Abstract
Microglia are the resident macrophage-like cells of the central nervous system (CNS) and, as such, have critically important roles in physiological and pathological processes such as CNS maturation in development, multiple sclerosis, and spinal cord injury. Microglia can be activated and recruited to action by neuronal injury or stimulation, such as axonal damage seen in MS or ischemic brain trauma resulting from stroke. These immunocompetent members of the CNS are also thought to have roles in synaptic plasticity under non-pathological conditions. We employ protocols for culturing microglia from the neonatal and adult tissues that are aimed to maximize the viable cell numbers while minimizing confounding variables, such as the presence of other CNS cell types and cell culture debris. We utilize large and easily discernable CNS components (e.g. cortex, spinal cord segments), which makes the entire process feasible and reproducible. The use of adult cells is a suitable alternative to the use of neonatal brain microglia, as many pathologies studied mainly affect the postnatal spinal cord. These culture systems are also useful for directly testing the effect of compounds that may either inhibit or promote microglial activation. Since microglial activation can shape the outcomes of disease in the adult CNS, there is a need for in vitro systems in which neonatal and adult microglia can be cultured and studied.
Keywords: Immunology, Issue 78, Neuroscience, Neurobiology, Cellular Biology, Molecular Biology, Medicine, Biomedical Engineering, Bioengineering, Anatomy, Physiology, immunosuppression, life sciences, animal biology, animal models, biochemistry, microglia, cortex, mouse, neonatal, cell culture, spinal cord, adult, tissue culture, animal model
Introduction
Microglia are the resident immune cells of the CNS, most closely resembling peripheral macrophages in structure and function 1. It has recently been demonstrated that postnatal microglial cells derive from primitive myeloid progenitors and are generated before the eighth day of embryogenesis, upending the previous notion that postnatal hematopoietic progenitors are the source of microglia in the adult brain 2. They play key roles in several neurological diseases and can quickly respond to infection or injury by releasing pro-inflammatory or anti-inflammatory cytokines 3. Thus microglia encompass a standalone unit in the CNS that can be manipulated to affect disease progression. Developing robust and reproducible methods to isolate and culture neonatal or adult microglial cells is important to future studies.
Microglia are known to be critical players in a number of brain pathologies. More recently, roles are emerging for the cells in normal brain development and function as microglia phagocytose excess neural progenitor cells from the dentate gyrus of the hippocampus 1, 4. Microglia can also modulate several neurological conditions that affect the spinal cord, such as MS, neuropathic pain, and spinal cord injury 5-7. Spinal cord microglia react differently compared to brain microglia in response to activation signals 8, 9, probably due to differences in the local environment. Thus it is important to establish an appropriate in vitro system to culture and study spinal cord microglia. Neonatal microglia produce significantly more of the pro-inflammatory cytokine nitric oxide compared to adult cells after in vitro stimulation with IFN-γ or TNF-a10,11 further highlighting the need to use adult cells to study microglia in the context of certain diseases.
The protocol we employ in the lab to culture neonatal microglia is a variation of recent methods which utilize shaking of mixed glial cultures in an effort to remove the microglia from the surface of the cell culture flask 12. We also describe a method to culture microglia from the adult mouse spinal cord based on a protocol first described by Yip, et al13. This method provides a quicker way to culture adult cells compared to other available protocols 14. The resulting preparation is 70% microglia; the remaining percentage is composed of astrocytes. Although the purity of our culture is lower compared to other published methods 13, this culture system is useful for exploring the microglial in culture response to various activating stimuli as well as for the study of diseases that mainly affect the spinal cord and in which a strong inflammatory response is a main feature.
All the protocols described have been approved by the Stony Brook University IACUC.
Protocol
1. Dissection (Day 0)
Induce anesthesia to p0-2 mouse pups with hypothermia. Wipe the heads of the pups with a Kimwipe soaked in 70% ethanol to disinfect the tissue.
Remove the heads with a pair of scissors, using 4 pups per 10 cm culture plate. Place the heads in a petri dish containing ice-cold Hank's buffer.
Anchor the heads using curved forceps through the eye sockets and carefully remove the skin covering the skull with straight microforceps.
Remove the cranial bones with straight microforceps, using caution to not puncture or damage the cortices. The most effective way is to begin the removal starting near the cerebellum, which is located at the base of the head, as this region will be discarded.
Remove the brain with a small spatula, and place the (4) brains in a fresh petri dish containing ice-cold Hank's buffer.
