Abstract
Objective:
We aimed to compare the rates of thrombolysis utilization for acute ischemic stroke in hospitals with neurology residency (NR) to those of other teaching (OT) and nonteaching (NT) hospitals.
Methods:
A retrospective serial cross-sectional cohort study of a nationally representative sample of stroke patients was conducted. Accreditation Council for Graduate Medical Education–accredited NR program–affiliated hospitals in the United States were cross-matched to the hospitals in the Nationwide Inpatient Sample from 2000 to 2010. ICD-9-CM codes were used for case ascertainment.
Results:
A total of 712,433 adult ischemic stroke patients from 6,839 hospital samples were included, of whom 10.1%, 29.1%, and 60.8% were treated in NR, OT, and NT hospitals, respectively. Stroke patients in NR received thrombolysis more frequently (3.74% ± 0.24% [standard error]) than in OT (2.28% ± 0.11%, p < 0.001) and NT hospitals (1.44% ± 0.06%, p < 0.001). The adjusted odds ratios (ORs) of thrombolysis rates in NR vs OT and NR vs NT increased with each decade increment in age. In multivariate analysis, NR was independently predictive of higher thrombolysis rate (adjusted OR 1.51; 95% confidence interval [CI] 1.44–1.59 [NR vs OT], and adjusted OR 1.82; 95% CI 1.73–1.91 [NR vs NT]).
Conclusions:
Acute stroke care in NR hospitals is associated with an increased thrombolytic utilization. The disparities between the thrombolysis rate in NR and that in OT and NT hospitals are greater among elderly patients.
IV thrombolysis using recombinant tissue plasminogen activator (tPA) within 3 hours of symptom onset has remained the only US Food and Drug Administration–approved therapy shown to improve outcomes in acute ischemic stroke (AIS) for more than a decade.1,2 Benefit is still seen when given from 3 to 4.5 hours after stroke onset.3 Despite significant increase in thrombolytic utilization over the last decade in the United States, the treatment is widely underutilized, with an estimated rate of 3.4% to 5.2% of all stroke cases during 2009.4,5 Identification of factors associated with thrombolytic utilization may lead to better understanding of barriers to the treatment and provide an opportunity for interventions to increase the utilization. Teaching hospitals may have greater adherence to evidence-based guidelines and have more resources for timely thrombolytic treatment, resulting in higher thrombolytic utilization compared to nonteaching (NT) hospitals.6,7 Hospitals with neurology residency (NR) training programs have physicians in training with focus in treating neurologic conditions. NR may also have a greater involvement of subspecialty-trained vascular neurologists and fellows in training compared to other teaching (OT) and NT hospitals, potentially influencing thrombolytic utilization. Therefore, we hypothesized that NR may have a stroke thrombolysis rate different from that in OT and NT hospitals.
METHODS
We compared the rate of thrombolytic utilization for AIS in NR to that in OT and NT hospitals in the United States in a retrospective serial cross-sectional cohort study from a national database.
Data source.
We used the Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP) from 2000 to 2010. NIS is sponsored by the Agency for Healthcare Research and Quality of the US Department of Health and Human Services (HHS). NIS is an approximately 20% sample of all admissions in nonfederal US hospitals. Hospitals are stratified based on the following 5 characteristics to ensure a sample representative of all hospitalizations in the United States: 1) geographic region: northeast, midwest, south, or west; 2) hospital ownership: public, private not-for-profit, or private investor-owned; 3) location: rural or urban; 4) teaching status: teaching or nonteaching, and 5) bed size: small, medium, or large. Primary and secondary diagnoses and in-hospital procedures are recorded using ICD-9-CM codes. Detailed information regarding the design and the contents of NIS can be obtained from the HCUP Web site http://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed December 1, 2012).8 NIS is publicly available and contains no patient-identifying information. Therefore, the database fulfills the requirements for exemption from a formal ethics committee review per HHS guidelines available at http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html (accessed December 1, 2012).9
Case selection.
