Abstract
In 2012, the Kaiser Permanente Area Medical Directors of Quality decided to sponsor analytic activities to improve shared decision making for patients with chronic kidney disease. The objective was to move shared decision making for renal replacement therapy or maximal conservative management upstream rather than waiting until the patient presented to the emergency room requiring acute dialysis. Nephrologists have multiple opportunities to discuss treatment options with patients throughout the course of their disease. However, despite these opportunities most patients beginning dialysis have not experienced shared decision making with their physicians. The shared-decision-making process may help patients understand the importance of being prepared to start dialysis and the benefits of maximal conservative management.
By having these discussions upstream we may be able to improve survival (save lives), slow down renal disease progression (save kidneys), preserve central veins for future vascular access (save veins), and be better stewards of finite resources needed to care for patients with end-stage kidney disease (save resources).
Introduction
In 2012, the American Society of Nephrology joined the American Board of Internal Medicine Foundation and Consumer Reports in the Choosing Wisely campaign. The purpose of this multiple-year campaign is to help physicians be better stewards of finite health care resources.1 The campaign strongly reflects a focus on high-quality and affordable care for all patients with chronic kidney disease (CKD). The campaign was designed to encourage shared decision making between patients and their physicians. Internal medicine specialists were asked to come up with five things physicians and patients should question. One of these questions from nephrologists was, “Should we initiate chronic dialysis without ensuring a shared-decision-making process between patients, their families, and their physicians?” In 2010, the Renal Physician Association published clinical practice guidelines on shared decision making for chronic kidney disease.2 They outlined three approaches to care for patients with end-stage kidney disease (ESKD) at the time of initiation of renal replacement therapy: 1) dialysis therapy without limitations on other treatments, 2) dialysis therapy without cardiopulmonary resuscitation, and 3) no dialysis therapy.
Because most nephrologists have been trained to use all therapies necessary to prolong life of patients with ESKD, they may hesitate to have an end-of-life discussion with patients who are preparing to start dialysis. Most patients will continue dialysis therapy until death, unless there is a paradigm shift regarding end-of-life care for patients with CKD. Recent surveys suggest that not all patients with ESKD want to preserve life by any means necessary.3 In addition, a study from the United Kingdom suggests dialysis may offer no survival advantage over 75 years for patients with stage 5 CKD (CKD5) and multiple comorbidities compared to CKD5 patients without multiple comorbidities.4 These studies suggest we have an opportunity to improve the process of shared decision making with CKD patients.5
Shared Decision-Making Process and Chronic Kidney Disease
Because renal function of patients with kidney disease usually declines gradually, nephrologists have multiple opportunities to discuss options for renal replacement therapy and end-of-life care. However, despite multiple visits to a nephrologist before starting dialysis therapy, less than 10% of ESKD patients reported a discussion about end-of-life care with their nephrologists in the last 12 months.3 There are at least 3 times when shared decision making with a CKD patient is critical: when the patient enters stage 4 (estimated glomerular filtration rate [eGFR] < 30 mL·min−1·1.73 m −2), when the patient is about to start renal replacement therapy (eGFR < 15 mL·min−1·1.73 m−2), or when the there is no evidence that further therapy will prolong life (eGFR < 5 mL·min−1·1.73 m −2, or age ≥ 75 years and multiple comorbidities). In addition to these 3 key times, progression to each substage of stage 4 CKD (CKD4) and CKD5 may prompt a nephrologist to discuss options for renal replacement therapy with the patient (Figure 1).
Figure 1.
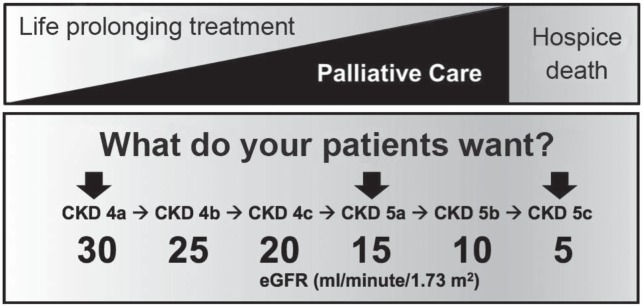
The top of the figure illustrates how the topic of life-sustaining treatment and the topic of palliative care can be integrated in discussions with the patient as the disease progresses. This approach is in contrast to management of end-stage kidney disease with dialysis until the very end of life, which leaves the patient unprepared for death and dying. The bottom of the figure relates stage of kidney disease and estimated glomerular filtration rate (eGFR). Patients enter stage 4 chronic kidney disease (CKD) when their eGFR decreases below 30 mL·min−1·1.73 m−2. Each additional decrease of approximately 5 mL·min−1·1.73 m−2 corresponds to the following substage. The arrows indicate 3 stages when shared decision making is critical.
