Abstract
Dengue virus (DENV) is considered to be the most important arthropod-borne viral disease and causes more than 100 million human infections annually. To further characterize primary DENV infection in vivo, rhesus macaques were infected with DENV-1, DENV-2, DENV-3, or DENV-4 and clinical parameters, as well as specificity and longevity of serologic responses, were assessed. Overt clinical symptoms were not present after infection. However, abnormalities in blood biochemical parameters consistent with heart, kidney, and liver damage were observed, and changes in plasma fibrinogen, D-dimers, and protein C indicated systemic activation of the blood coagulation pathway. Significant homotypic and heterotypic serum immunoglobulins were present in all animals, and IgG persisted for at least 390 days. Serum neutralizing antibody responses were highly serotype specific by day 120. However, some heterotypic neutralizing activity was noted in infected animals. Identification of serotype-specific host responses may help elucidate mechanisms that mediate severe DENV disease after reinfection.
Introduction
Dengue virus (DENV) is a mosquito-transmitted, positive-sense, single-stranded RNA virus in the family Flaviviridae and genus Flavivirus.1 An estimated 2.5 billion persons reside in DENV-endemic or DENV-epidemic regions, and more than 100 million DENV infections occur each year, ranking it among the most medically significant arthropod-borne diseases.2,3 Dengue virus is divided into four distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) and each has a worldwide distribution coexistent with the mosquito transmission vectors Aedes aegypti and Ae. albopictus as a secondary vector.3,4 Urbanization, international travel, insufficient public health measures, and decreasing vector control programs have facilitated the rapid expansion of the Ae. aegypti vector in urban areas and an increasing number of DENV infections each year.3 Southeast Asia is among the regions most affected by DENV, and data suggest symptomatic cases of DENV have increased four-fold over 30 years with a cost of illness estimated to be U.S. $56 million annually.5
Non-human primates (NHPs) are the only other known mammalian reservoir of DENV and clinical isolates from the human transmission cycle do not require adaptation to replicate in rhesus macaques; however, in all cases, overt clinical disease is not present in infected animals.6–8 After primary DENV challenge, rhesus macaques developed low-grade transient viremia, moderate lymphadenopathy, and robust immune responses.9 Secondary DENV challenge studies have demonstrated that lymphadenopathy, splenomegaly and hepatomegaly develop in rhesus macaques, and some animals have rash, subcutaneous bleeding, and increased levels of liver enzymes.10 To date, there are limited reports detailing complete clinical and immunologic responses in DENV-infected NHPs, and most do not include data for animals infected with all four DENV serotypes or data documenting temporal changes in clinical responses for individual animals. Few studies detail the longevity of primary and secondary immune responses to all four DENV serotypes, and in most studies, antibody responses have typically been evaluated for 21 days post-challenge. Considering the wide spectrum of DENV disease in humans and the varied host response in outbred animals, such as NHPs, further studies examining each DENV serotype in individual animals could help further elucidate subtle serotype-specific differences in vivo. Moreover, more complete temporal clinical and immunologic data sets from individual DENV-infected NHPs would be useful for understanding how each DENV serotype affects overall host homeostasis.
Because DENV infection of NHPs induces immune responses that are similar to human anti-DENV humoral responses, these animals have been used extensively to study the best DENV isolates for vaccine studies and potential immunogenicity of various DENV isolates or vaccine candidates.4,11–15 Specifically, in rhesus macaque studies, vaccines for all DENV serotypes have been evaluated. However, viremia and neutralizing antibody immediately after challenge have been used as correlates of protection, but host immunity over longer periods of time remains largely unknown.16,17 Other NHP models have also been investigated. However, most of the studies evaluated viremia after DENV-1 and DENV-2 challenge aiming to identify the best strain for vaccine studies.18–20 In all of these NHP studies, comprehensive clinical responses to each of the DENV serotypes have not been described after DENV vaccination and challenge. Although cell-mediated responses and inflammatory cytokine transcription has been assessed in DENV-infected cynomolgus macaques,21,22 a more in-depth understanding of serotype-specific immunologic and clinical responses to DENV infection in vivo may identify useful clinical measures relevant to evaluating efficacy of potential DENV vaccine and therapeutic candidates.
In this study, rhesus macaques were infected with human DENV isolates of each serotype and immune responses were monitored for 13 months post-infection (pi). Clinical parameters were measured for each animal after DENV challenge including, hematology, serum clinical biochemistry, plasma coagulation assays, systemic viral loads, and plasma cytokine levels. Clinical responses to DENV were highly similar among groups of infected animals. However, serotype-specific differences in viremia and blood homeostatsis were observed. For all DENV-infected animals, high levels of homotypic neutralizing antibody and low levels of heterotypic non-neutralizing antibodies persisted for over a year. These data suggest that infection with each serotype may lead to slightly different responses in vivo. However, host antibody responses to DENV serotypes are similar and long-lived.
Materials and Methods
Tissue culture.