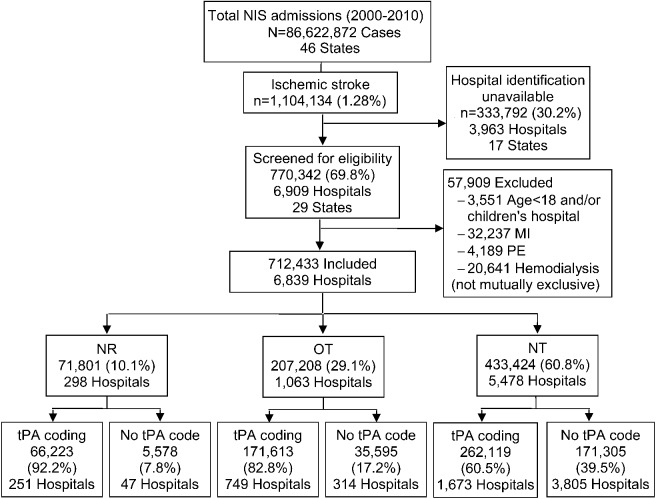
Figure 1 shows the case selection flowchart of the study. We used ICD-9 codes 433.x1, 434.x1, and 436 as a primary or secondary diagnosis to identify AIS.10–13 Thrombolytic infusion was ascertained by ICD-9 volume 3 procedure code 99.10.4,14,15 To avoid the uncertainty of indication for thrombolysis, we excluded the cases with acute myocardial infarction or pulmonary embolism and those on hemodialysis (with possibly clotted access) from the analyses. Cases from all children's hospitals and those aged <18 years were also excluded. Hospitalizations for short-term inpatient rehabilitation are excluded from the NIS. Hospitals from states participating in NIS that suppressed the hospital identifiers in the database were also excluded from the analyses due to inability to ascertain NR vs OT status (table e-1 on the Neurology® Web site at www.neurology.org).
Figure 1. Case selection.
Tissue plasminogen activator (tPA) coding indicates the hospitals coding at least one thrombolytic infusion for ischemic stroke during the sampled year. MI = myocardial infarction; NIS = Nationwide Inpatient Sample; NR = neurology residency; NT = nonteaching; OT = other teaching; PE = pulmonary embolism.
Hospital academic status.
Teaching hospitals are defined in the NIS as hospitals meeting any of these 3 criteria: 1) American Medical Association–approved residency program, 2) member of the Council of Teaching Hospitals, or 3) ratio of full-time equivalent interns and residents to beds of 0.25 or higher. We identified all accredited NR training programs in the United States from the Accreditation Council for Graduate Medical Education Web site (http://www.acgme.org/acgmeweb) (accessed December 1, 2012).16 All hospitals affiliated with these programs that are staffed with neurology residents were classified as NR (list available from the authors upon request). The remaining teaching hospitals were defined as OT hospitals and all other hospitals were classified as NT.
Stratification variables.
We calculated the thrombolysis rates among NR, OT, and NT hospitals stratified by age, sex, ethnicity, health insurance, modified Charlson comorbidity index (CCI),17,18 stroke case volume of the hospital, bed size, location, US geographic region, and Joint Commission–certified Primary Stroke Center (JC-PSC) status. Age was divided into 18–44, 45–54, 55–64, 65–74, 75–84, and ≥85 years.19,20 Ethnicity was divided into Caucasian, African American, Hispanic, Asian/Pacific Islander, other, and missing ethnicity data. CCI was calculated by using HCUP comorbidity software available at http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp (accessed December 1, 2012)21 and categorized into 4 groups: 0, 1, 2, and ≥3.22,23 Hospital stroke case volume was divided into the following quartiles: 1st (1–20 cases/year), 2nd (21–66 cases/year), 3rd (67–164 cases/year), and 4th (>164 cases/year). We identified the JC-PSCs from the JC Web site http://www.jointcommission.org (accessed December 1, 2012) and active JC-PSC certification status at the time of the admission was determined based on the initial certification date.24 The Web site currently provides the certification history from 2006 onwards and therefore, all analyses involving JC-PSC were limited to 2006–2010.
