Abstract
Objectives:
To evaluate the effect of intensive care unit continuous EEG (cEEG) monitoring on inpatient mortality, hospital charges, and length of stay.
Methods:
A retrospective cross-sectional study was conducted using the Nationwide Inpatient Sample, a dataset representing 20% of inpatient discharges in nonfederal US hospitals. Adult discharge records reporting mechanical ventilation and EEG (routine EEG or cEEG) were included. cEEG was compared with routine EEG alone in association with the primary outcome of in-hospital mortality and secondary outcomes of total hospital charges and length of stay. Demographics, hospital characteristics, and medical comorbidity were used for multivariate adjustments of the primary and secondary outcomes.
Results:
A total of 40,945 patient discharges in the weighted sample met inclusion criteria, of which 5,949 had reported cEEG. Mechanically ventilated patients receiving cEEG were younger than routine EEG patients (56 vs 61 years; p < 0.001). There was no difference in the 2 groups in income or medical comorbidities. cEEG was significantly associated with lower in-hospital mortality in both univariate (odds ratio = 0.54, 95% confidence interval 0.45–0.64; p < 0.001) and multivariate (odds ratio = 0.63, 95% confidence interval 0.51–0.76; p < 0.001) analyses. There was no significant difference in costs or length of stay for patients who received cEEG relative to those receiving only routine EEG. Sensitivity analysis showed that adjusting for diagnosis-related groups (DRGs) for any neurologic diagnoses, DRGs for neurologic procedures, and specific DRGs for epilepsy/convulsions did not substantially alter the association of cEEG with reduced inpatient mortality.
Conclusions:
cEEG is favorably associated with inpatient survival in mechanically ventilated patients, without adding significant charges to the hospital stay.
Continuous EEG (cEEG) is increasingly utilized in critically ill patients with abnormal neurologic function. cEEG can detect convulsive and nonconvulsive seizures, brain ischemia, and other disturbances as they occur, prompting adjustment of anticonvulsants1,2 or interventions to reverse focal ischemia.3,4 For seizures, only cEEG can provide this diagnostic information; for detection of focal ischemia, cEEG may be more sensitive than imaging5 and gives uninterrupted bedside appraisal. Encephalopathic patients may benefit from cEEG6 even in the absence of known acute brain injury.7
The evidence for cEEG has focused on rates of seizure detection in specific patient populations,8 and the significance of particular EEG patterns.9–11 Meaningful improvement in patient outcomes has yet to be demonstrated.12,13
The literature regarding costs, charges, or cost-effectiveness of cEEG is more limited. A single-center study showed that cEEG was responsible for only 1% of total hospital charges for neuro–intensive care unit (ICU) patients, and found that both length of stay and total charges markedly declined from the period before incorporation of cEEG into ICU practice.14
Data from real-world administrative claims and reported public datasets, although limited in clinical information, can help determine impact and outcomes of existing technologies using large sample sizes.15–18 Therefore, we used a cross-sectional study design from a large public inpatient dataset to test the hypothesis that outcomes from ICU stays reporting cEEG monitoring are different from those with routine EEG alone.
METHODS
Data source.
We examined standardized hospital-coded discharge data from the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, maintained by the Agency for Healthcare Research and Quality, to examine national health care usage and quality trends. The NIS is the largest all-payer dataset of inpatient hospitalizations in the United States, comprising a 20% stratified sample of nonfederal community hospitals. For the year 2009, more than 8 million discharges from 1,050 hospitals in 44 states were reported.19 A 5-year span beginning in 2005 was chosen based on the authors' personal experience of increased utilization of the technology within that period, and data availability.
Standard protocol approvals, registrations, and patient consents.
The Human Subjects Division of the Institutional Review Board of the University of Washington has determined that the NIS is a dataset that does not require associated research projects to be reviewed for exemption or approval.20 The data contained in the NIS are neither identifiable nor private and do not meet the federal definition of “human subjects research.”
Inclusion criteria.
Adults (aged 18 years or older) identified with continuous mechanical ventilation (ICD-9-CM codes 967, 9670, 9671, 9672) and either routine EEG (ICD-9-CM code 8914) or cEEG (ICD-9-CM code 8919) reported in inpatient discharge records from January 1, 2005 to December 31, 2009 were included.21
Patient and hospital characteristics.