The African green monkey kidney (Vero; ATCC CCL81) cell line was obtained from M.J. Cardosa (Penang, Malaysia). Cells were maintained in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY) supplemented with 10% calf serum (Hyclone, Logan, UT), Glutamax (Gibco), and 50 U/mL penicillin/50 μg/mL streptomycin (Gibco) (DMEM-10) at 37°C in a humidified 5% CO2 chamber.
Viruses.
DENV-1 (strain Western Pacific), DENV-2 (strain NGC), DENV-3 (strain Sleman/78), and DENV-4 (strain 814669) were low-passage virus isolates provided by Dr. Steve Whitehead (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD and have been described.15 Briefly, DENV-1 and DENV-4 isolates had been amplified twice on Vero cells, and DENV-2 was amplified once on Vero cells and again with C6/36 mosquito (Ae. albopictus) cells. DENV-3 was passaged twice on C6/36 cells, five times on Vero cells, and again on C6/36 cells before use. These DENV stocks have been used in DENV vaccine studies, and all four stocks have been shown to lead to productive infection in NHPs.15 Japanese encephalitis virus (JEV; vaccine strain SA14-14-2) was obtained during participation in the study reported by Ferguson and others23 and amplified once in C6/36 cells before use in the animals.
For in vitro studies, virus stocks were further amplified by inoculating Vero cell monolayers (70–80% confluence in T-25 flasks) with 200-400 μL of DENV or JEV stock. Infected cell monolayers were incubated with continuous rocking in a 37°C in a humidified 5% CO2 chamber. After 1 hour, cultures were supplemented with 4 mL of DMEM medium containing Glutamax, penicillin/streptomycin, and 5% heat-inactivated fetal bovine serum (Gibco) (DMEM-5). Virus infected cell monolayers were cultured at 37°C in a humidified 5% CO2 chamber and medium was refreshed with DMEM-5 after four days. For DENV, culture supernatant was harvested 6–7 days pi and clarified by centrifugation (15 min at 1,100 × g). For JEV, culture supernatant was harvested day 5 pi and clarified by centrifugation. Aliquots of amplified virus stocks were maintained at −80°C until use.
Virus titer was determined by standard virus titration assay using Vero cell lines. In brief, cells were grown to 80% confluence in 24-well plates (Fisher, Pittsburgh, PA). Culture medium was replaced with 100 μL of virus stock serially diluted (log dilutions) in DMEM medium supplemented with Glutamax, penicillin/streptomycin, and 3% heat-inactivated fetal bovine serum (DMEM-3). Flavivirus infections were maintained at 37°C in a humidified 5% CO2 chamber with continuous rocking. After 1 hour, an additional 500 μL of DMEM-3/1% carboxymethylcellulose sodium salt (Sigma, St. Louis, MO) (DMEM-3/1% CMC) was added to each well. Virus titration plates were incubated at 37°C in a humidified 5% CO2 chamber for 5 days. Titer plates were washed twice in phosphate-buffered saline (PBS) and fixed in 80% methanol for 20 minutes at room temperature. Fixed plates were stored at −20°C and foci were stained as described below.
Animals.
Animal studies were performed in accordance with guidelines promulgated by the Animal Welfare Act, state and Federal statue, Guide for the Care and Use of Laboratory Animals,24 National Research Council, and Boston University Institutional Animal Care and Use Committee (approved protocol #AN-15148.2012.10). The research facility was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Serum samples were collected from adult rhesus macaques (Macaca mulatta) and screened by using an enzyme-linked immunsorbent assay (ELISA) for pre-existing virus-specific antibody against DENV (DENV-1, DENV-2, DENV-3, DENV-4), JEV), yellow fever virus (YFV), and West Nile virus (WNV) as described below. Twenty juvenile seronegative animals with weights of 4–6 kg were selected for the study. Animals were pair-housed unless suitable matches could not be made because of temperament. Health, diet, and behavior were monitored daily, and animals were provided continual access to environmental enrichment. All animals were sedated for DENV inoculations and phlebotomy and all procedures were performed by Boston University laboratory animal veterinarians and qualified veterinary technical staff.
Prescreening flavivirus ELISAs.
For screening rhesus macaques serum samples before challenge, commercially available ELISA kits for DENV-1, DENV-2, DENV-3, JEV and WNV from Calbiotech (DENV; Spring Valley, CA) and Diagnostic Automation, Inc. (JEV and WNV; Calabasas, CA) were used, and each ELISA was preformed according to the manufacturer's recommendations. For DENV-4 and YFV, undiluted viral supernatants were coated directly on 96-well microtiter plates (Nunc, Roskilde, Denmark) at room temperature for 2 hours. Virus was removed and wells were blocked with 200 μL 5% milk/PBS for 2 hours. Excess blocking solution was removed and 100 μL of the serum sample diluted 1:5 in 5% milk/PBS was added to the assay plate in triplicate and incubated for at least 2 hours with shaking at room temperature. Assay plates were washed four times with 0.05% Tween–20 (Sigma-Aldrich, St. Louis, MO)/PBS and 100 μL of horseradish peroxidase (HRP)–conjugated goat anti-human antibodies (Pierce, Rockford, IL) diluted 1:5,000 in 5% milk/PBS was added to each well and plates were incubated for 1 hour at room temperature. Assay plates were washed four times and 100 μL of colorimetric substrate 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] diammonium salt (Roche Applied Sciences, Indianapolis, IN) was added to each well. After 10–15 minutes of color development, optical density (OD) was measured (405 nm wavelength) by using the Versamax microplate reader (Molecular Devices Corp., CA) with SoftMax Pro version 5.3 software (Molecular Devices).
