Abstract
Objective:
The current study examined the association between pulse pressure (PP) and CSF-based biomarkers for Alzheimer disease, including β-amyloid 1–42 (Aβ1–42) and phosphorylated tau (P-tau) protein, in cognitively normal older adults.
Methods:
One hundred seventy-seven cognitively normal, stroke-free older adult participants (aged 55–100 years) underwent blood pressure assessment for determination of PP (systolic − diastolic blood pressure) and lumbar puncture for measurement of CSF Aβ1–42 and P-tau. Pearson correlations and multiple linear regression, controlling for age, sex, APOE genotype, and body mass index, evaluated the relationship between PP and Alzheimer disease biomarkers.
Results:
PP elevation was associated with increased P-tau (r = 0.23, p = 0.002), reduced Aβ1–42 (r = −0.19, p = 0.01), and increased P-tau to Aβ1–42 ratio (r = 0.27, p < 0.001). After controlling for covariates, PP remained associated with P-tau (β = 0.18, p = 0.0196) and P-tau to Aβ1–42 ratio (β = 0.0016, p < 0.001) but was no longer associated with Aβ1–42 (β = −0.1, p = 0.35). Post hoc multivariate analyses indicated that increased PP was associated with all biomarkers in younger participants (aged 55–70 years) (Aβ1–42: p = 0.050; P-tau: p = 0.003; P-tau to Aβ ratio: p = 0.0007) but not older participants (aged 70–100 years).
Conclusions:
PP elevation is associated with increased CSF P-tau and decreased Aβ1–42 in cognitively normal older adults, suggesting that pulsatile hemodynamics may be related to amyloidosis and tau-related neurodegeneration. The relationship between PP and CSF biomarkers is age-dependent and observed only in participants in the fifth and sixth decades of life.
The relationship between blood pressure (BP) and both cognitive decline and Alzheimer dementia has been extensively investigated.1 Fewer studies have investigated the biological mechanisms underlying the association between BP and cognitive decline. Pulse pressure (PP) displays a linear increase with age and represents an index of vascular aging. Alterations in pulse wave velocity and wave reflection timing associated with age-related arterial stiffening are responsible for the increased systolic and decreased diastolic pressure (i.e., increased PP) characteristic of vascular aging. PP elevation is associated with increased risk of Alzheimer dementia, independent of clinical stroke and hypertension, even in patients older than 75 years.2 It remains unclear whether the association between PP and Alzheimer dementia is entirely mediated by its impact on subclinical cerebrovascular disease or whether there is a direct link with Alzheimer pathophysiology (i.e., amyloidosis and tau-related neurodegeneration).
It has been hypothesized that age-related arterial stiffening, marked by PP elevation, may have a role in cerebral small-vessel disease because of its association with increased distal pressure oscillation and pulse wave energy.3 In support of this hypothesis, PP elevation has been associated with white matter lesions in older adults without dementia and postmortem neuropathologic measures of cerebrovascular disease in autopsy-confirmed Alzheimer disease (AD) patients.4,5 Alternatively, it has been proposed that PP elevation may directly affect Alzheimer pathology itself by increasing β-amyloid (Aβ) accumulation, and possibly tau phosphorylation.6 To investigate this possibility, we examined the relationship between PP and AD biomarkers in a sample of cognitively normal older adults.
METHODS
Participants and procedures.
One hundred seventy-seven cognitively normal older adults were recruited at 3 centers: University of California, San Diego (UCSD) Alzheimer's Disease Research Center, University of Washington, and Oregon Health and Sciences University, as previously reported.7 Participants underwent detailed clinical and laboratory evaluation and had no clinically significant abnormalities. Further specific exclusionary criteria included history of stroke, TIA, myocardial infarction, diabetes, or body mass index (BMI) ≥30. Study entry also required Mini-Mental State Examination scores ≥26, Clinical Dementia Rating Scale score of 0, and no evidence or history of cognitive or functional decline. Additionally, scores on delayed recall were higher than a cutoff of 1.5 SD below age-adjusted means for both the Logical Memory and New York University Paragraph tests.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the UCSD, University of Washington, and Oregon Health and Sciences University institutional review boards, and all participants provided written informed consent before being enrolled.