Sensitivity analysis.
A total of 29.8% of all cases were treated in hospitals not reporting any thrombolytic infusion code during the sampled year. As this may represent either underreporting or actual nonutilization by the hospital in a given year, we conducted a separate analysis using only the hospital samples coding at least one thrombolytic infusion in a given year to verify the internal validity of the primary results.
Statistical analysis.
Baseline patient and hospital characteristics are described by using median and interquartile range for numeric variables and percent proportions for categorical variables. The thrombolysis rates are presented as the percent of all AIS cases treated. Comparisons were made by using Wilcoxon rank-sum test for numeric and χ2 test for categorical variables. Standard errors of population estimates were calculated as recommended by HCUP.25 We calculated secular trends of thrombolytic utilization in all 3 hospital types by using χ2 test for linear association. Multivariate logistic regression was used to assess independent effect of hospital academic status on thrombolytic utilization. Model building based on statistically significant univariate association of the predictor variables was not used because of the large sample size resulting in significant univariate associations even with small absolute differences. The following prespecified confounders were identified based on prior studies7,26–30 and were controlled for in the regression models: age, sex, ethnicity, health insurance, CCI, hospital stroke case volume, urban vs rural location, geographic region, calendar year, and JC-PSC status. All analyses were performed using Statistical Package for the Social Sciences version 17.0 (SPSS Inc., Chicago, IL) with statistical significance set at p < 0.05.
RESULTS
A total of 712,433 AIS cases in 6,839 hospital samples, representing 64.5% of all stroke cases treated during the study period in the United States, were included in the analysis. Of these, 71,801 (10.1%) were treated in 298 NR and 207,208 (29.1%) were treated in 1,063 OT hospitals. Of the total stroke cases, 499,955 (70.2%) cases were treated in 2,673 hospitals coding at least one thrombolytic infusion during the sampled year (figure 1). Patients in NR were younger, less likely to be female or Caucasian, and had lower CCI. NR were more likely to be urban and large hospitals and have JC-PSC certification. NR also had higher stroke and thrombolytic case volumes compared to OT and NT hospitals. Proportionately more patients in NR were treated in the northeast United States compared to those in OT or NT hospitals (table 1).
Table 1.
Baseline patient and hospital characteristics

The overall thrombolytic utilization rate was 1.92% ± 0.06% (standard error) during the 11-year study period. The unadjusted thrombolysis rate was higher in NR (3.74% ± 0.24%) compared to OT (2.28% ± 0.11%, p < 0.001) and NT hospitals (1.44% ± 0.06%, p < 0.001). All 3 hospital types showed trends of increasing thrombolytic utilization between 2000 and 2010 (p < 0.001 for all 3 trends) (figure e-1). The thrombolysis rate increased in NR from 0.96% in 2000 to 6.25% in 2010 while the rate of increase was lower during the same period in OT (from 0.82% to 4.86%) and NT hospitals (from 0.82% to 3.83%).
Age group 75–84 years had the highest rate of thrombolysis (4.09% ± 0.29%) in NR among all age groups, while the rates were the highest in age group 18–44 in OT (3.40% ± 0.23%) and NT hospitals (2.60% ± 0.15%). The thrombolysis rate decreased with each decade increment in age from 18 to 44 years both in OT and NT hospitals (figure 2A). The adjusted odds ratios (ORs) of thrombolysis rates in NR vs OT and NR vs NT increased with each decade increment in age from 18 to 44 years, indicating higher discrepancy in thrombolysis rates in more elderly patients (figure 2B). The increase in adjusted ORs with advancing age was also found after controlling for JC-PSC certification during 2006–2010 (figure e-2).
Figure 2. Thrombolysis rates by age.