DENV inoculations.
Groups of animals (n = 4) were given 105 plaque-forming units (PFU) of DENV-1, DENV-2, DENV-3, DENV-4 or JEV. For DENV, isolates received from Dr. Whitehead (National Institute of Allergy and Infectious Diseases, National Institutes of Health) were not passaged or cultured before use in rhesus macaque. Sterile virus inoculum was delivered by subcutaneous inoculation to sedated animals. Thereafter, veterinary and husbandry staff monitored animals at least twice a day for health and behavioral changes for two weeks after challenge and then daily thereafter.
Specimen collection.
Whole blood samples were collected in EDTA, sodium citrate, and untreated vacutainer blood collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) 22 or 10 days preceding challenge (denoted as day 0 in text and figures), as well as on days 5, 6, 7, 8, 10, and 14 pi. The total volume of whole blood sample obtained did not exceed the maximum volume recommended by the Guide for the Care and Use of Laboratory Animals,24 the Boston University Institutional Animal Care and Use Committee, and laboratory animal care veterinarians. Untreated whole blood samples were allowed to clot at room temperature, and blood fractions were separated by centrifugation at 700 × g for 15 minutes. The serum fraction was harvested, aliquoted, and used immediately or stored at −80°C. The blood plasma fraction was isolated from sodium citrate–treated whole blood by centrifugation (900 × g for 15 minutes) and used immediately to assess coagulation homeostasis. Separate 140-μL aliquots of EDTA-treated whole blood sample were diluted in either 600 μL AVL (QIAGEN, Valencia, CA) for RNA extraction or 600 μL of sterile PBS (Invitrogen, Carlsbad, CA) for virus isolation. Diluted samples for virus isolation were immediately stored at −80°C. Samples for RNA isolation were homogenized by using a mini-bead beater as described,25 and RNA was purified by using the QIAmp Viral RNA Mini Kit (QIAGEN) according to the manufacturer's directions. Blood RNA isolations were performed with QIACube automated systems (QIAGEN), and purified RNA product was stored at −80°C. Additional serum samples were collected at 30-day intervals (days 30, 60, 90, 120, 135, 180, 210, 237, 270, 300, 360, and 390) pi.
Blood biochemical and hematologic analysis.
Samples of serum and EDTA-treated whole blood were submitted to two veterinary diagnostic laboratories (Idexx, Westbrook, ME or Cummings School of Veterinary Medicine at Tufts University, Grafton, MA) for clinical biochemistry analysis and complete blood counts, respectively. Analysis included measurement of serum concentration of albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), amylase (AMY), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatine kinase (CK), γ-glutamyltransferase (GGT), glucose (GLU), globulin (GLOB), total protein (tPRO), cholesterol (CHOL), urea, calcium (Ca), carbon dioxide (tCO2), chloride (Cl), sodium (Na), potassium (K), and phosphorus (Phos), as well as absolute white blood cell count (WBC), neutrophil (NEU) count, NEU %, lymphocyte (LYM) count, LYM %, monocyte (MONO) count, MONO %, eosinophil (EOS) count, EOS %, basophil (BAS) count, BAS %, red blood cell count (RBC), hemoglobin (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW), platelet count (PLT), and mean platelet volume (MPV). Blood measures were defined by comparison with prebleed values and previous described reference hematologic ranges for rhesus macaques.26
Blood coagulation factors prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), d-dimer (D-Di), fibrinogen (FIB), protein C (PROC), and protein S (PROS) were determined by using an STA Compact Blood Analyzer and instrument-specific reagents (Stago, Parsippany, NJ) according to the manufacturer's recommendations.
Taqman reverse–transcriptase polymerase chain reaction.
