Commentary
Levetiracetam in Pregnancy: Results From the UK and Ireland Epilepsy and Pregnancy Registers.
Mawhinney E, Craig J, Morrow J, Russell A, Smithson WH, Parsons L, Morrison PJ, Liggan B, Irwin B, Delanty N, Hunt SJ. [Published online ahead of print January 9, 2013.] Neurology 2013;80(4):400–405. 10.1212/WNL.0b013e31827f0874.23303847
OBJECTIVES: Levetiracetam is a broad-spectrum antiepileptic drug (AED) which is currently licensed in the United States and the United Kingdom and Ireland for use as adjunctive treatment of focal-onset seizures and myoclonic seizures or generalized tonic-clonic seizures, occurring as part of generalized epilepsy syndromes. In the United Kingdom and Ireland, it is also licensed as monotherapy treatment for focal-onset seizures. Previous small studies have suggested a low risk for major congenital malformations (MCM) with levetiracetam use in pregnancy. METHODS: The UK and Ireland Epilepsy and Pregnancy Registers are prospective, observational registration and follow-up studies that were set up to determine the relative safety of all AEDs taken in pregnancy. Here we report our combined results for first-trimester exposures to levetiracetam from October 2000 to August 2011. RESULTS: Outcome data were available for 671 pregnancies. Of these, 304 had been exposed to levetiracetam in monotherapy, and 367 had been exposed to levetiracetam in combination with at least one other AED. There were 2 MCM in the monotherapy group (0.70%; 95% confidence interval [CI] 0.19%–2.51%) and 19 in the polytherapy group 5.56% (3.54%–8.56%) [corrected]. The MCM rate in the polytherapy group varied by AED regimen, with lower rates when levetiracetam was given with lamotrigine (1.77%; 95% CI 0.49%–6.22%) than when given with valproate (6.90%; 95% CI 1.91%–21.96%) or carbamazepine (9.38%; 95% CI 4.37%–18.98%). CONCLUSION: This study, in a meaningful number of exposed pregnancies, confirms a low risk for MCM with levetiracetam monotherapy use in pregnancy. MCM risk is higher when levetiracetam is taken as part of a polytherapy regimen, although further work is required to determine the risks of particular combinations. With respect to MCM, levetiracetam taken in monotherapy can be considered a safer alternative to valproate for women with epilepsy of childbearing age.
In utero exposure to first-generation antiepileptic drugs (AEDs) has been shown to increase the risk of congenital malformations and cognitive deficits (1). The risk of major congenital malformations with AED exposure is estimated to be between 4 and 9 percent, compared with the background risk of 1 to 2 percent (2). Prenatal exposure to different older-generation AEDs has been associated with different malformations. For example, the risk of spina bifida increases with exposure to valproate, digit hypoplasia with phenytoin, oral clefts with phenobarbital, and neural tube defects with carbamazepine (3). Moreover, recent data have suggested that fetal exposure to valproate can increase the risk of autism (4) and can affect the child's cognitive abilities (1). However, until recently, data on the safety of newer-generation AEDs have been limited, except for lamotrigine (5). Indeed, for quite a few years, lamotrigine has been increasingly prescribed for women with epilepsy who are of childbearing age, while data on other new AEDs, especially levetiracetam, have been accumulating.
Levetiracetam is approved as an add-on treatment of myoclonic, primary generalized, and partial-onset seizures with or without secondary generalization. Its pharmacokinetic attributes have facilitated its wide clinical use. For example, levetiracetam has linear pharmacokinetics and rapid onset of action, is totally excreted by the kidneys, does not interact with other drugs, can be loaded intravenously or orally (6), is weight neutral, has no cognitive side effects, and does not require blood-level monitoring. These attributes have made levetiracetam a preferable first line AED treatment for many physicians despite its indication as adjunctive therapy in the United States. Thus, it is of great importance to assess the safety of levetiracetam in pregnancy.
Mawhinney et al. recently published important data about the safety of levetiracetam during pregnancy from the UK and Ireland Epilepsy and Pregnancy Registers, which complement other reports published since 2010 (Table). The authors collected outcome data on 671 women who became pregnant while receiving levetiracetam as monotherapy (304 pregnancies) or as part of AED polytherapy (367 pregnancies). They did not record serum AED levels or smoking and alcohol use as part of this study. In the monotherapy group, there were two cases with major congenital malformations (0.7%; 95% confidence interval [CI], 0.19–2.51%), and in the polytherapy group, there were 19 (5.56%; CI, 3.54–8.56%). Polytherapy, including levetiracetam, was associated with increased chances of caesarian delivery (126 cases) compared with levetiracetam monotherapy (73 cases) (p < 0.05, χ2). In addition, the dose of levetiracetam in the monotherapy group did not influence mean birth weight or mean gestational age. The mean daily dose of levetiracetam was 3,000 mg for cases with major malformations, 1,148 mg for cases of minor malformations, and 1,680 mg for those with no malformations. Although there is a clear trend for higher doses to be associated with major malformations, these numbers were not statistically significantly different (p = 0.09). Additionally, higher levetiracetam doses in the polytherapy group were associated with increased risk of spontaneous abortion (p = 0.02), but not of major malformations (p = 0.19). In the polytherapy group, seizure control, including specifically generalized tonic–clonic seizures in the first trimester, did not correlate with the risk of major congenital malformations. As regards different AED combinations in those on polytherapy, major malformations occurred in only 1.8% of those exposed to levetiracetam and lamotrigine compared with 6.9% in those exposed to levetiracetam and valproate, 9.4% in those exposed to levetiracetam and carbamazepine, and none in 20 pregnancies exposed to levetiracetam and topiramate. One of the children exposed to levetiracetam and lamotrigine has multiple digit abnormalities. Of note, while rat and rabbit data on fetal exposure to levetiracetam demonstrated increased risks of skeletal abnormalities, these abnormalities were observed with levetiracetam doses per surface area that are 12 times the maximum recommended dose in humans (2). In humans, of all the cases reported in different registries (Table), only three cases of skeletal abnormalities related to fetal exposure to levetiracetam in the first trimester were reported by the Union Chimique Belge (UCB) registry (7).