Patient age, sex, race, comorbidities, year of discharge, diagnosis-related group (DRG), epilepsy/convulsion diagnosis, hospital discharge volume, hospital teaching status, and urban vs rural hospital status were abstracted as independent variables from the NIS. A comorbidity score was calculated using the Elixhauser-Coffey method,22 with 5 dummy categories (0, 1, 2, 3, >3) created from the score, an ordinal grouping reflected in prior analyses of inpatient data.23 DRG was dichotomized into those DRGs associated with CNS disorders or injury (including seizures), and those without. Diagnosis of epilepsy or convulsions was reported using the Agency for Healthcare Research and Quality Clinical Classification Software ICD-9-CM grouping.24 Those EEG studies reportedly performed on the day of discharge (including day of death) were identified to allow for separate analysis of this group, for whom EEG performance may have been for supportive evidence of brain death. Discharge years were treated as dummy variables. Hospital discharge volume was included as a continuous measure while other hospital-specific variables were categorical terms.
Outcomes of interest and model specification.
In-hospital mortality was the primary outcome. Length of stay (in days) and hospitalization charges (in yearly inflation-adjusted 2009 US dollars) were secondary outcomes. For inferential analysis, discharge records, which reported routine EEG but not cEEG in mechanically ventilated patients, were the reference. Covariates were determined by Anderson-Newman criteria of enabling, predisposing, and need factors of health care utilization.25 The neurologic DRG and epilepsy/convulsions dummy variables were not used in the main analysis because the NIS does not identify whether conditions existed before admission or arose during hospitalization, making it unclear whether these variables were important in selecting the intervention, or occurred as a downstream effect. The category indicating studies performed on the day of discharge was suspected a priori to be highly correlated with in-hospital death. These categories excluded from the main analysis were analyzed in separate sensitivity analyses (see below).
Statistical analyses.
All analyses used NIS complex sample design elements (probability weights, stratification, and clustering) for national-level estimates unless otherwise stated. Descriptive analysis of reported variables (totals, proportions, or means) was performed, with differences between exposure groups (cEEG and routine EEG only) evaluated through 2-sample t test, as the large sample sizes in each group permitted use of the t distribution.26 Univariate and multivariate regression analyses were performed for association of cEEG with the primary and secondary outcomes, comparing with observations reporting only routine EEG as a reference. Logistic regression models were used for the dichotomous outcome of in-hospital death. Multivariate regressions included patient demographic, comorbidity, and hospital variables. In-hospital death was an additional covariate in multivariate analysis of length of stay and hospital charges. Hospital charges were inflated to 2009 dollar levels using the medical consumer price index,27 then evaluated using a generalized linear model with log link for best fit. Length of stay was evaluated with ordinary least-squares analysis, reporting difference in days. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported for the results of the logistic regressions, while the attributable charges were reported as percentage of overall charges. Missing data were analyzed for bias and excluded if randomly distributed between exposures and outcomes. Significance was set at p ≤ 0.05 for all analyses. All statistical testing was performed using STATA 12.1 (Stata Corp, College Park, TX).
Sensitivity analyses.
The effects of subgroups for 1) epilepsy/convulsions diagnosis, 2) neurologic DRG, and 3) routine EEG or cEEG performed on the day of discharge were analyzed separately. Univariate logistic regressions were performed for association of each subgroup with cEEG and independently for the outcome of in-hospital mortality. Each subgroup was then added as a covariate to multivariate regressions for cEEG with the outcome of death to determine the effect of adjustment by subgroup. Lastly, the multivariate regression was rerun, excluding the subgroup observations.
As a further sensitivity analysis, we ran the multivariate logistic regression on the primary outcome using interaction terms for 1) cEEG with teaching hospitals, and 2) cEEG with hospital volume (dichotomizing hospital volume to high or low relative to the mean) to ascertain whether either of these hospital characteristics was responsible for differences in survival over routine EEG.
RESULTS
Study characteristics and descriptive analysis.