Reverse-transcriptase real-time PCR (RT-PCR) was performed as described.25 Samples, no-template control, and DENV RNA standards derived from infectious virus of known titer were assessed in triplicate by using the one-step RT–PCR QuantiFast Probe PCR Kit (QIAGEN). For each serotype-specific DENV RNA standard and JEV RNA standards, RNA was extracted from infectious virus, eluted in a volume of 60 μL, and serially diluted such that 2 μL represented genomic material from 105, 103 or 101 PFU. All reactions contained 2 μL of purified RNA diluted in 23 μL of RT-PCR master mixture consisting of 12.5 μL of Quantifast master mixture, 0.625 μL of Quantifast RT enzyme, 5.75 μL of distilled water, 1.25 μL each of 18 μM DENV or JEV forward and reverse primer (Integrated DNA Technologies, Coralville, IA), and 1.25 μL of 5 μM DENV or JEV FAM-labeled probe (Integrated DNA Technologies). Primer/probe sets were developed specifically for each DENV serotype and JEV by using primer/probe design software (Integrated DNA Technologies). Specific primer and probe sequences are shown in Table 1. Each assay was performed by using optical 96-well microtiter assay plates (BioRad, Hercules, CA) sealed with optical clear film (BioRad) and run on the CXF96 Real-time PCR Detection System (BioRad) with the following parameters: 50°C for 15 minutes, 95°C for 5 minutes, and 40 cycles of 95°C for 5 seconds and 60°C for 10 seconds. To control for RT-PCR plate-to-plate variability, DENV or JEV RNA standards were run on each plate, representing 105, 103 and 101 PFU, and cycle threshold (Ct) values were set to 100, 10 and 1 relative units, respectively. For biological samples, CFX software (BioRad) calculated the relative amount of RNA per milliliter by using linear regression based on Ct values for purified DENV or JEV RNA standards and their assigned relative values. The limit of detection for each RT-PCR, based on RNA extracted from infectious virus, was the equivalent of 100.01 PFU.
Table 1.
Serotype-specific DENV and JEV oligonucleotide primer and probe sequences*
| Virus | Forward primer, 5′→3′ | Reverse primer, 5′→3′ | Probe, 5′→3′ |
|---|---|---|---|
| DENV-1 | TCAAAGGAGAAGACGGGTGTTGGT | TGACCCTGCAGAGACCAATGACTT | FAM-ACGGCATGGAAATCAGACCAGTCAAGGA-TAM |
| DENV-2 | GAAATGGGTGCCAACTTCAAGGCT | TCTTTGTGCTGCACTAGAGTGGGT | FAM-ACTAACAGATGGTGAAGAGCGGGTGA-TAM |
| DENV-3 | AGAAGGTCAGAACCAACGCAGCTA | TGTAAACGCAGCTTCCACACTTGC | FAM-AAGAGAACCAATGGGACAGTGCGAGA-TAM |
| DENV-4 | ATCTCTAGCGGCCATTGCTAACCA | AATAGCATCCCATTGCCAACAGCG | FAM-AAAGGATGGCCGCTCCACAGAATGGA-TAM |
| JEV | ACCTGAGACAAAGGAATGCCCTGA | CACACGGGTTGATGTGATGCCAAA | FAM-AGCACAGAGCTTGGAACAGCATGCAA-TAM |
DENV = dengue virus; JEV = Japanese encephalitis virus.
Multiplexed serum cytokine assay.
Cytokines assayed included interleukin (IL)-2, IL-8, IL-10, IL-12, interferon (INF)-γ, monocyte chemotactic protein-1(MCP-1), and tumor necrosis factor (TNF)-α. Antibody-coated magnetic microspheres optimized for quantifying nonhuman primate specific cytokines (Invitrogen) were premixed and the multiplexed assay was performed according the manufacturer's instructions with the following specifics: 50 μL of serum was used per sample and the cytokine capture was performed overnight at 4°C. Biotinylated cytokine detection antibodies (Invitrogen) and streptavidin–phycoerythrin (Invitrogen) were used at the manufacturer's recommended dilution. Assays were performed on a Luminex® 200 IS™ machine equipped with Bio-Plex Manager Software (version 5.0) (Bio-Rad). Median fluorescent intensity across 50 beads was determined for each sample. Cytokine concentration was determined by regression analysis using observed M.F.I values from standard curves of each cytokine. Regression analysis was performed by using logistic-5PL regression analysis in Bio-Plex Manager Software.
DENV focus reduction neutralization test.
The DENV focus reduction neutralization test (FRNT) procedure was based on methods described.14,27 Briefly, rhesus immune serum and negative control serum were heat-inactivated at 56°C for 45 minutes. Each serum sample was serially diluted from 1:16 to 1:16,384 in DMEM-3 and mixed with DENV (approximately 35 focus forming units) in equal volumes. Mixtures were incubated at 37°C for 45 minutes in a humidified 5% CO2 chamber with continuous rocking. Growth media (DMEM-10) was removed from Vero cell monolayers cultured overnight to 80% confluence in 24-well culture plates (BD Falcon, Franklin Lakes, NJ). Duplicate wells of Vero cells were inoculated with 100 μL of serum/virus dilution, incubated at 37°C for 1 hour in a humidified 5% CO2 chamber with continuous rocking, and subsequently overlayed with 500 μL of DMEM-3/1% CMC/well. Plates were maintained at 37°C in a humidified 5% CO2 chamber for five days. Plates were washed twice in PBS and fixed in 80% methanol for 20 minutes at room temperature. Fixed plates were stored at −20°C until staining.