(A) Unadjusted thrombolysis rates by age. Error bar indicates ±1 standard error of the population estimate. (B) Adjusted odds ratios (ORs) of thrombolysis rates by age. Adjusted for sex, ethnicity, health insurance, Charlson comorbidity index, location, geographic region, calendar year, and hospital stroke case volume. Error bar indicates 95% confidence interval of the OR. *p < 0.05; **p < 0.001. NR = neurology residency; NT = nonteaching; OT = other teaching; tPA = tissue plasminogen activator.
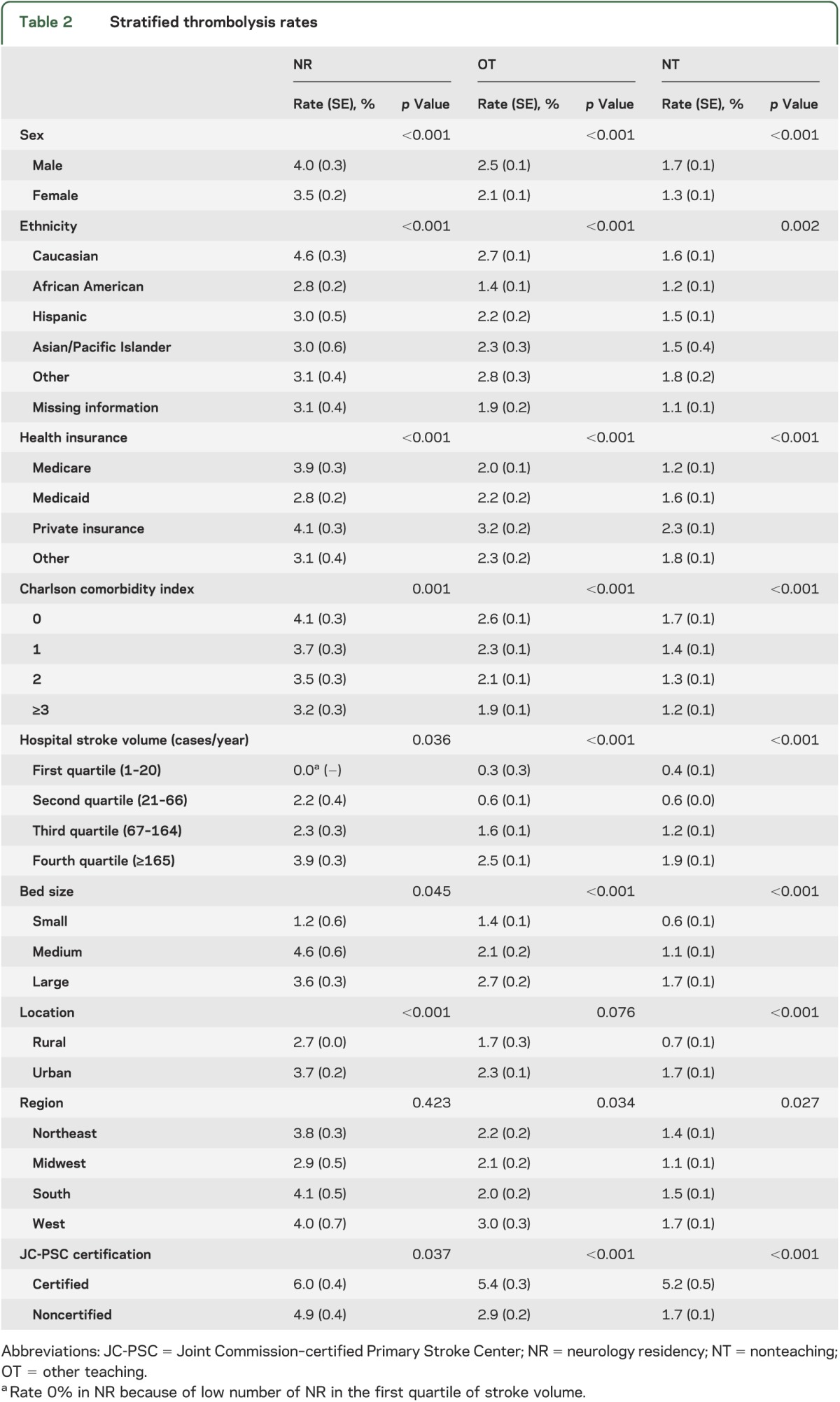
Table 2 summarizes the thrombolysis rates stratified by sex, ethnicity, health insurance, CCI, hospital stroke case volume, bed size, location, geographic region, and JC-PSC certification among all 3 hospital types. The rates were higher in male patients, Caucasian patients, private insurance, lower CCI, higher stroke case volume, medium and large bed size, urban location, and JC-PSCs. The rates were the highest in the western United States in OT and NT hospitals, while the geographic differences were not significant in NR hospitals.