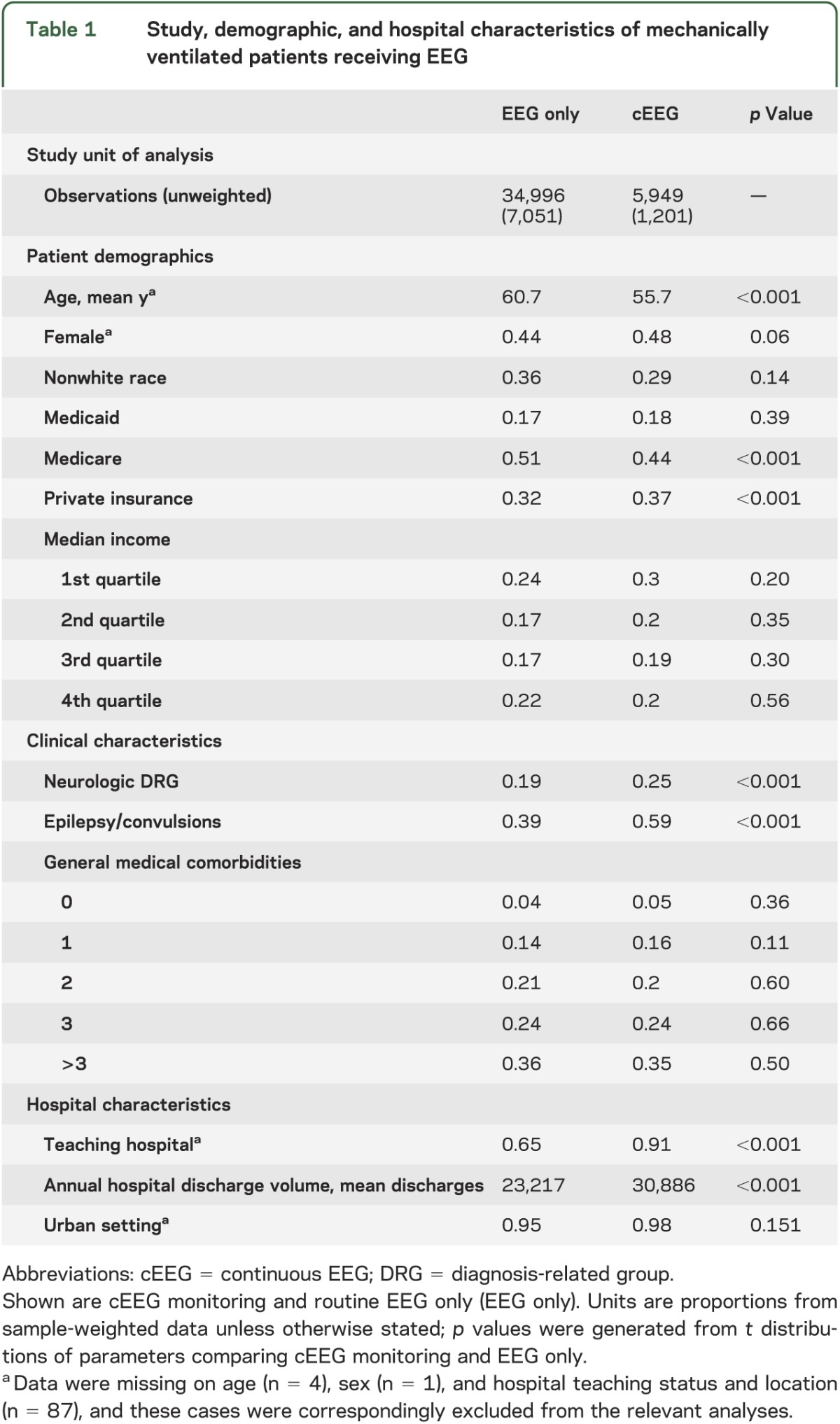
A total of 40,945 inpatient discharges (8,252 in unweighted sample) reporting ICD-9 codes for mechanical ventilation and either type of EEG were included, grouped into cEEG and routine EEG categories (figure). Of these, 5,949 (1,201 unweighted) were coded for cEEG monitoring. Those receiving cEEG were younger (56 vs 61 years; p < 0.001) and more likely to have private insurance and less likely Medicare. There was no significant difference in the distribution of income by quartile. cEEG patients were more likely to have a DRG indicating a primarily neurologic procedure or CNS problem (25% vs 19%; p < 0.001), and an epilepsy/convulsions diagnosis as part of the discharge record (59% vs 39%; p < 0.001). There was no difference in distribution among general medical comorbidity severity levels (0, 1, 2, 3, >3) between the 2 groups. Hospitals reporting cEEG in ventilated persons had a larger volume of total annual inpatient discharges, and were more likely to be teaching hospitals. See table 1 for details.
Figure. Study participants flow diagram.
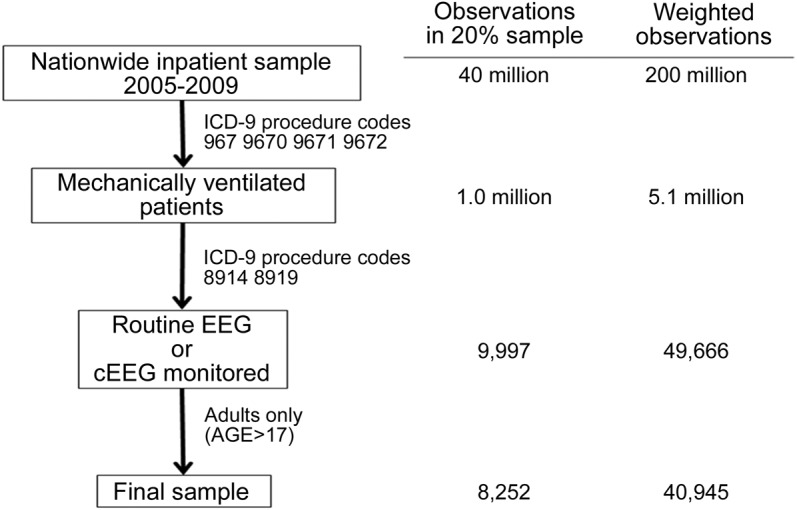
Nationwide Inpatient Sample search strategy for adult, mechanically ventilated patients receiving either continuous EEG (cEEG) monitoring or routine EEG.