DENV focus staining protocol.
Fixed plates were warmed to room temperature just before staining. Methanol fix was removed, and each well was blocked in a 5% milk/PBS solution for 10 minutes at room temperature. Blocking solution was replaced with 200 μL of 5% milk/PBS containing 4 μg/mL of purified mouse monoclonal antibody (mAb) 2H2 (DENV premembrane protein specific;28 American Type Culture Collection,, Manassas, VA) and plates were incubated for 2 hours at 37°C with continuous rocking. Plates were washed 3 times in PBS and incubated for 1 hr at 37°C with continuous rocking in 200 μL 5% milk/PBS solution containing HRP conjugated goat anti-mouse antibodies (Sigma Aldrich) diluted 1:500. Plates were washed three times in PBS and rocked continuously with 150 μL of TrueBlue peroxidase colorimetric substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). After sufficient color development (usually ≤ 8 minutes), substrate was removed and plates were washed once with distilled water. The total number of DENV foci for each well were counted, and the 50% reduction in virus foci (FRNT50) titer was determined by regression analysis relative to total count for wells incubated with test serum or virus alone wells (seronegative control serum diluted 1:16). The FRNT50 titer was calculated by using Graphpad Prism 6 software (Graphpad Software, La Jolla, CA).
Antibody-capture DENV ELISAs.
Serotype-specific DENV IgM and IgG responses were measured by using components of the DENV-JEV MACE Kit (Venture Technologies, Penang, Malaysia) and anti-human IgG capture antibodies. In brief, 96-well microtiter plates (Nunc) were coated overnight with 100 μL of rabbit polyclonal anti-human IgM or IgG (Dako, Glostrup, Denmark) diluted 1:1,000 in 0.045 M sodium bicarbonate/0.018 M sodium carbonate solution. Coat solution was removed and the wells were blocked with 200 μL 0.5% bovine serum albumin (BSA; American Bioanalytical, Inc., Natick, MA)/PBS for 2 hours. Excess blocking solution was removed, and 100 μL of the serum sample diluted 1:50 in 0.1% BSA/PBS was added to the assay plate in triplicate and incubated for at least 2 hours with shaking at room temperature. Assay plates were washed six times with 0.05% BSA/PBS. To each well, 100 μL of inactivated DENV-1, DENV-2, or DENV-3 antigen (Venture Technologies) diluted 1:2 in 0.1% BSA/PBS or stock DENV-4 virus diluted 1:2 in 0.1% BSA/PBS was added, and plates were incubated overnight at 4°C. Plates were washed as described above and incubated for 1 hour at room temperature with 100 μL of DE1 + JE1 mouse mAb mixture (Venture Technologies) diluted 1:5 in 0.1% BSA/PBS (DENV-1, DENV-2, and DENV-3 assays) or DENV-4 specific mouse mAb E2 (BEI Resources, Manassas, VA) diluted 1:100 in 0.1% BSA/PBS (DENV-4 assays). Assay plates were washed as before, and 100 μL of HRP-conjugated rabbit polyclonal anti-mouse antibodies (Dako) diluted 1:1,000 in 0.1% BSA/PBS was added to each well, and plates were incubated for 1 hour at room temperature. Antigen captured by test serum samples was detected by using 100 μL/well of colorimetric substrate 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] diammonium salt (Roche Applied Sciences). After 10–15 minutes of color development, OD was measured (405 nm wavelength) by using the Versamax microplate reader (Molecular Devices Corp.) with SoftMax Pro version 5.3 software (Molecular Devices).
Serum library repository.
Serum samples obtained before infection and selected serum samples from days 30, 120, 150, 180, 210, 237, 270, 300, 330, 360, and 390 were deposited in the Biodefense and Emerging Infections Research Resources Repository (BEI Resources).
Results
Non-human primate infections.
Prior to challenge, rhesus macaques were assessed for general health and evidence of previous/current exposure to DENV, WNV, JEV, and YFV. All animals included in the study were in good general health, seronegative by ELISA, and negative for DENV, JEV, and YFV genomic RNA as determined by real-time PCR. Groups of four animals were inoculated subcutaneously with 105 PFU of DENV-1 (strain Western Pacific), DENV-2 (strain NGC), DENV-3 (strain Sleman/78), DENV-4 (strain 814669), or JEV (strain SA 14-14-2). Animal health, blood biochemistry, complete blood counts, viral loads, cytokine levels, and blood coagulation were determined for two weeks after challenge. Serum IgM, IgG, and neutralizing antibodies were also quantified after infection. Animal numbers, sample collection, virus challenge, and study timeline are summarized in Figure 1.
Figure 1.