Table 2.
Stratified thrombolysis rates
In multivariate analysis, NR was independently predictive of higher thrombolysis rate (adjusted OR 1.51; 95% confidence interval [CI] 1.44–1.59, p < 0.001 [NR vs OT], and adjusted OR 1.82; 95% CI 1.73–1.91, p < 0.001 [NR vs NT]). Other factors associated with higher thrombolysis rate in regression analyses were younger age, male sex, Caucasian ethnicity, private insurance vs Medicare, Medicare vs Medicaid or other insurance, lower CCI, urban location, higher hospital stroke case volume, and more recent calendar year. The JC-PSC certification was also independently associated with increased thrombolytic utilization during 2006–2010 (adjusted OR 1.62; 95% CI 1.54–1.70, p < 0.001) (table 3).
Table 3.
Multivariate analysis: Predictors of thrombolytic utilization

The thrombolysis rate was also higher in NR (4.05% ± 0.24%) compared to OT (2.75% ± 0.12%, p < 0.001) and NT hospitals (2.39% ± 0.09%, p < 0.001) among the hospitals coding at least one thrombolytic infusion during the sampled year. Baseline characteristics and univariate/multivariate analyses of thrombolytic utilization in thrombolysis coding hospitals (tables e-2, e-3, and e-4, and figure e-3) were comparable to the primary analysis of all included hospitals.
DISCUSSION
IV thrombolysis, the standard of care in AIS, is underutilized in the United States. NR training programs in our study showed higher thrombolysis utilization compared to OT and NT hospitals. Several unmeasured factors may explain the differences. The presence of residents with focus on treating neurologic conditions may have influenced the thrombolysis rates directly or indirectly. Physicians in NR may have better awareness of or be more inclined to follow evidence-based guidelines for AIS treatment. NR may also have higher number of in-training vascular neurology fellows and fellowship-trained vascular neurologists, potentially contributing to the higher thrombolysis rates. NR also usually have 24/7 availability of in-hospital neurology residents or vascular neurology fellows, making thrombolytic treatment possible during off hours and weekends. They may have greater experience in thrombolytic treatment due to higher volumes and be more comfortable using a treatment that has serious side effects if used inappropriately.
Hospital teaching status has been shown to affect thrombolysis rate in prior studies using NIS.6,7 Adjusted odds of thrombolytic treatment in all teaching hospitals was found to be 27% higher compared to NT hospitals after controlling for demographic and hospital-related factors.7 A recent study of thrombolysis rate in young stroke patients between 2001 and 2009 showed higher rate among urban teaching hospitals compared to urban NT and rural hospitals.6 However, to our knowledge, no previous large study has explored the association of NR training program with thrombolytic utilization.