Table 1.
Study, demographic, and hospital characteristics of mechanically ventilated patients receiving EEG
Utilization totals by year.
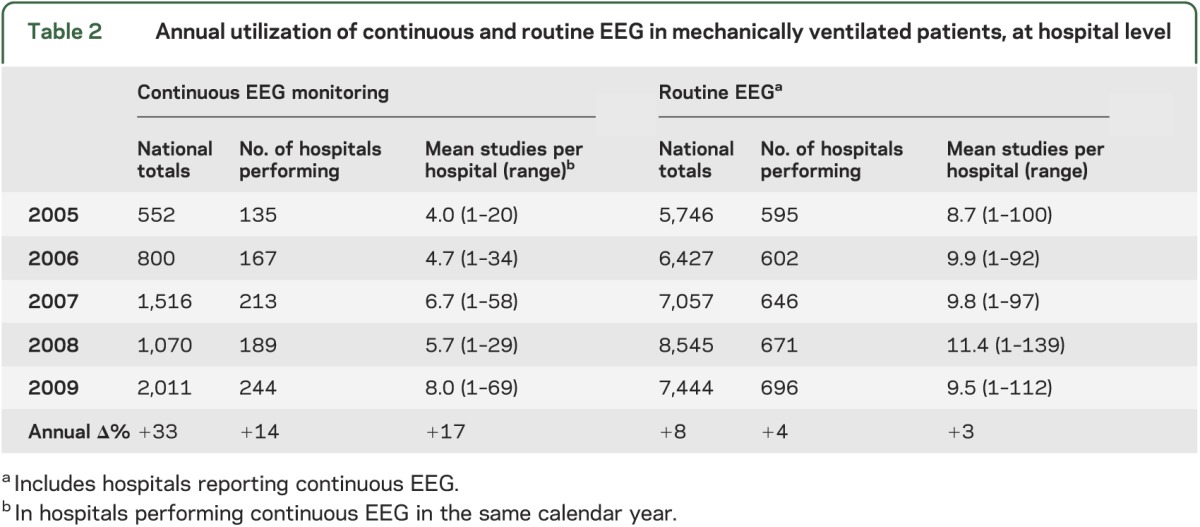
Totals of mechanically ventilated patients receiving cEEG increased dramatically in the study period (table 2), by 263% over 4 years, a mean of 33% annually. In contrast, routine EEG use in these patients grew an average of 8% per year. By hospital, the mean annual number of ventilated patients with cEEGs (in hospitals reporting cEEG use) doubled from 4 to 8, while those receiving routine EEGs showed little growth. Hospitals reporting cEEG in ventilated patients nearly doubled from 135 to 244. The top 20 hospitals in the unweighted sample (14%) provided 54% of the cEEGs in the study.
Table 2.
Annual utilization of continuous and routine EEG in mechanically ventilated patients, at hospital level
Primary outcome.