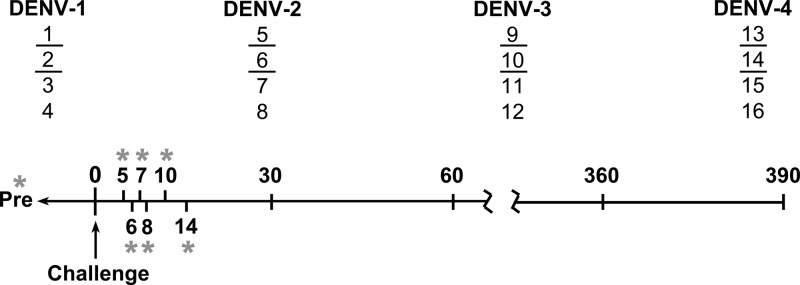
Schematic timeline summarizing dengue virus (DENV) challenge study and clinical sampling schedule. Sixteen DENV-seronegative rhesus macaques were divided into four groups, each consisting of two females (underlined numbers) and two males as indicated. Each group was inoculated subcutaneously with 105 plaque-forming units of DENV-1 (strain Western Pacific), DENV-2 (strain NGC), DENV-3 (strain Sleman/78), or DENV-4 (strain 814669), and clinical and antibody responses were evaluated over time. Blood samples were collected repeatedly over the first two weeks post-infection (asterisks), including before challenge (Pre) and on days 5, 6, 7, 8, 10, and 14 as indicated. Additional serum samples were collected on day 30 and at 30-day intervals thereafter until day 390.
A previous study reported clinical symptoms in rhesus macaques including rash and hemorrhage after DENV challenge with 1×107 PFU; however, hemorrhagic symptoms have not been observed at lower inoculating doses9,11 In our study, overt symptoms, including rash or visible signs of hemorrhagic illness, did not develop in inoculated rhesus macaques. Furthermore, animal behavior remained consistent during the weeks after challenge. These results are consistent with prior reports of experimental infections of NHPs with lower doses of DENV.
Quantitation of DENV genomic RNA in blood.
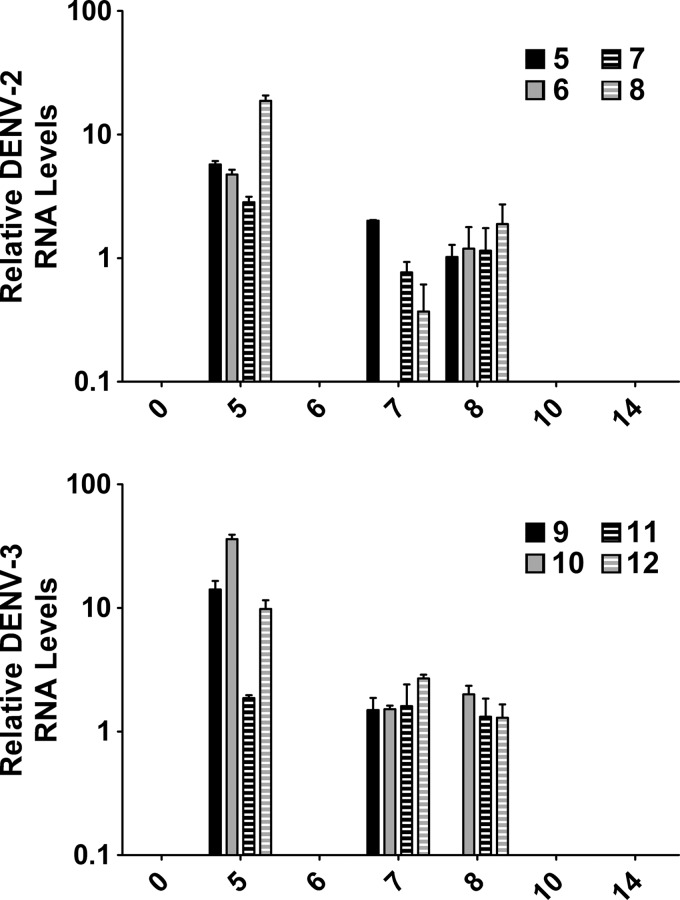
One-step Taqman real-time RT-PCRs were developed with DENV serotype-specific primer/probe combinations and validated by using a range of DENV RNA standards derived from infectious virus with known titer. All primer and probe sequences are shown Table 1. For each blood sample, RNA was column extracted immediately after collection and used in RT-PCR. To control for RT-PCR plate-to-plate variability, DENV RNA standards were run on each plate, representing 105, 103 and 101 PFU, and Ct values were set to 100, 10, and 1 relative units, respectively. For each blood sample, the Ct value was converted to a relative quantity of DENV genomic RNA by using linear regression based on Ct values from DENV RNA standard curves and their relative units. For DENV-2- or DENV-3-infected animals, peak detection of DENV genomic RNA was on day 5 pi and persisted in most animals until day 8 (Figure 2). Although virus-specific RNA was not detected in samples from day 6 pi, daily variation in detection of RNA genome in the blood may occur during viral infection.29 Surprisingly, DENV genomic RNA was not detected in any blood samples from DENV-1- or DENV-4 infected animals. Viremia in DENV-1- and DENV-4 infected animals may have been undetectable because of possible differences in the route of infection compared with those in previous DENV vaccine studies15 and differences in virus passage history as compared with DENV-2 and DENV-3. Alternatively, the DENV genomic RNA may have peaked and resolved before day 5 or levels of RNA were below the sensitivity of the RT-PCR.