Consistent with previous reports from different data sources,4,26,31 we also found significant increase in thrombolytic utilization in recent years. Factors contributing to this trend include the American Heart Association's Get With the Guidelines quality improvement campaign (April 2003),32 the JC-PSC certification program (December 2003),33 approval of new diagnosis-related group 559 (AIS with use of thrombolytic agent) with increased payment by Centers for Medicare & Medicaid Services (August 2005),34 the European Cooperative Acute Stroke Study 3 publication3 with subsequent science advisory from American Heart Association/American Stroke Association recommending IV tPA up to 4.5 hours after stroke onset (May 2009),35 expansion of telestroke networks across the country,36 and increasing comfort level among physicians for using thrombolytic treatment with increasing experience and accumulating evidence of safety and efficacy of tPA. It is known that JC-PSCs use tPA more frequently than noncertified centers33 and in our study, there is an association between JC-PSC certification and NR. However, NR was associated with increased tPA use independent of JC-PSC status in adjusted analysis, thus indicating that other unmeasured factors are also contributing to the thrombolysis rates.
Similar to previous reports, the overall thrombolysis rate decreased with age in our study.7,26 However, the elderly stroke patients in NR had relatively high thrombolysis rates. Elderly patients have more severe stroke37 and patients with more severe strokes have shorter onset-to-door time,31 which may explain why elderly patients had high thrombolysis rate in NR in our study. Physicians in NR may be more aware of poor outcomes in elderly stroke patients without thrombolytic treatment, with fewer than one-third of patients achieving independent ambulation and a similar proportion discharged to home.37 Notably, there was an increasing discrepancy in thrombolysis rates in NR vs OT and NR vs NT with advancing age. This finding may indicate that the physicians in OT and NT hospitals are more cautious using thrombolytic treatment in elderly patients. Exclusion of age >80 from early tPA trials1,3 may have contributed to this finding. The recently published International Stroke Trial 3 showing safety and efficacy of tPA in age >80 years is expected to increase thrombolysis rates among the elderly population.2 Comparison of thrombolysis rates among elderly in NR vs OT and NR vs NT in upcoming years may provide important insight into the effect of large clinical trials on change in clinical practice in OT and NT hospitals.
Analysis of 536,328 cases from the Premier Hospital database in 2 separate studies showed that ICD-9 codes underestimated thrombolytic utilization compared to pharmacy records.4,15 Combining the results of these studies, we calculated that ICD-9 code 99.10 identified 73.5% (range 57.6% to 81.9%) of pharmacy billing–verified total thrombolytic cases. Extrapolating this finding to our study, a weighting factor of 1.36 may correct for the ICD-9 coding–related underestimation of thrombolysis rates in our study. Based on the adjusted ORs of tPA rates in different age groups and the incidence of AIS in the United States,19 we estimated that more than 11,000 additional stroke patients would be treated annually with tPA if the thrombolysis rates were similar in OT and NT hospitals to those in NR (table e-5).
The results of this study should be interpreted with the following cautions due to inherent limitations of administrative datasets such as NIS. Using ICD-9 codes to define cases may have introduced ascertainment bias. However, the ICD-9 codes used to define AIS have been validated to have high specificity (>90%) and positive predictive value (>85%) both in academic as well as nonacademic hospital settings.11,13,38,39 Though procedure code 99.10 has modest sensitivity,15 it has high specificity of >95%.40 Therefore, we may have underestimated the tPA rates, but the case identification is likely to be accurate. Moreover, inaccuracies in ICD-9 coding are expected to be distributed randomly across all age groups and academic types and would bias the results toward null; therefore, the differences in thrombolysis rates found in this study are valid. We did not correct for the underreporting of the thrombolysis by ICD-9 code. This approach resulted in more conservative estimation of the differences in thrombolysis rate between hospitals of different academic status. Of note, the thrombolysis code does not distinguish the mode of thrombolytic agent delivery, different thrombolytic agents, and the dosing. Approximately one-third of all cases were treated at hospitals not coding thrombolytic infusion. This may represent coding omission or actual nonutilization of thrombolysis in these hospitals. Therefore, we conducted a separate analysis using thrombolysis coding hospitals only to assess the validity of the study results and found comparable results between the 2 types of analyses. As NIS does not have important clinical elements such as time of onset and stroke severity, it was not possible to calculate the thrombolysis rates among the eligible cases. NIS also lacks information regarding variations in Emergency Medical Services triage practice, which could potentially affect the transport of thrombolysis-eligible patients. Also of interest would be comparing the thrombolysis rates in different NR hospitals based on whether the treatment decision is made primarily by the neurology residents, vascular neurology fellows, general neurologists, or vascular neurologists. Additionally, an analysis of the trend of thrombolytic rates in each hospital academic type based on the time since first thrombolytic treatment by the hospital as soon as it has treatment capability would allow a comparison of learning curves of the hospitals for thrombolysis; however, as a result of the NIS sampling strategy, the majority of the hospitals did not get sampled for enough consecutive years to allow for this analysis. Despite these limitations, large-scale studies using clinical database or chart abstraction to calculate population estimates of thrombolytic utilization with high generalizability may not be feasible and national or regional administrative databases such as NIS provide a useful tool to study such large-scale population phenomena.