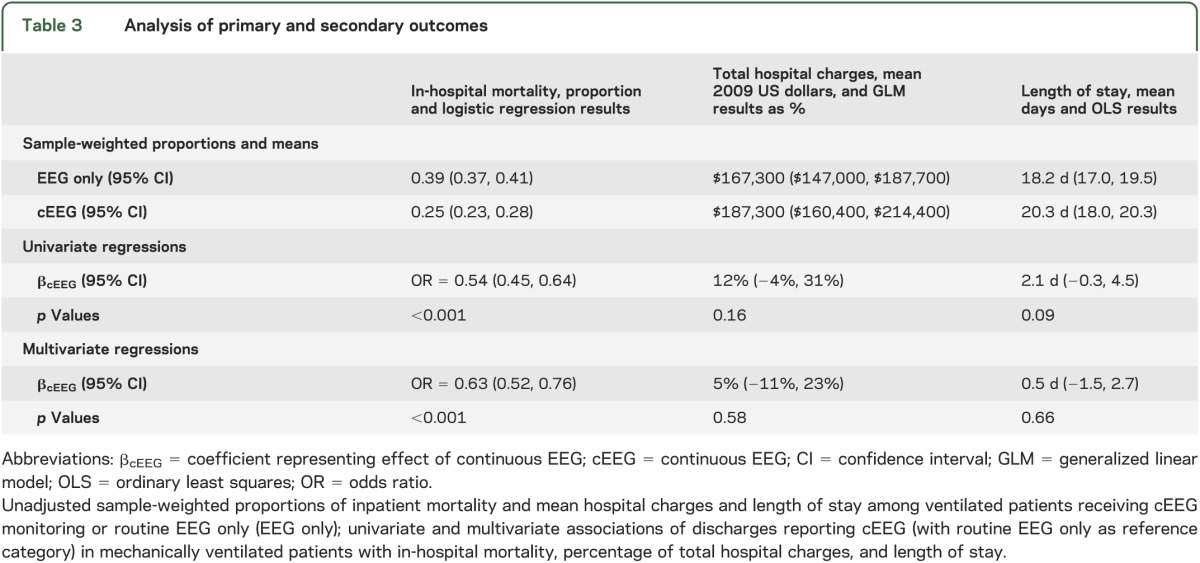
The unadjusted in-hospital mortality for ventilated patients who received cEEG was 25%, vs 39% for those receiving routine EEG only, for an OR of 0.54 (95% CI 0.45–0.64; p < 0.001) (table 3). In the multivariate regression model, including patient demographic and hospital characteristics, as well as comorbidity and year categories, the adjusted OR comparing in-hospital mortality for cEEG vs routine EEG alone was 0.63 (95% CI 0.52–0.76; p < 0.001). Age was highly associated with mortality in the multivariate analysis (OR = 1.03 per year over mean age, 95% CI 1.02–1.03; p < 0.001; see table e-1 on the Neurology® Web site at www.neurology.org).
Table 3.
Analysis of primary and secondary outcomes
Secondary outcomes.
cEEG accounted for 12% (95% CI −4%, 31%; p = 0.16) of the total hospital charges in univariate analysis, but only 5% (95% CI −11%, 23%; p = 0.58) of total charges after multivariate adjustment (table 3). Length of stay was 2.1 days longer (95% CI −0.3, 4.5 days; p = 0.09) in cEEG patients than EEG patients, but was only 0.5 days longer (95% CI −1.5, 2.7 days; p = 0.66) after multivariate regression. Higher income quartiles had longer stays and greater hospital charges in multivariate analysis, as did persons of nonwhite race, while accounting for the effect of in-hospital death (tables e-2 and e-3).
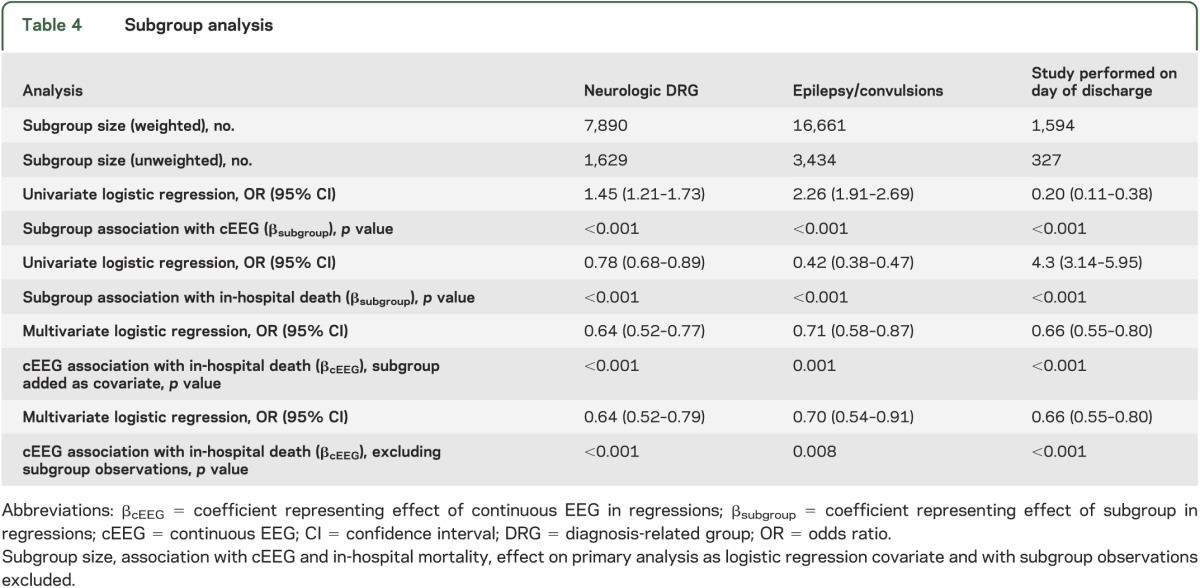
Subgroup analyses.