Figure 2.
Relative quantitation of dengue virus (DENV) genome in blood after challenge. RNA was purified from whole blood and DENV RNA genome was quantitated for each sample in triplicate by using the Quantifast Probe one-step reverse transcription–polymerase chain reaction. DENV genomic RNA was detected by using the CFX96 polymerase chain reaction system, and relative genomic RNA quantity was calculated by using instrument software and linear regression based on RNA standards extracted from infectious virus with known titer. The mean and SEM are shown on the y-axis for positive samples that only included DENV-2-infected and DENV-3-infected animals. Day post-infection is shown on the x-axis and animal numbers for each serotype are indicated.
Primary IgM response after DENV infection.
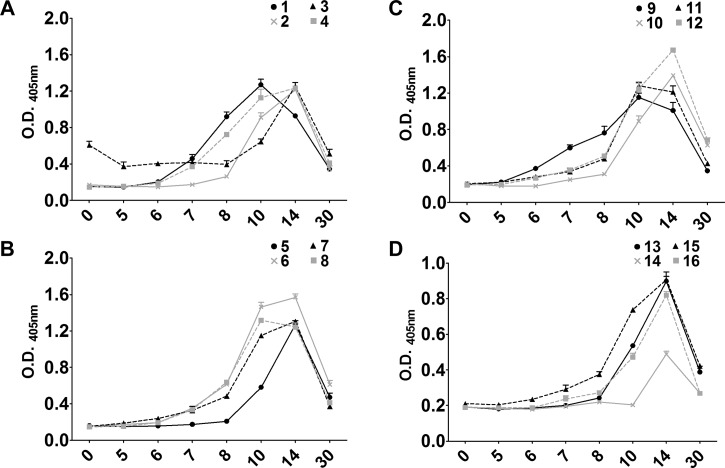
To further assess infection in DENV-2- and DENV-3-infected animals and determine if animals were infected with DENV-1 and DENV-4, serum IgM levels were determined. Serum IgM responses to primary DENV exposure were detected by using an IgM-capture ELISA as detailed in the Materials and Methods. Despite serotype differences in viremia, similar IgM responses were detected in all animals after infection (Figure 3). The DENV homotypic IgM responses were detected between day 8 and day 10 pi and peaked at days 10–14. IgM was still detectable on day 30; however, levels had decreased significantly. The time to detection of initial IgM responses differed between groups, which could result from differences in individual serotype immunogenicity, virus kinetics in the host, or host variability. Not unexpectedly, in all animals, serum IgM responses exhibited high cross-reactivity with heterotypic DENV serotypes. These results suggest a productive infection in all animals after DENV inoculation and activation of the host adaptive immune response. Animals challenged with 105 PFU of JEV did not show development of a productive infection or JEV-specific neutralizing antibody responses and served as essentially uninfected control animals for baseline coagulation and cytokine profiles after repeated phlebotomy.
Figure 3.
Homotypic serum IgM levels after primary dengue virus (DENV) infection. Serum samples from days 0 (prebleed), 5, 6, 7, 8, 10, 14, and 30 were diluted 1:50 and assayed in triplicate by using IgM capture enzyme-linked immunosorbent assay reagents from Venture Technologies. Optical density (O.D.) was determined by measuring the absorbance at 405 nm, and the mean and SEM of each sample is shown on the y-axis. Day post-infection is shown on the x-axis, and animal numbers for each DENV serotype are indicated at the top right of each graph. A, DENV-1- infected animals; B, DENV-2-infected animals; C, DENV-3-infected animals; D, DENV-4 infected animals.
Temporal changes in white blood cell and platelet counts during DENV infection.