The new paradigm suggests that chronic disease is a journey of many months or years. During this journey the nephrologist and patient are in constant communication about prognosis and treatment options.6
The Optimal Start Initiative
In 2012, the Kaiser Permanente Associate Medical Directors of Quality decided to sponsor analytic activities to improve shared decision making with CKD patients. The goal was to move shared decision making about renal replacement therapy or maximal conservative management upstream, rather than waiting until the patient presented to the emergency room requiring acute dialysis. As outlined above, nephrologists have multiple opportunities to discuss treatment options with patients throughout the course of their disease. However, despite these opportunities, most patients do not have an optimal start to renal replacement therapy.
An optimal start of dialysis means that the patient begins dialysis without a vascular catheter. Vascular catheters are associated with increased infectious complications, central vein stenosis, mortality, and greater cost compared with arteriovenous fistula.7 For this reason, CKD patients are encouraged to start renal replacement therapy with a preemptive transplant, arteriovenous fistula, peritoneal dialysis, or maximal conservative management. Despite our best efforts to reduce the number of patients who start dialysis with a vascular catheter, only 27% of ESKD patients in the US start dialysis without a vascular catheter.7 This represents a significant opportunity for improvement. To improve outcomes, we should consider intervening earlier and have upstream discussions with our patients about risks of nonoptimal start of renal replacement therapy. We should be able to decrease the risk of nonoptimal start by initiating life-plan conversations when CKD patients first present to the renal clinic.
We would also like our patients to have an optimal start on their journey toward end-of-life care. Two recent studies suggest we may not be accomplishing this effectively. In 2012, Wong et al8 reported that 76% of dialysis patients were hospitalized in their final month of life. On average, these patients were in the hospital for 9.8 days. Forty-eight percent were admitted to the intensive care unit, and 29% had an intensive procedure performed during the hospital stay. Only 20% were admitted to a hospice. Forty-four percent of these patients died in the hospital. In comparison, 39% of cancer patients and 55% of heart failure patients were admitted to a hospice. Only 35% of cancer patients and 29% of heart failure patients died in the hospital during their last month of life. Davison3 surveyed a total of 584 CKD4 and CKD5 patients as they presented to dialysis, transplantation, or predialysis clinics in Canada. Participants reported poor knowledge of palliative care options and their illness trajectory. Sixty-one percent of patients regretted their decision to start dialysis. More patients wanted to die at home (36.1%) or in a hospice (28.8%) compared with a hospital (27.4%).
The latter studies suggest that we are not having effective conversations with our patients about end-of-life care until their death is very near. Our current paradigm for care needs to be questioned. We propose a new paradigm that encourages shared decision making and development of life-care plans. In this paradigm, conversation with CKD patients would shift from planning short-term goals to charting a course to deliver what is best for patients by reducing risk and maximizing the potential for effective, proactive care. These conversations can focus on survival and action plans to improve the health of the CKD patient’s mind, body, and spirituality.
Stage 4 Chronic Kidney Disease
The optimal time to begin the “life with kidney disease” discussion is when a patient presents to the renal clinic with CKD4.9 These initial discussions should include 1) major causes of kidney disease, 2) stages of kidney disease, and 3) treatment of kidney disease. It may be appropriate to discuss 5-year survival rates for CKD4 and the risk of starting dialysis in the next 5 years. In 2004, Keith et al published a longitudinal follow-up and outcomes study of patients with CKD in a large managed-care organization.10 The study reported the percentage of patients who started renal replacement therapy or died before starting dialysis or transplantation. The risk of starting dialysis over a 5-year period was 1.1%, 1.3%, and 19.9% for CKD2, CKD3, and CKD4 patients, respectively. The study also reported that the risk of death over a 5-year period was 19.5%, 24.3%, and 45.7% for CKD2, CKD3, and CKD4 patients, respectively. This information may help patients with CKD understand their prognosis. These data can be integrated into their life plan to help them develop a strategy with their nephrologist that will decrease their risk of dialysis and death.11 Nephrologists can initiate these discussions, and educational shared-decision-making classes can reinforce them.