Neurologic DRG and epilepsy/convulsions diagnoses were more common in the cEEG group, and associated with survival (p < 0.001 for all analyses). Approximately 5% of observations reported studies performed on the day of discharge, which were highly associated with in-hospital mortality (OR = 4.3, 95% CI 3.1–6.0; p < 0.001) and not cEEG (OR = 0.2, 95% CI 0.1–0.4; p < 0.001). The effect of including these subgroups as covariates or excluding their observations in the primary outcome multivariate logistic regression equation did not substantially alter the association of cEEG with in-hospital mortality or its significance (see table 4). The effects of interaction of hospital characteristics (academic status and high discharge volume) with cEEG on in-hospital mortality were not significant (OR = 1.36, 95% CI 0.90–2.05; p = 0.15 and OR = 1.23, 95% CI 0.78–1.92; p = 0.92, for academic status and high hospital discharge volume, respectively).
Table 4.
Subgroup analysis
DISCUSSION
In this analysis of ICU outcomes in a large, observational dataset, cEEG is associated with a substantial and robust improvement in in-hospital survival compared with routine EEG alone. The effect does not lessen accounting for patient age, burden of comorbidities, hospital discharge volume, and academic status, and holds for patients with nonneurologic DRGs. While adjusted hospital charges and length of stay were greater for those with cEEG monitoring, these were not significantly more than patients receiving routine EEG alone.
ICU cEEG monitoring is performed largely in urban academic centers, but total annual use has increased dramatically through growth in the number of performing hospitals and per-hospital utilization. At the same time, per-hospital utilization of routine EEGs in the ICU was mostly flat. The NIS consists of only one-fifth of the hospitals in the United States in any given year, so numbers reported may underestimate the total number of cEEGs if particular hospitals with very high utilization were not included in the sample.
Prior studies have emphasized the inaccuracy of routine EEG in seizure detection in critically ill patients and the influence of cEEG on daily ICU management as a proxy for outcomes. In a study of 169 comatose patients in the ICU,28 a standard 20-minute EEG had both low seizure detection rate and no correlation of findings with outcomes. cEEG monitoring over 24 hours is more likely to detect episodic or intermittent brain dysfunction including seizures than a limited selection of 20 to 40 minutes of EEG, facilitating more responsive clinical decision-making. Seven months after discharge from the ICU, daily management using cEEG was cited as contributing to the excellent outcome of 15 of 84 patients hospitalized for status epilepticus or severe encephalopathy.29 In 2 large series of monitored ICU patients,1,14 cEEG was reported to guide changes in antiepileptic medications (initiation, dose titration, or switch), as well as other daily management decisions, in a majority of patients.
Our study extends these results by considering an important clinical outcome, in-hospital mortality. In the light of prior literature, cEEG more accurately and rapidly diagnoses evolving pathologic brain activity, permitting better and more responsive management of brain dysfunction, and thereby improves mortality. In our analysis, patient age was an independent predictor of in-hospital death in multivariate logistic regression, but cEEG reduction in mortality remained significant after adjustment (details in table e-1). Hospital academic status and high hospital discharge volume were not associated with survival.
In subgroup analysis, although patients with neurologic DRGs were more likely to receive cEEG, the benefit to in-hospital survival was preserved in patients for whom the primary discharge diagnosis was a condition other than neurologic dysfunction. Likewise, an epilepsy/convulsions diagnosis was associated with both less in-hospital death and cEEG, but significant mortality benefit was maintained when excluding these patients from the analysis. This suggests possible utility of cEEG for mechanically ventilated patients outside the neuro-ICU, even when seizures may not be a major concern.
The commitment of equipment, time, and personnel required for cEEG in the ICU is not insubstantial. Infrastructure for machines, high-speed data transfer, and multimodality simultaneous review requires considerable capital expenditure. cEEG monitoring is labor-intensive, with trained technologists and physicians reviewing large volumes of traces, potentially throughout the 24-hour cycle. The increased costs for cEEG are acknowledged in part by Medicare Relative Value Unit (RVU) reimbursement of the technical component of EEG Current Procedural Terminology (CPT)-4 coding.30 For 2013, the technical component for “EEG for coma or sleep only” (CPT 95822) is reimbursed for 10.9 RVUs, but “EEG monitoring with technologist or nurse in attendance, each 24 hours” (CPT 95956) receives 45.67 RVUs. For our analysis, the mean unadjusted difference in charges between the 2 groups was substantial, but adjusting for in-hospital mortality (which lowers charges by necessarily decreasing hospital stay length), patient demographics, and clinical and hospital characteristics, resulted in a residual adjusted increase of 5% for the cEEG group, which was not significant.