Table.
Major Congenital Malformations Associated With Exposure to Levetiracetam
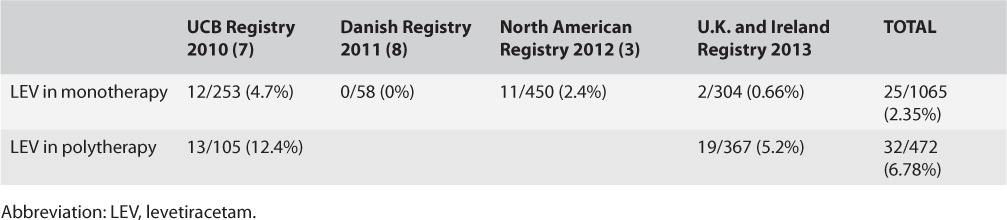
The rate of major congenital malformations associated with exposure to levetiracetam monotherapy reported by Mawhinney et al. (0.7%) is comparable with that in the non-epilepsy population, and the dose of levetiracetam did not correlate with the risk. Demonstrating the safety of levetiracetam in pregnancy is of great clinical utility. Currently, many epileptologists try to switch to lamotrigine during or before planned pregnancy because of its known relative safety during pregnancy. Indeed, the North American registry reported comparable rates of major malformations between levetiracetam (11 of 450) and lamotrigine (31 of 1562) (p = 0.56, χ2). However, levetiracetam is easier to use because of known pharmacokinetic differences between the two drugs. For example, levetiracetam can be orally loaded (6), whereas lamotrigine requires complex titration schedules. Also, levetiracetam is 100% excreted by the kidneys, thus requiring less-frequent dose alterations during pregnancy than lamotrigine whose level continues to drop drastically during pregnancy. Moreover, in addition to its efficacy in focal epilepsy, levetiracetam is an excellent AED to use in myoclonic epilepsies, where lamotrigine may actually worsen the myoclonus.
Future studies should assess the relative safety of various AED combinations. Also, major congenital malformations may not be the only consequence of in utero exposure to AEDs, since the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study demonstrated long-term cognitive adverse events related to in utero exposure to valproic acid (1). Thus, such longer term studies will further shed light on the longer term effects of in utero exposure to AEDs. The Liverpool and Manchester Neurodevelopment group partially answered this question when they reported a safer neurodevelopment profile of levetiracetam than valproate (9). Mawhinney et al. acknowledge the limitation of lacking a control, but unfortunately most registries do not contain a control group. It would be ideal for future studies to include a control group and to document serum levels of AEDs, as well as the intake of other medications and substances of abuse.
Footnotes
Editor's Note: Authors have a Conflict of Interest disclosure which is posted under the Supplemental Materials (208.7KB, docx) link.
Abbreviation: LEV, levetiracetam.
References
- 1.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt S, Craig J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, Morrison PJ, Morrow J. Levetiracetam in pregnancy: Preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology. 2006;67:1876–1879. doi: 10.1212/01.wnl.0000244491.48937.55. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Holmes LB. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78:1692–1699. doi: 10.1212/WNL.0b013e3182574f39. [DOI] [PubMed] [Google Scholar]
- 4.Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 5.Cunnington M, Tennis P. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955–960. doi: 10.1212/01.WNL.0000154515.94346.89. [DOI] [PubMed] [Google Scholar]
- 6.Koubeissi MZ, Amina S, Pita I, Bergey GK, Werz MA. Tolerability and efficacy of oral loading of levetiracetam. Neurology. 2008;70:2166–2170. doi: 10.1212/01.wnl.0000313151.64005.c0. [DOI] [PubMed] [Google Scholar]
- 7.Montouris G, Harden C, Alekar S, Leppik I. UCB Antiepileptic Drug Pregnancy Registry— Keppra® data. Presented at American Epilepsy Society, December, 2010, San Antonio, TX: Abst. 1.257. [Google Scholar]
- 8.Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305:1996–2002. doi: 10.1001/jama.2011.624. [DOI] [PubMed] [Google Scholar]
- 9.Shallcross R, Bromley RL, Irwin B, Bonnett LJ, Morrow J, Baker GA. Child development following in utero exposure: Levetiracetam vs sodium valproate. Neurology. 2011;76:383–389. doi: 10.1212/WNL.0b013e3182088297. [DOI] [PMC free article] [PubMed] [Google Scholar]