US hospitals with neurology trainees are associated with higher thrombolytic utilization for AIS compared to OT and NT hospitals and the differences in thrombolysis rates are higher in older patients. Efforts to increase utilization among elderly in OT and NT hospitals by interventions such as in-service educational programs for physicians treating AIS can potentially have a large impact on stroke system of care. Future studies should explore the factors responsible for higher thrombolysis utilization in NR and barriers to treatment in OT and NT hospitals.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Jihan Grant from the Department of Neurology at SUNY Downstate Medical Center and Pareshbhai Bharodiya from the Department of Internal Medicine at the Brooklyn Hospital Center for their help in verifying the accuracy of manual crosslinking of the neurology residency hospitals in NIS and ACGME datasets.
GLOSSARY
- AIS
acute ischemic stroke
- CCI
Charlson comorbidity index
- CI
confidence interval
- HCUP
Healthcare Cost and Utilization Project
- HHS
Health and Human Services
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- JC-PSC
Joint Commission–certified Primary Stroke Center
- NIS
Nationwide Inpatient Sample
- NR
neurology residency
- NT
nonteaching
- OR
odds ratio
- OT
other teaching
- tPA
tissue plasminogen activator
Footnotes
Editorial, page 1972
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Moradiya: study concept and design, acquisition, analysis, and interpretation of data, statistical analysis, writing the first draft of the manuscript, and making subsequent revisions. Dr. Crystal: study design and critical revision of the manuscript for important intellectual content. Dr. Valsamis: critical revision of the manuscript for important intellectual content. Dr. Levine: study design, interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
STUDY FUNDING
Supported in part by NIH grants U10NS077378, R25NS079211, U10NS080377, and R01HL096944 to Dr. Steven R. Levine.
DISCLOSURE
Y. Moradiya, H. Crystal, and H. Valsamis report no disclosures. S. Levine is Associate Editor of MEDLINK, receives research funding from the NIH and Genentech, Inc., and has served as an expert witness in acute stroke cases. Go to Neurology.org for full disclosures.