The limitations of this study are largely a function of retrospective analysis of the NIS, a dataset derived from inpatient coding records. We are dependent on accurate coding for EEG, and we assume that coding for mechanical ventilation is a proxy for intensive care. Although cEEG is recorded in real-time, patient discharge records reporting cEEG do not indicate whether cEEG was read and interpreted in real-time as true neuro-telemetry, or hours later. Therefore, the beneficial effect of cEEG observed in this analysis may be an underestimate of the potential effect from real-time brain monitoring.31 We can state based on our results that there is strong association between decreased in-hospital mortality and cEEG use relative to routine EEG alone, but the retrospective, cross-sectional nature of the data does not permit inferences of causation.
Additional caution should be exercised insofar as our comparator was the use of routine EEG, indicating that at some point during management of these patients, determination of electrocerebral activity was deemed important. The presence of an epilepsy/convulsions diagnosis in both groups is higher than in some series that examined de novo seizure activity in the ICU,8,32 suggesting that monitoring was performed to assess function in ICU patients with known seizure disorders. Selection of cEEG over EEG alone in the ICU may indicate a higher pretest likelihood of patient survival, as reflected in the younger mean age of persons receiving cEEG monitoring. However, adjustment of outcomes for age did not alter the direction or statistical significance of the survival benefit for cEEG. Other methods such as propensity score matching33 may reduce this bias, but also may limit the sample size and statistical power of the analysis through exclusion of cases. The robustness of our findings to multivariate analysis suggests that selection bias alone is not responsible for the results of the primary analysis. Also, comparison of in-hospital survival rates of cEEG recipients to ICU mortality in patients who did not receive any EEG at all is beyond the scope of this study. The general ICU population is enormous relative to the number of ICU patients receiving cEEG (approximately 1,000:1 as depicted in the figure), and multivariate adjustment alone would likely be insufficient to control for the bias in selection of cEEG. Lastly, our analysis is limited to adults and cannot be generalized to the pediatric and/or neonatal ICU population, and because the NIS does not follow patients longitudinally, the effect of cEEG monitoring after hospital discharge cannot be tracked in these data.
Limitations aside, our analysis has important implications for clinical care and health care policy. These results provide further support that cEEG should be considered for intensive care patients for whom EEG is indicated. Our results should assist neurologists in advocating to insurers and policymakers that cEEG is a potentially valuable procedure in mechanically ventilated patients warranting reimbursement policies that preserve its use while the technology and its applications continue to evolve and are critically scrutinized. If trends continue, the proportion of hospitals offering cEEG will continue to rise, creating increased demand for trained electroencephalographers and technologists; if, however, recent Centers for Medicare & Medicaid Services’ payment changes enacted for other neurophysiologic tests are applied to cEEG, a potentially consequential tool for improving ICU survival may be threatened.
Our study demonstrates that in a large, nationally representative dataset of inpatient discharges between 2005 and 2009, cEEG monitoring in mechanically ventilated patients was utilized at prodigiously growing rates, and was significantly associated with in-hospital survival relative to routine EEG, without a statistically significant effect on hospital charges. These outcomes are consistent with earlier analyses and, if confirmed in well-designed, prospective clinical studies, would indicate that the use of this emerging technology should be encouraged by health care policymakers.
Supplementary Material
ACKNOWLEDGMENT
Dr. Ney had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
GLOSSARY
- CD-9-CM
International Classification of Diseases, ninth revision, Clinical Modification
- cEEG
continuous EEG
- CI
confidence interval
- CPT
Current Procedural Terminology
- DRG
diagnosis-related group
- ICD-9-CM
International Classification of Diseases, Ninth revision, Clinical Modification
- ICU
intensive care unit
- NIS
Nationwide Inpatient Sample
- OR
odds ratio
- RVU
Relative Value Unit
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Ney: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. van der Goes: acquisition of data, analysis and interpretation, analysis and interpretation, analysis and interpretation. Dr. Nuwer and Dr. Nelson: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Dr. Eccher: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J. Ney is the medical director for Surgical Neuromonitoring Associates and Surgical Neuromonitoring, PLLC. D. van der Goes is the recipient of a grant from the AAN for establishing the value of neurology. M. Nuwer is the medical director for Sleep Med. L. Nelson and M. Eccher report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol 2009;66:723–728 [DOI] [PubMed] [Google Scholar]