All steps from this point on utilize microforceps and are performed under a dissection microscope. Starting on the ventral side of the brain, anchor the tissue by holding the cerebellum and make two small incisions on either side of the midbrain. Be careful not to cut all the way through the tissue, as the cortices cover the midbrain in this region.
Gently tease the midbrain and cerebellum in one piece from the two cortices. The two cortices should form a concave shape.
Separate the two cortices and orient one single cortex with the medial side up for further dissection. The hippocampus can be difficult to observe, but it is located opposite of the olfactory bulb which appears as a small nodule on the pointed end of the cortex.
Remove the hippocampus, which has a crescent-shape. Flip the cortex over to view the dorsal side.
Using the olfactory bulb as a starting point, remove all meninges and the olfactory bulb itself from the cortex.
The dissected cortices should be placed into 15 ml conical tubes with 14 ml of cold Hank's buffered saline on ice.
2. Cell Culture (Day 0)
Pre-coat 10 cm tissue culture dishes with 5 μg/ml of Poly-D-Lysine (PDL) diluted in autoclaved water for 3 hr at 37 °C.
Aspirate PDL, and wash plates once with autoclaved water. Dry plate under the UV in the tissue culture hood for 20 min. This step should be performed just prior to the dissection process.
Aspirate Hank's buffer from tubes containing the cortices from 4 brains each, apply 4 ml of 1x trypsin/EDTA solution, triturate tissue with p1000 tip, and place tubes in 37 °C incubator for 15 min.
Add 4 ml of complete microglial media per tube to stop the enzymatic digestion, mix, and spin down contents at 1.5K rpm for 5 min.
Aspirate supernatant and repeat wash with 4 ml of complete media. Resuspend cells in 10 ml of complete microglial media (DMEM with 10% FBS, 1% sodium pyruvate, 0.08% gentamycin), and filter through a 40 micron mesh cell strainer.
Plate at a density of 8 cortices per 10 ml in a 10 cm tissue culture plate and place in a tissue culture incubator at 37 °C and 5% CO2 (See Adult Microglia protocol).
(Day 3)
Change media in all cell culture dishes with complete microglial media.
(Day 10)
Add 400 microliters of 60 mM Lidocaine in HBSS (to detach microglia) into medium of 10-cm tissue culture plates and incubate at room temperature (RT) for 10-15 min.
Collect the media/cell suspension from the plate, and wash the plate once with Hank's buffer. Collect the wash buffer to recover remaining microglia.
Add 5 mM EDTA (pH 8.0) to the cell suspension to a final concentration of 50 μM or at a 1/100 dilution.
Spin down cell suspension at 1,000 x g (1,500 rpm) for 5 min and re-suspend in 1 ml DMEM with 1% FBS.
Count viable cell number (as per Adult Microglia 3.1/3.2) and split cells into tissue culture plates at the desired experimental density. Approximately 1x106 microglia are harvested from a 10 cm plate containing the cortices from 4 brains. For immunofluorescence, a density of 2.5x104 cells per 18 mm coverslip is recommended.
Allow at least 24 hr for microglial cells to fully return to their ramified, resting state prior to use.
Protocol Text: (Adult Microglia)
1. Tissue Collection
Coat tissue culture plates with Poly-D-Lysine (PDL) for 3 hr at 37 °C or overnight at 4 °C. Wash the plates once with autoclaved water immediately prior to use.
Euthanize mouse by CO2 asphyxiation and ascertain that euthanasia is complete. Clean spinal cord area with 70% ethanol.
Using a pair of scissors cut the skin on top of the spinal cord; then proceed to cut the spinal cord out from region T1 to T12. Cut the remaining muscle off of the sides of the spinal cord.
Slowly cut the vertebrae using microscissors as you gently hold the spinal cord with your fingertips. Be careful not to puncture the spinal cord as the tissue is very soft. Slowly extract the spinal cord using microforceps.
Submerge the spinal cord in a petri dish containing ice-cold HBSS. Using a light microscope remove all visible meninges using microforceps
Cut the spinal cord into transversal segments small enough to generate several fragments. The thickness of the fragments should not be more than 2 mm in order to facilitate efficient digestion. Transfer the spinal cord pieces to a 15 ml conical tube containing 1 ml ice-cold HBSS. Keep the tube on ice until the digestion step.