CSF biomarker assessment.
Participants underwent early morning lumbar puncture by experienced clinicians, after an overnight fast. CSF samples were aliquoted into 0.5-mL fractions in polypropylene microtubes, flash-frozen, and stored at −80°C until assessment of CSF Aβ1–42 and P-tau181 proteins. CSF samples were assayed by multiplex bead-based immunoassay (INNO-BIA AlzBio3; Innogenetics, Ghent, Belgium) at a single laboratory per the manufacturer's protocol. Three internal standard CSF samples were used across each plate to assist in calibration, and intra- and interplate coefficients of variation were less than 10%.
BP assessment.
Brachial artery BP measures were obtained using a sphygmomanometer and a stethoscope. Participants were seated comfortably for a few minutes before BP assessment. Systolic BP was recorded at the first Korotkoff sound and diastolic BP was recorded at the cessation of Korotkoff sounds (i.e., fifth Korotkoff sound). Two recordings were taken from each arm while the participant was seated. These measures were averaged to obtain an estimate of resting BP. PP was calculated as follows: PP = systolic BP − diastolic BP. Height and weight were recorded, and BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
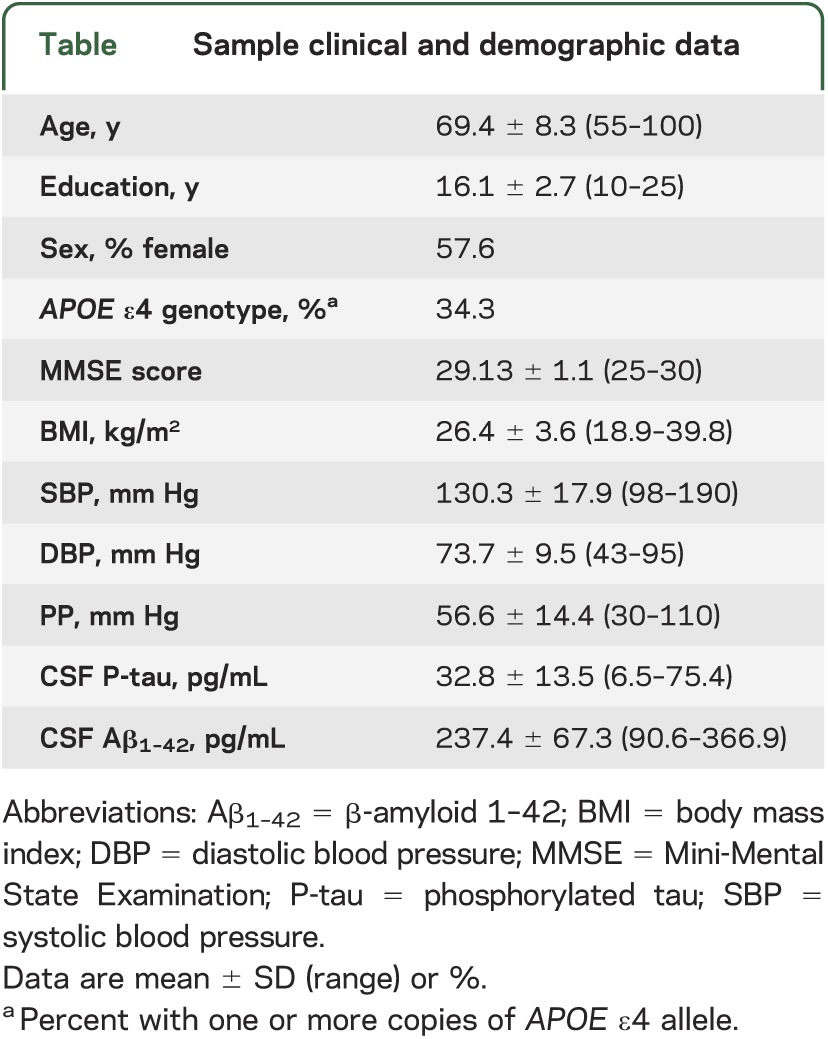
The table displays sample clinical and demographic data, as well as means and SDs for BP measures and CSF biomarkers.