REFERENCES
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 4.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011;42:1952–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakaran S, McNulty M, O'Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke 2012;43:875–877 [DOI] [PubMed] [Google Scholar]
- 6.Kansara A, Chaturvedi S, Bhattacharya P. Thrombolysis and outcome of young stroke patients over the last decade: insights from the nationwide inpatient sample. J Stroke Cerebrovasc Dis 2012;22:799–804 [DOI] [PubMed] [Google Scholar]
- 7.Schumacher HC, Bateman BT, Boden-Albala B, et al. Use of thrombolysis in acute ischemic stroke: analysis of the Nationwide Inpatient Sample 1999 to 2004. Ann Emerg Med 2007;50:99–107 [DOI] [PubMed] [Google Scholar]
- 8.Overview of the nationwide inpatient sample (NIS) [online]. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 1, 2012
- 9.Human subject regulations decision charts [online]. Available at: http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html. Accessed December 1, 2012
- 10.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke 1998;29:1602–1604 [DOI] [PubMed] [Google Scholar]
- 11.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke 2005;36:1776–1781 [DOI] [PubMed] [Google Scholar]
- 12.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf 2008;17:20–26 [DOI] [PubMed] [Google Scholar]
- 13.Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol 2001;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubinsky R, Lai SM. Mortality of stroke patients treated with thrombolysis: analysis of Nationwide Inpatient Sample. Neurology 2006;66:1742–1744 [DOI] [PubMed] [Google Scholar]
- 15.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008;39:924–928 [DOI] [PubMed] [Google Scholar]
- 16.Accredited programs and sponsoring institutions [online]. Available at: http://www.acgme.org/acgmeweb/acgmeweb/tabid/172/GraduateMedicalEducation/AccreditedProgramsandSponsorSearch.aspx. Accessed December 1, 2012
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke 2004;35:1941–1945 [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation 2013;127:e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke 2009;40:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HCUP comorbidity software [online]. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed December 1, 2012
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 23.Saposnik G, Baibergenova A, O'Donnell M, Hill MD, Kapral MK, Hachinski V. Hospital volume and stroke outcome: does it matter? Neurology 2007;69:1142–1151 [DOI] [PubMed] [Google Scholar]
- 24.Joint Commission certified organizations [online]. Available at: http://www.qualitycheck.org/help_certified_orgs.aspx. Accessed December 1, 2012
- 25.Houchens R, Elixhauser A. Final report on calculating nationwide inpatient sample (NIS) variances, 2001 [online]. Available at: http://www.hcup-us.ahrq.gov/reports/methods/CalculatingNISVariances200106092005.pdf. Accessed December 1, 2012
- 26.Rost NS, Smith EE, Pervez MA, Mello P, Dreyer P, Schwamm LH. Predictors of increased intravenous tissue plasminogen activator use among hospitals participating in the Massachusetts primary stroke service program. Circ Cardiovasc Qual Outcomes 2012;5:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:154–160 [DOI] [PubMed] [Google Scholar]
- 28.Keyhani S, Arling G, Williams LS, et al. The use and misuse of thrombolytic therapy within the Veterans Health Administration. Med Care 2012;50:66–73 [DOI] [PubMed] [Google Scholar]
- 29.Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines–Stroke 2003–2010. Stroke 2012;43:1858–1864 [DOI] [PubMed] [Google Scholar]
- 30.Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke 2009;40:3580–3584 [DOI] [PubMed] [Google Scholar]
- 31.Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US academic medical centers, 2001–2004. Stroke 2009;40:3845–3850 [DOI] [PubMed] [Google Scholar]
- 32.Smaha LA. The American Heart Association Get With the Guidelines program. Am Heart J 2004;148:S46–S48 [DOI] [PubMed] [Google Scholar]
- 33.Mullen MT, Kasner SE, Kallan MJ, Kleindorfer DO, Albright KC, Carr BG. Joint Commission primary stroke centers utilize more rt-PA in the nationwide inpatient sample. J Am Heart Assoc 2013;2:e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demaerschalk BM, Durocher DL. How diagnosis-related group 559 will change the US Medicare cost reimbursement ratio for stroke centers. Stroke 2007;38:1309–1312 [DOI] [PubMed] [Google Scholar]
- 35.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009;40:2945–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey JL, Jahnke HK, Goslar PW, Partovi S, Flaster MS. tPA by telephone: extending the benefits of a comprehensive stroke center. Neurology 2005;64:154–156 [DOI] [PubMed] [Google Scholar]
- 37.Fonarow GC, Reeves MJ, Zhao X, et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation 2010;121:879–891 [DOI] [PubMed] [Google Scholar]
- 38.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–485 [DOI] [PubMed] [Google Scholar]
- 39.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):100–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi AI, Harris-Lane P, Siddiqi F, Kirmani JF. International Classification of Diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. J Clin Epidemiol 2006;59:856–858 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.