- 2.Nuwer MR. Electroencephalograms and evoked potentials: monitoring cerebral function in the neurosurgical intensive care unit. Neurosurg Clin N Am 1994;5:647–659 [PubMed] [Google Scholar]
- 3.de Vos CC, van Maarseveen SM, Brouwers PJ, van Putten MJ. Continuous EEG monitoring during thrombolysis in acute hemispheric stroke patients using the brain symmetry index. J Clin Neurophysiol 2008;25:77–82 [DOI] [PubMed] [Google Scholar]
- 4.Vespa PM, Nuwer MR, Juhasz C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol 1997;103:607–615 [DOI] [PubMed] [Google Scholar]
- 5.Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol 2004;21:341–352 [PubMed] [Google Scholar]
- 6.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med 2009;37:2051–2056 [DOI] [PubMed] [Google Scholar]
- 7.Kamel H, Betjemann JP, Navi BB, et al. Diagnostic yield of electroencephalography in the medical and surgical intensive care unit. Neurocrit Care Epub 2012 Jul 21 [DOI] [PubMed]
- 8.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748 [DOI] [PubMed] [Google Scholar]
- 9.Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology 2013;80:339–344 [DOI] [PubMed] [Google Scholar]
- 10.Hirsch LJ, Laroche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27 [DOI] [PubMed] [Google Scholar]
- 11.Alvarez V, Oddo M, Rossetti AO. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin Neurophysiol 2013;124:204–208 [DOI] [PubMed] [Google Scholar]
- 12.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg 2009;109:506–523 [DOI] [PubMed] [Google Scholar]
- 13.Rossetti AO, Oddo M. The neuro-ICU patient and electroencephalography paroxysms: if and when to treat. Curr Opin Crit Care 2010;16:105–109 [DOI] [PubMed] [Google Scholar]
- 14.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol 1999;16:1–13 [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Andrews RM. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg 2010;145:1201–1208 [DOI] [PubMed] [Google Scholar]
- 16.AbuSalah AM, Melton GB, Adam TJ. Patient-specific surgical outcomes assessment using population-based data analysis for risk model development. AMIA Annu Symp Proc 2012;2012:1089–1098 [PMC free article] [PubMed] [Google Scholar]
- 17.Shivaraju A, Patel V, Fonarow GC, Xie H, Shroff AR, Vidovich MI. Temporal trends in gastrointestinal bleeding associated with percutaneous coronary intervention: analysis of the 1998–2006 Nationwide Inpatient Sample (NIS) database. Am Heart J 2011;162:1062.e5–1068.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walcott BP, Kuklina EV, Nahed BV, et al. Craniectomy for malignant cerebral infarction: prevalence and outcomes in US hospitals. PLoS One 2011;6:e29193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project, 2005–2009. Rockville, MD: Agency for Healthcare Research and Quality; Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed August 10, 2013 [PubMed] [Google Scholar]
- 20.SOP Public Data Sets, version 2.0 [online]. Human Subjects Division, Institutional Review Board, University of Washington; Available at: http://www.washington.edu/research/hsd/docs/1125. Accessed February 10, 2013 [Google Scholar]
- 21.Classification of Diseases, Functioning, and Disability: International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [online]. Atlanta: Centers for Disease Control; Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed July 18, 2011 [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 23.Kalanithi PS, Patil CG, Boakye M. National complication rates and disposition after posterior lumbar fusion for acquired spondylolisthesis. Spine 2009;34:1963–1969 [DOI] [PubMed] [Google Scholar]
- 24.HCUP: Clinical Classifications Software (CCS) for ICD-9-CM; Healthcare Cost and Utilization Project (HCUP) [online]. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March 10, 2013 [Google Scholar]
- 25.Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Mem Fund Q Health Soc 1973;51:95–124 [PubMed] [Google Scholar]
- 26.Hansen JP. CAN'T MISS: conquer any number task by making important statistics simple. Part 6. Tests of statistical significance (z test statistic, rejecting the null hypothesis, p value), t test, z test for proportions, statistical significance versus meaningful difference. J Healthc Qual 2004;26:43–53 [DOI] [PubMed] [Google Scholar]
- 27.Bureau of Labor and Statistics; Consumer Price Index [online]. Available at: http://www.bls.gov/cpi/. Accessed August 10, 2013 [Google Scholar]
- 28.Scozzafava J, Hussain MS, Brindley PG, Jacka MJ, Gross DW. The role of the standard 20 minute EEG recording in the comatose patient. J Clin Neurosci 2010;17:64–68 [DOI] [PubMed] [Google Scholar]
- 29.Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol 2004;61:1090–1094 [DOI] [PubMed] [Google Scholar]
- 30.Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule, DME Face-to-Face Encounters, Elimination of the Requirement for Termination of Non-Random Prepayment Complex Medical Review and Other Revisions to Part B for CY 2013. Federal Register 2012:68891–69373 [PubMed] [Google Scholar]
- 31.Hirsch LJ. Brain monitoring: the next frontier of ICU monitoring. J Clin Neurophysiol 2004;21:305–306 [PubMed] [Google Scholar]
- 32.Jordan KG. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin 1995;13:579–626 [PubMed] [Google Scholar]
- 33.Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 2005;24:1563–1578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