Table.
Sample clinical and demographic data
Statistical analyses.
Pearson product moment correlations were used to examine the univariate relationship between PP and CSF biomarkers. Multiple linear regression was used to test the relationship between PP and CSF biomarkers independent of the potential confound effects of aging, sex, APOE genotype (using terms for a heterozygous ε4 and homozygous ε4 genotype), and BMI. All statistical tests were 2-tailed with a significance cutoff of p < 0.05.
RESULTS
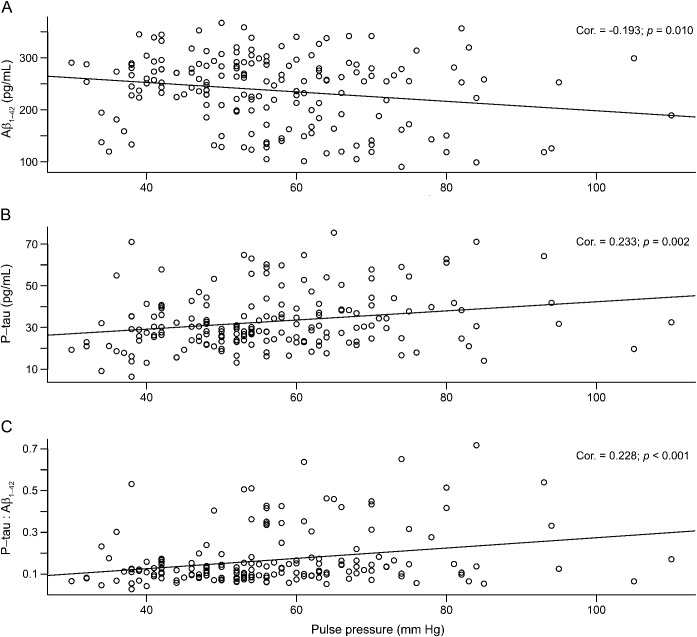
In univariate analyses, PP elevation was associated with reduced Aβ1–42 level (r = −0.19, p = 0.01; figure, A), increased P-tau concentration (r = 0.23, p = 0.002; figure, B), and increased P-tau to Aβ1–42 ratio (r = 0.27, p < 0.001; figure, C). After controlling for age, sex, APOE genotype, and BMI, the relationship between PP and P-tau remained (β = 0.15, p = 0.044), as did the relationship with P-tau to Aβ ratio (β = 0.0016, p < 0.001), but the correlation between PP and Aβ1–42 was no longer present (β = −0.1, p = 0.35).
Figure. Pulse pressure correlates with Alzheimer disease biomarkers.
Scatterplots display the association between elevated pulse pressure and (A) reduced CSF β-amyloid 1–42 (Aβ1–42), (B) increased phosphorylated tau (P-tau), and (C) increased P-tau to Aβ1–42 ratio.
Post hoc analyses indicated that the relationships between PP and all biomarkers were present among those aged 55 to 70 years (Aβ1–42: β = −0.87, p = 0.050; P-tau: β = 0.28, p = 0.006; P-tau to Aβ ratio: β = 0.0023, p = 0.002), but not among those aged 71 years and older (Aβ1–42: β = 0.20, p = 0.67; P-tau: β = −0.01, p = 0.89; P-tau to Aβ ratio: β = 0.0005, p = 0.66).
