy in both these lesional forms represents only one facet of a larger spectrum of symptoms, signs, and diseases. These patients demand a wholistic approach to their management and treatment. Remembering these factors and caring for the psychosocial, psychological, psychiatric conditions, and comorbidities that occur will help ensure improved quality of life for those individuals with either form of lesional epilepsy.
References
- 1.Van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 2.Smits A, Duffau H. Seizures and the natural history of World Health Organization Grade II gliomas: A review. Neurosurg. 2011;68:1326–1333. doi: 10.1227/NEU.0b013e31820c3419. [DOI] [PubMed] [Google Scholar]
- 3.You G, Sha Z, Jiang T. The pathogenesis of tumor-related epilepsy and its implications for clinical treatment. Seizure. 2012;21:153–159. doi: 10.1016/j.seizure.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR, Forsyth P, Rossetti AO, Lacombe D, Mirimanoff RO, Vecht CJ, Stupp R. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, Solheim O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308:1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 6.Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, Berger MS. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 7.Sherman JH, Moldovan K, Yeoh HK, Starke RM, Pouratian N, Shaffrey ME, Schiff D. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114:1617–1621. doi: 10.3171/2010.12.JNS101602. [DOI] [PubMed] [Google Scholar]
- 8.Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9:453–457. doi: 10.1053/seiz.2000.0458. [DOI] [PubMed] [Google Scholar]
- 9.Annegers JF, Hauser WA, Lee JR, Rocca WA. Secular trends and birth cohort effects in unprovoked seizures: Rochester, Minnesota 1935–1984. Epilepsia. 1995;36:575–579. doi: 10.1111/j.1528-1157.1995.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 10.Appleton RE. Seizure-related injuries in children with newly diagnosed and untreated epilepsy. Epilepsia. 2002;43:764–767. doi: 10.1046/j.1528-1157.2002.41101.x. [DOI] [PubMed] [Google Scholar]
- 11.Armed Forces Epidemiological Board. Traumatic brain injury in military service members—2006–02. http://www.health.mil/dhb/2006/2006-02.pdf. Accessed January 4, 2012. [Google Scholar]
- 12.Armed Forces Health Surveillance Center. TBI numbers by severity: All Armed Forces. http://www.dvbic.org/pdf/dod-tbi-2000-2011Q4-as-of-120210.pdf. Accessed February 24, 2012. [Google Scholar]
- 13.Bruns J, Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;44(suppl):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 14.Bryant RA. Disentangling mild traumatic brain injury and stress reactions. N Engl J Med. 2008;358:525–527. doi: 10.1056/NEJMe078235. [DOI] [PubMed] [Google Scholar]
- 15.Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- 16.Gilchrist J, Thomas KE, Xu L, McGuire LC, Coronado V. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤ 19 years—United States, 2001–2009. MMWR. 2011;60:1337–1342. [PubMed] [Google Scholar]
- 17.Goldberg MS. Death and injury rates of U.S. military personnel in Iraq. Mil Med. 2010;175:220–226. doi: 10.7205/milmed-d-09-00130. [DOI] [PubMed] [Google Scholar]
- 18.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429–434. doi: 10.1056/NEJM199802123380704. [DOI] [PubMed] [Google Scholar]
- 20.Herman ST. Epilepsy after brain insult: Targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 21.Hernández AV, Steyerberg EW, Taylor GS, Marmarou A, Habbema JDF, Maas AIR. Subgroup analysis and covariate adjustment in randomized clinical trials of traumatic brain injury: A systematic review. Neurosurg. 2005;57:1244–1253. doi: 10.1227/01.neu.0000186039.57548.96. [DOI] [PubMed] [Google Scholar]
- 22.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 23.Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: A report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- 24.Salinsky M, Spencer D, Boudreau E, Ferguson F. Psychogenic nonepileptic seizures in U.S. veterans. Neurology. 2011;77:945–950. doi: 10.1212/WNL.0b013e31822cfc46. [DOI] [PubMed] [Google Scholar]
- 25.Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148:255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- 26.Tanielian T, Jaycox LH, Schell TL, Marshall GN, Burnam MA, Eibner C, Karney BR, Meredith LS, Ringel JS, Vaiana ME, The Invisible Wounds Study Team Invisible Wounds of War: Summary and Recommendations for Addressing Psychological and Cognitive Injuries. Arlington, VA: RAND Corporation; 2008. [Google Scholar]
- 27.Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, Lucas T, Newell DW, Mansfield PN, Machamer JE, Barber J, Dikmen SS. Magnesium sulfate for neuroprotection after traumatic brain injury: A randomised controlled trial. Lancet Neurol. 2007;6:29–38. doi: 10.1016/S1474-4422(06)70630-5. [DOI] [PubMed] [Google Scholar]
- 28.Temkin NR, Dikmen SS, Anderson GD, Wilensky AJ, Holmes MD, Cohen W, Newell DW, Nelson P, Awan A, Winn HR. Valproate therapy for prevention of posttraumatic seizures: A randomized trial. J Neurosurg. 1999;91:593–600. doi: 10.3171/jns.1999.91.4.0593. [DOI] [PubMed] [Google Scholar]
- 29.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 30.Wojcik BE, Stein CR, Bagg K, Humphrey RJ, Orosco J. Traumatic brain injury hospitalizations of U.S. Army soldiers deployed to Afghanistan and Iraq. Am J Prev Med. 2010;38:S108–S116. doi: 10.1016/j.amepre.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Bernard S. Chang, Lowenstein Daniel H. Subcomittee of the American Academy of Neurology: Report of the Quality Standards Practice parameter: Antiepileptic drug prophylaxis in severe traumatic brain injury. Neurology. 2003;60:10–16. doi: 10.1212/01.wnl.0000031432.05543.14. [DOI] [PubMed] [Google Scholar]