End-Stage Kidney Disease
Shared decision making should occur between patients and their families and physicians before chronic dialysis begins. The discussions should focus on 4 goals: 1) save lives, 2) save kidneys, 3) save veins, and 4) save resources (Table 1). The US Renal Data System 2012 reported that there were no significant differences between 5-year survival rates for patients treated with peritoneal dialysis and those treated with hemodialysis.7 Transplantation, however, has a significant survival benefit compared with dialysis and should be the preferred form of renal replacement therapy. The main problem with renal transplantation is the risk of death while waiting for a renal transplant. Data from the US Renal Data System 2012 showed that there are currently about 86,000 ESKD patients on the waiting list, and we only perform about 17,000 renal transplantations per year in the US. The median time patients spend on a waiting list for a renal transplant is 2.6 years. The proportion of patients who die after 1, 3, and 5 years waiting for a renal transplant is 1.7%, 9.6%, and 20.3%, respectively.
Table 1.
Shared decision making during chronic kidney disease
| Stage | eGFR | Plan |
|---|---|---|
| 4 | < 30 | Shared decision making |
| 4a | 26–30 | Reduce risk of cardiovascular disease |
| Reduce risk of kidney disease progression | ||
| 4b | 21–25 | Upstream optimal start initiative |
| 4c | 16–20 | Preemptive renal transplantation |
| 5 | < 15 | Optimal start |
| 5a | 10–15 | Peritoneal dialysis bridge therapy |
| 5b | 5–9 | Home dialysis bridge therapy |
| 5c | < 5 | In-center hemodialysis |
eGFR = estimated glomerular filtration rate, mL·min−1·1.73 m−2
The next important goal of ESKD management is to preserve existing renal function.12 Wang and Lai reviewed the importance of saving kidney function during dialysis.13 Preserving residual renal function has always been the primary clinical goal of nephrology, and there is no reason why this goal should not extend to patients on dialysis. Since we do not routinely report survival outcomes of patients on dialysis by eGFR or CKD5 sub-stage, there is no clear evidence that preserving residual renal function remains important after dialysis therapy commences. However, residual renal function contributes significantly to the overall health of dialysis patients. A patient with an eGFR of 10 mL·min−1·1.73 m −2 is better off than a patient with an eGFR of 5 mL·min−1·1.73 m −2 in terms of maintaining fluid balance, phosphorus control, removal of uremic toxins, and prevention of vascular calcifications. In addition, a decline in residual renal function may contribute significantly to anemia and malnutrition in patients on dialysis. We can prevent loss of residual renal function with many of the treatments we use to prevent loss of residual function during CKD4. These include angiotensin-converting enzyme inhibitors and avoidance of acute kidney injury caused by dehydration, high doses of antibiotics, non-steroidal anti-inflammatory agents, or contrast dye.
The third goal for patients starting dialysis is to save their veins. Central veins are the lifeline for patients on hemodialysis. Creation of a successful arteriovenous fistula for dialysis depends on the central veins being healthy. Use of central vein catheters may increase the risk of central vein stenosis caused by scarring and infection. Once the central vein is stenosed, creating a functioning arteriovenous fistula will be more difficult. Vascular catheters should be avoided for dialysis. A good way to save the veins is to start with peritoneal dialysis instead of hemodialysis with a vascular catheter.14 The benefits of peritoneal dialysis have been outlined by Chaudhary15 and include similar survival rate, lower cost, and improved quality of life compared with hemodialysis.