2. Digestion of Tissue
Aspirate HBSS from 15 ml conical tube containing spinal cord tissue. Be careful not to aspirate any piece of tissue.
Add 1 ml of trypsin (2.5 g/L irradiated porcine trypsin and 0.2 g/L EDTA in HBSS) and incubate at 37 °C for 30 min.
To stop the digestion remove the trypsin and add 3 ml of primary microglia medium which contains serum to halt the enzymatic digestion.
Pipet up and down to dislodge the tissue. Filter the cell suspension using a 40 μm cell strainer.
3. Cell Counting and Plating
Mix equal volume of cell suspension and DAPI solution.
Count cells using a hemocytometer.
Plate at a density between 5-7x105 cells/ml in PDL-coated 35 x 10 mm dishes . Plating at a lower density prevented cell growth even when cells were cultured in 12 well plates which have a smaller area compared to 35 x 10 mm dishes.
Representative Results
An example of resting and activated microglial cells is shown in Figure 1. The microglia were visualized 24 hr after plating (Figure 1a) and exhibit ramified (resting) morphology. Exposure to the priming reagent, bacterial lipopolysaccharide (LPS) results in changes in microglial morphology as the cells become activated (Figure 1b).
An example of counting the cells for plating is shown in Figure 2. Due to the presence of cell debris, DAPI Fluoromount (instead of Trypan Blue) was added to an equal volume of the cell suspension and placed in a hemocytometer for cell counting as described in 4, 13. DAPI positive cells were visualized using a fluorescent microscope. Use of the bright field setting facilitated an accurate count of the cells present (Figure 2). Around 7x105 cells were obtained per mouse spinal cord.
The cells were plated in PDL-coated 35 x 10 mm tissue culture dishes. We found that coating with PDL was a critical step, as cells did not adhere/grow unless the plates were coated. Microglia fully attach to the culture plate after approximately two days in culture (Figure 3). The spinal cord of MacGreen mice, which express GFP under the control of the microglia/macrophage promoter CSF-1R, was used for this experiment.
 Figure 1. Representative image of neonatal microglia after two days in culture. Microglia were cultured from p0 C57/BL6 mouse pups, and plated on uncoated coverslips at a density of 2.5 x 105 cells/ml. a, c. Untreated microglia that have returned to a resting, ramified state; b, d. Microglia treated with 100 ng/ml LPS for 4 hr and show an activated, amoeboid shape; Iba1 (green, a marker specific for a macrophages/microglia cell surface protein) has been used to visualize the cells, while bright field images were used to show overall cell populations. DAPI Fluoromount was used to visualize nuclei and indicate the purity of the culture.
Figure 1. Representative image of neonatal microglia after two days in culture. Microglia were cultured from p0 C57/BL6 mouse pups, and plated on uncoated coverslips at a density of 2.5 x 105 cells/ml. a, c. Untreated microglia that have returned to a resting, ramified state; b, d. Microglia treated with 100 ng/ml LPS for 4 hr and show an activated, amoeboid shape; Iba1 (green, a marker specific for a macrophages/microglia cell surface protein) has been used to visualize the cells, while bright field images were used to show overall cell populations. DAPI Fluoromount was used to visualize nuclei and indicate the purity of the culture.
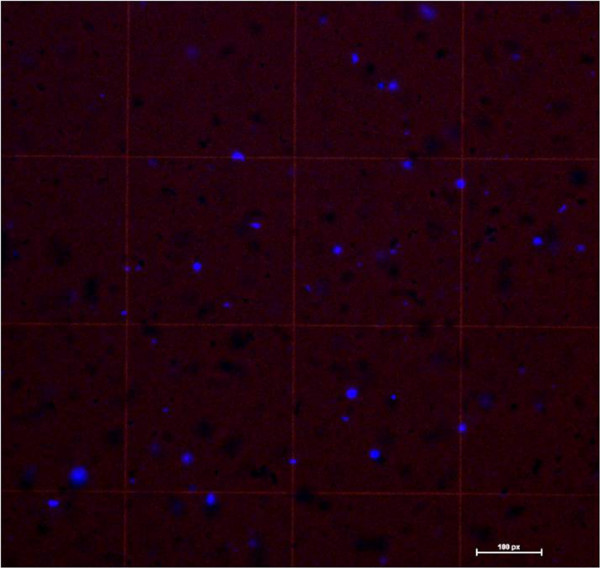 Figure 2. Cell counting. DAPI Fluoromount was mixed with an equal volume of cell suspension and placed in a hemocytometer. We combined the brightfield and the fluorescence setting to visualize DAPI positive cells and the hemocytometer grid for an accurate cell count.