DISCUSSION
The findings of the present study indicate that PP elevation in cognitively normal older adults is associated with increased CSF P-tau, indicating that pulsatile hemodynamics may be related to subclinical neurodegeneration. Post hoc analyses revealed that increased PP was associated with both elevated P-tau and reduced Aβ1–42, exclusively among participants aged 70 years or younger, further suggesting an association with AD biomarkers in middle-aged participants, specifically during the fifth and sixth decades of life. This is consistent with findings indicating that midlife BP elevation is more predictive of later neurodegeneration and cognitive decline than late-life BP elevation.1
Midlife hypertension has also been linked to subsequent neuropathologic evidence of both neurofibrillary tangles and amyloid plaques,8 and a recent PET imaging study reported an association between PP and increased Aβ1–42 deposition with Pittsburgh compound B (PiB-PET) among middle-aged adults.9 This is consistent with the hypothesis that PP elevation may interfere with the clearance of Aβ1–42 because of its association with alterations in pulse wave amplitude and timing.6 Specifically, these hemodynamic changes lead to increased resistance and decreased compliance of arteriolar resistance vessels,3,4 which may ultimately lead to structural changes that could impair Aβ1–42 clearance along the perivascular spaces.6 It is also possible that these changes in vascular function could lead to arteriolar hypercontractility and reduced tissue perfusion, or blood-brain barrier dysfunction and leakage of plasma constituents, either of which may result in neurodegeneration and increased P-tau.10 The association between PP and Aβ1–42 was only found in our post hoc analysis and there was a greater association between PP and P-tau, suggesting that the latter mechanism may be more salient. Although AD is characterized by both amyloid- and tau-based pathologies, P-tau is more strongly associated with neurodegeneration and cognitive decline, suggesting that PP elevation could convey increased risk of dementia through its association with tau phosphorylation.
Whether the association between PP and P-tau represents the downstream impact of an association between PP on Aβ1–42, or a more direct, amyloid-independent, association between pulsatile hemodynamics and P-tau itself, remains unclear. It is also unclear why these observations are limited to younger individuals. One possibility is that although PP may be among the few factors modifying cerebral amyloidosis and tau-related neurodegeneration in younger individuals, additional emergent factors related to brain aging itself may modify these pathophysiologic processes in older adults, making the effects of PP less relevant in a more aged sample. The current study is limited by cross-sectional design and the lack of cerebrovascular biomarkers. Future studies examining the longitudinal association between PP and markers of cerebrovascular disease, blood-brain barrier dysfunction, and AD may clarify the relationship between vascular aging and AD.
GLOSSARY
- Aβ1–42
β-amyloid 1–42
- AD
Alzheimer disease
- BMI
body mass index
- BP
blood pressure
- PP
pulse pressure
- P-tau
phosphorylated tau
- UCSD
University of California, San Diego
AUTHOR CONTRIBUTIONS
Dr. Nation contributed to the data analysis/interpretation and manuscript preparation. Dr. Edland contributed to the study design, data analysis/interpretation, and manuscript preparation. Dr. Bondi, Dr. Salmon, and Dr. Delano-Wood contributed to the manuscript preparation. Dr. Peskind and Dr. Quinn contributed to the study design and manuscript preparation. Dr. Galasko contributed to the study design, data interpretation, and manuscript preparation.
STUDY FUNDING
The current study was supported by the Alzheimer's Association (grant IIRG 07-59343 to M.W.B.), and the NIH (grants R01 AG012674 to M.W.B., K24 AG026431 to M.W.B., and P50 AG05131). CSF sample collection was supported by NIH grants AG05131 (UCSD), AB005136 (University of Washington), and AG010129 (Oregon Health Sciences University).
DISCLOSURE
D. Nation reports no disclosures. S. Edland served on data safety monitoring boards for Eli Lilly (2010–2012), Pfizer (2012–2013), and Eisai (2012–2013), and as consultant to Janssen Research & Development, LLC (2012–2013). M. Bondi, D. Salmon, L. Delano-Wood, E. Peskind, J. Quinn, and D. Galasko report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499 [DOI] [PubMed] [Google Scholar]
- 2.Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke 2003;34:594–599 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik Study. Brain 2011;134(pt 11):3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nation DA, Delano-Wood L, Bangen KJ, et al. Antemortem pulse pressure elevation predicts cerebrovascular disease in autopsy-confirmed Alzheimer's disease. J Alzheimers Dis 2012;30:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol 2009;118:87–102 [DOI] [PubMed] [Google Scholar]
- 7.Peskind ER, Li G, Shofer J, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol 2006;63:936–939 [DOI] [PubMed] [Google Scholar]
- 8.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57–62 [DOI] [PubMed] [Google Scholar]
- 9.Langbaum JB, Chen K, Launer LJ, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging 2012;33:827.e11–827.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011;12:723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]