The final goal is to save resources. In 2012, we spent almost $30 billion treating ESKD. This is almost 8% of total Medicare spending.7 Medicare spending per patient per year by type of renal replacement therapy is $32,914 for renal transplantation, $66,751 for peritoneal dialysis, and $87,561 for hemodialysis. About a third of Medicare ESKD costs is for inpatient treatment, a third is for dialysis therapy, and a third is for outpatient treatment. These data indicate an opportunity to reduce costs by treating more patients with peritoneal dialysis and transplantation. In addition, readmission rates for dialysis patients significantly exceed those for Medicare patients without ESKD.7 Reducing avoidable readmissions should be associated with better proactive management of CKD.
Maximum Conservative Management
The final critical time to have a shared-decision-making discussion with a patient is when there may be no survival benefit from renal replacement therapy. Murtagh et al16 performed a retrospective analysis of the survival of 129 CKD5 patients older than 75 years; the patients attended a dedicated multidisciplinary predialysis clinic. The dialysis group had 1- and 2-year survival rates of 84% and 76%, respectively, compared with 68% and 47% for the conservative management (no dialysis therapy) group. However, this survival advantage was lost in those patients with high comorbidity scores, especially when the comorbidity included ischemic heart disease. The authors conclude that CKD5 patients older than 75 years who receive specialist nephrology care and follow a planned management pathway have a substantially reduced survival advantage on dialysis if they have multiple comorbidities. These data suggest that comorbidity should be a major consideration when advising elderly patients for or against dialysis. Dialysis is prescribed for many patients who may not have a survival advantage attributable to dialysis. This is a good example of a situation where shared decision making may help inform a patient of the risks and benefits of dialysis. In patients older than 75 years with multiple comorbidities, the risk of dialysis may outweigh the benefits. Why should patients undergo surgery for arteriovenous fistula and go to therapy for up to 12 hours per week if there is no good evidence that this type of therapy will increase survival?
Maximal conservative management programs are developing around the country to help care for patients who choose no dialysis therapy. These programs are in their infancy but are projected to increase in size over time and may care for an estimated 10% to 20% of the ESKD population.17 Models of care are still developing, but they may follow 3 patterns: renal palliative care programs run by nephrology teams trained in palliative care; palliative care programs run by palliative care physicians trained in CKD5 care; or a combined program where patients are seen by both a nephrology team and a palliative care team. These teams will work together to provide individualized, patient-focused care. They will have discussions that focus on listening to and understanding the patient while providing prognostic information. Important to these discussions will be the balancing of expectations with anticipated complications that are associated with disease progression. Synergy between the nephrology team and the palliative care team will provide the expertise needed to achieve these goals.
… patients [with chronic kidney disease stage 5] who choose conservative therapy will have extensive health care needs, including control of symptoms, measures to retard disease progression, and management of complications of renal disease.
Conclusion
Shared decision making is critical to the long-term outcome of the patient with kidney disease. New information provided by the leadership of the American Board of Internal Medicine and the American Society of Nephrology has laid the groundwork for the Choosing Wisely initiative. This initiative encourages nephrologists to have shared-decision-making discussions with their patients with kidney disease. If the physician and patient work together as a team, they maximize the patient’s ability to develop an effective life plan that improves survival and prepares the patient for end-of-life care (Table 1).
Increasing numbers of cases of CKD5 may be managed without dialysis. Dialysis may offer no survival advantage to high-risk, older patients with CKD5 and multiple comorbidities.16 CKD5 patients who choose conservative therapy will have extensive health care needs, including control of symptoms, measures to retard disease progression, and management of complications of renal disease. Meeting the palliative care needs of CKD5 patients who choose conservative therapy will require both that nephrologists learn about palliative care and that palliative care physicians learn about nephrology.
A collaborative approach that includes the patient and physician can improve services for all patients with CKD, although models of care implementing an upstream shared-decision-making process need further evaluation and development. These therapy pathways are being developed to help nephrologists develop effective service delivery programs for all CKD patients. Future efforts in nephrology should implement a broad, patient-centered, upstream shared-decision-making process focused on saving lives, saving kidneys, saving veins, and efficient use of resources needed to care for all patients with kidney disease. Our overall goal should be to treat the patient, not just the disease.