Figure 2. Cell counting. DAPI Fluoromount was mixed with an equal volume of cell suspension and placed in a hemocytometer. We combined the brightfield and the fluorescence setting to visualize DAPI positive cells and the hemocytometer grid for an accurate cell count.
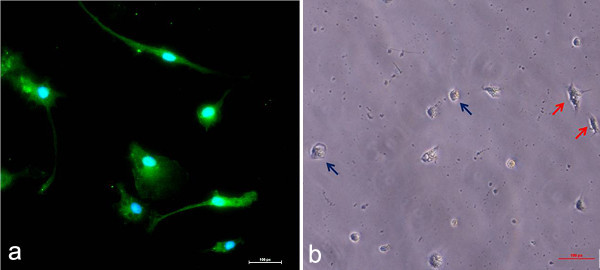 Figure 3. Representative images of adult spinal cord microglia after two days in culture. a. The spinal cord of a MacGreen mouse was used for the culture procedure. Cells were plated in a PDL-coated 35 x 10 mm tissue culture dish at a density of 7x105 cells/ml. b. Bright field image of spinal cord microglia after two days in culture. Microglia (blue arrows) and astrocytes (red arrows) are shown. Click here to view larger figure.
Figure 3. Representative images of adult spinal cord microglia after two days in culture. a. The spinal cord of a MacGreen mouse was used for the culture procedure. Cells were plated in a PDL-coated 35 x 10 mm tissue culture dish at a density of 7x105 cells/ml. b. Bright field image of spinal cord microglia after two days in culture. Microglia (blue arrows) and astrocytes (red arrows) are shown. Click here to view larger figure.
Discussion
Microglia modulate CNS normal functioning as well as inflammatory responses to various pathologies. Functional synaptic remodeling by microglia has been implicated in the maintenance of normal brain homeostasis 15. During the neurogenic cascade they participate in the clearance of neural progenitor cells from the dentate gyrus of the hippocampus 4, 16. Therefore, it is necessary to develop a culture system in which to study neonatal and adult microglia, which will cover the vast swath of development and adulthood, throughout which microglia have multiple discrete functions. We have outlined a quick and efficient method to culture microglia from the adult spinal cord and neonatal brain. We were able to obtain cell preparations in the adult that were 70% microglia with the remaining percentage consisting of astrocytes, and the purity of the neonatal microglial preps approaching 100%.
Given the role that astrocytes have in regulating the activation state of microglia 17-19, it is important to optimize the culture conditions to improve the purity of the culture. Although combining the cell suspension with an equal volume of DAPI Fluoromount allows for an accurate cell count, it is not possible to distinguish microglia from other cell types using this method. To improve the purity of the adult microglial preparation, GFP- coated Dynabeads can be incubated with the cell suspension derived from MacGreen mice, whose myeloid cells already express GFP 20. Another suggestion would be to plate the mixed cell suspension in culture dishes that have not been previously coated with PDL to prevent astrocytic attachment 13. However, we have found that no cells grow on the plate unless they have been previously coated, which in our hands is applicable to both neonatal and adult cells.
Many protocols describing methods for isolation and plating of primary microglia from either rat or mouse brain tissue utilize shaking of mixed glial cultures to detach the microglia and thereby obtain a purified population for downstream experiments 12. Our protocol differs most noticeably in excluding the shaking step, and instead focusing on the purification of a microglial-only population through precisely titrated culturing conditions. In our experience, shaking mixed glial cultures in order to remove the microglia is effective, but has one significant drawback - the fact that the shaken microglia take many days to return to a complete resting morphology 12. This may be problematic given that activated microglia have a considerably different cytokine profile 21. Our protocol, while taking slightly longer to obtain pure microglia, maintains the cells in a resting state throughout. As with the adult prep discussed in the next paragraph, the one drawback of our method is a possibility of small contamination of other cell types, namely astrocytes.