Acknowledgments
Leslie Parker, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Kidney Philosophy
Superficially, it might be said that the function of the kidneys is to make urine; but in a more considered view one can say that the kidneys make the stuff of philosophy itself.
— Homer W Smith, 1895–1962, American physiologist and science advocate
References
- 1.Choosing wisely [Web site on the Internet] Philadelphia, PA: ABIM Foundation; c2013 [cited 2012 Nov 26]. Available from: www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx. [Google Scholar]
- 2.Recommendations summary [monograph on the Internet] 2nd ed. Rockville, MD: Renal Physicians Association; 2010. Oct, Shared decision-making in the appropriate initiation of and withdrawal from dialysis: clinical practice guideline. [cited 2012 Nov 26]. Available from: www.renalmd.org/catalogue-item.aspx?id=682. [Google Scholar]
- 3.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010 Feb;5(2):195–204. doi: 10.2215/CJN.05960809. DOI: http://dx.doi.org/10.2215/CJN.05960809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtagh FR, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FR. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007 Jul;22(7):1955–62. doi: 10.1093/ndt/gfm153. DOI: http://dx.doi.org/10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 5.Williams AW, Dwyer AC, Eddy AA, et al. American Society of Nephrology Quality, and Patient Safety Task Force Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012 Oct;7(10):1664–72. doi: 10.2215/CJN.04970512. DOI: http://dx.doi.org/10.2215/CJN.04970512. [DOI] [PubMed] [Google Scholar]
- 6.Burns A, Davenport A. Maximum conservative management for patients with chronic kidney disease stage 5. Hemodial Int. 2010 Oct;14(Suppl 1):S32–7. doi: 10.1111/j.1542-4758.2010.00488.x. DOI: http://dx.doi.org/10.1111/j.1542-4758.2010.00488.x. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System [homepage on the Internet] Minneapolis, MN: USRDS Coordinating Center; [cited 2012 Nov 26]. Available from: www.usrds.org. [Google Scholar]
- 8.Wong SP, Kreuter W, O’Hare AM. Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med. 2012 Apr 23;172(8):661–4. doi: 10.1001/archinternmed.2012.268. DOI: http://dx.doi.org/10.1001/archinternmed.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abboud H, Henrich WL. Clinical practice. Stage IV chronic kidney disease. N Engl J Med. 2010 Jan 7;362(1):56–65. doi: 10.1056/NEJMcp0906797. DOI: http://dx.doi.org/10.1056/NEJMcp0906797. [DOI] [PubMed] [Google Scholar]
- 10.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004 Mar 22;164(6):659–63. doi: 10.1001/archinte.164.6.659. DOI: http://dx.doi.org/10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 11.Obrador GT, Arora P, Kausz AT, Pereira BJ. Pre-end-stage renal disease care in the United States: a state of disrepair. J Am Soc Nephrol. 1998 Dec;9(12 Suppl):S44–54. [PubMed] [Google Scholar]
- 12.Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009 Jun;53(6):1068–81. doi: 10.1053/j.ajkd.2009.02.012. DOI: http://dx.doi.org/10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006 May;69(10):1726–32. doi: 10.1038/sj.ki.5000382. DOI: http://dx.doi.org/10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- 14.Ghaffari A. Urgent-start peritoneal dialysis: a quality improvement report. Am J Kidney Dis. 2012 Mar;59(3):400–8. doi: 10.1053/j.ajkd.2011.08.034. DOI: http://dx.doi.org/10.1053/j.ajkd.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol. 2011 Feb;6(2):447–56. doi: 10.2215/CJN.07920910. DOI: http://dx.doi.org/10.2215/CJN.07920910. [DOI] [PubMed] [Google Scholar]
- 16.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin N, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007 Jul;22(7):1955–62. doi: 10.1093/ndt/gfm153. DOI: http://dx.doi.org/10.1093/ndt/gfm153. [DOI] [PubMed] [Google Scholar]
- 17.Swidler MA. Geriatric renal palliative care. J Gerontol A Biol Sci Med Sci. 2012 Dec;67(12):1400–9. doi: 10.1093/gerona/gls202. DOI: http://dx.doi.org/10.1093/gerona/gls202. [DOI] [PubMed] [Google Scholar]