Our technique for culturing adult microglia varies considerably from preexisting methods. Many protocols call for the use of density gradient centrifugation (Percoll) as well as the use of flow cytometry to obtain a pure microglial population 22. In our method of creating a stable microglial culture from adult spinal cord we do not utilize either technique, which makes for a considerably less time consuming and complicated protocol for the researcher to follow. While the vast majority of adult microglial preparations come from brain tissue, the spinal cord is comprised of the same cell types - and therefore our protocol presents a fair comparison to previously described methods 22. One tradeoff in our protocol is a slightly less pure microglial preparation, consisting of 70% microglia, with the remaining fraction of cells being mostly of astrocytic origin. This discrepancy can potentially be overcome using an additional step of isolation of more pure microglia using very specific cell type markers of the microglial lineage such as Iba-1 and F4/80 conjugated to magnetic beads.
Most studies that assess the role of microglia in neurological diseases that affect the spinal cord have been conducted using cells from the neonatal brain, and while this is a solid approach for answering questions about a role for microglia in normal development as well as pathologies affecting the brain specifically, we felt that a more specific method had to be established to study microglia function in the adult spinal cord. Our neonatal method is a very efficient way to answer most questions regarding the normal functioning of the brain as well as in certain pathological conditions, and the adult method is a good alternative to the use of neonatal cells for such studies given the variability in immunological responses between neonatal microglia and adult cells and between microglia from the brain and the spinal cord 6-9. Microglia obtained from both methods described are also appropriate for in vitro co-culture studies (we primarily culture them with neurons or oligodendrocytes in our lab) that address the effect of microglia on other cell types as well as the neonatal and adult microglial response to activating stimuli.
Disclosures
Authors have nothing to disclose.
Acknowledgments
We would like to thank members of the Tsirka lab for their advice and helpful comments. This work was supported by R01NS42168 to SET, 12PRE12060489 to RB, an NSF-3MT IGERT and a Turner Dissertation fellowship to LT, and NSF-3MT IGERT to JCN.
References
- Ransohoff RM, Perry VH. Microglial Physiology: Unique Stimuli, Specialized Responses. Ann. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler M, Conway S, Ng L, Stanley E, Samokhavalov I, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330 doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Env. Res. Pub. Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero J, Chancey J, Enikolopov G, Overstreet-Wadiche L, Tsirka S, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmetsberger J, Tsirka S. Microglial inhibitory factor (MIF/TKP) mitigates secondary damage following spinal cord injury. Neurobiol. Dis. 2012;47:295–309. doi: 10.1016/j.nbd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tsirka SE. Animal Models of MS Reveal Multiple Roles of Microglia in Disease Pathogenesis. Neurol Res. Int. 2011. p. 383087. [DOI] [PMC free article] [PubMed]
- Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nature Neurosci. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK. Immune response by microglia in the spinal cord. Ann. N.Y. Acad. Sci. 2010;1198:271–278. doi: 10.1111/j.1749-6632.2010.05536.x. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Tan S, Wills TE, Porritt MJ, Howells DW. Comparison of inflammation in the brain and spinal cord following mechanical injury. J. Neurotrauma. 2008;25:1217–1225. doi: 10.1089/neu.2007.0308. [DOI] [PubMed] [Google Scholar]
- Schell JB, Crane CA, Smith MF, Roberts MR. Differential ex vivo nitric oxide production by acutely isolated neonatal and adult microglia. J. Neuroimmunol. 2007;189:75–87. doi: 10.1016/j.jneuroim.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan CA, Roberts MR. Resident microglia from adult mice are refractory to nitric oxide-inducing stimuli due to impaired NOS2 gene expression. Glia. 2004;48:120–131. doi: 10.1002/glia.20066. [DOI] [PubMed] [Google Scholar]
- Tamashiro TT, Dalgard CL, Byrnes KR. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp. 2012. p. e3814. [DOI] [PMC free article] [PubMed]
- Yip PK, Kaan TK, Fenesan D, Malcangio M. Rapid isolation and culture of primary microglia from adult mouse spinal cord. J. Neurosci. Methods. 2009;183:223–237. doi: 10.1016/j.jneumeth.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. J. Immunol. Meth. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M-È, Majewska A. A role for microglia in synaptic plasticity. Comm. Integr. Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Expt. Neurol. 1998;149:329–340. doi: 10.1006/exnr.1997.6738. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Cerase J, Menéndez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- Min K-J, Yang M-S, Kim S-U, Jou I, Joe E-H. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J. Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmono R, Oceandy D, Pollard J, Tong W, Pavli P, Wainwright B, Ostrowski M, Himes S, Hume D. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- Ekdahl C. Microglial activation - tuning and pruning adult neurogenesis. Front Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, McEwen B, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]