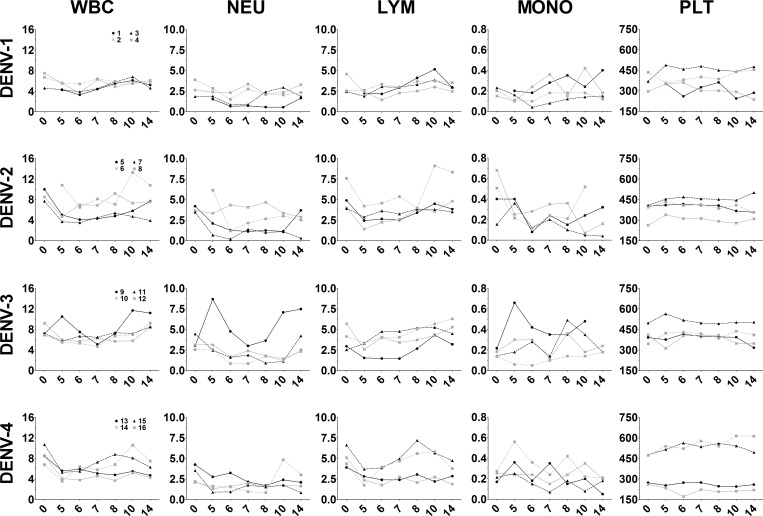
To assess changes in blood cell populations after DENV infection, complete blood counts were performed on whole blood samples from infected animals and results are shown in Figure 4. Leukopenia was observed in most infected animals and WBC absolute numbers were lowest during days 5–8 pi. Leukopenia was most evident in DENV-2-, DENV-3-, and DENV-4-infected animals and, although WBC levels increased in most animals by day 14, WBC absolute counts remained low in two DENV-2-infected and three DENV-4-infected animals. Not unexpectedly, the absolute number of neutrophils and lymphocytes were also decreased in all infected animals, except two DENV-3-infected animals, #9 and #11, respectively (Figure 4). Monocyte numbers fluctuated over the course of infection and varied greatly between animals and serotype. Nonetheless, these data suggest activation of macrophage populations in response to infection. Absolute platelet counts remained unchanged among infected animals. The WBC percentages also changed after infection with percent NEU decreasing, suggesting either virus-induced cell death or extravasation to sites of inflammation, and percent LYM increasing suggesting an ongoing immune response (Supplemental Figure 1). Similar to absolute number, MONO percentages fluctuated over the course of infection and varied greatly between animals and serotype.
Figure 4.
Temporal changes in white blood cells and platelets after dengue virus (DENV) challenge. Whole blood collected on days 0 (prebleed), 5, 6, 7, 8, 10, and 14 post-infection were submitted to a veterinary diagnostic service laboratory for analysis. The absolute cell counts (×106 cells/mL) for each animal are shown on the y-axis. WBC = white blood cells, NEU = neutrophils; LYM = lymphocytes; MONO = monocytes; PLT = platelets. Day post-infection is shown on the x-axis. Animal numbers for each DENV serotype are indicated in the left column and animal numbers are the same for each panel in the same row.
Serum cytokine changes during DENV infection.
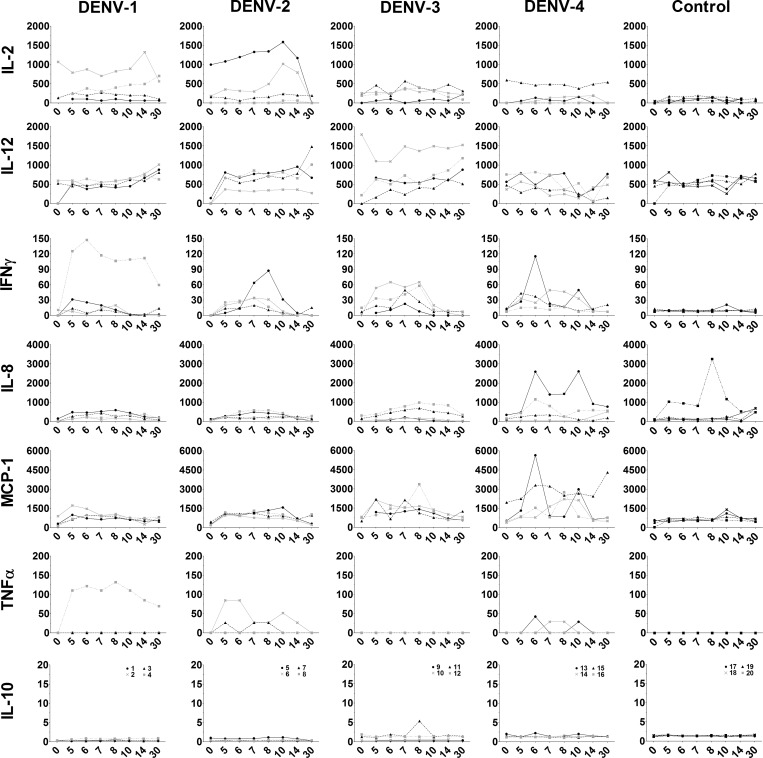
To further characterize the initial immune response to DENV, serum cytokine responses were measured by using multiplexed microsphere protein-based cytokine assays. Cytokines assayed included IL-2, IL-8, IL-10, IL-12, INF-γ, MCP-1, and TNF-α. As shown in Figure 5, uninfected control animals had detectable IL-12; however, concentrations did not change over time. One control animal had increased levels of IL-8 but all other cytokines assayed were undetectable in control animals. Conversely, increased serum levels of IL-2, INF-γ, IL-8, MCP-1, and TNF-α were observed 5–14 days after DENV infection, which is consistent with temporal detection of DENV genome in blood and observed WBC changes (Figure 5). For DENV-1-infected animals, one animal showed increased concentrations of IL-2, INF-γ, and TNF-α; however, cytokines were nearly undetectable in all other animals in this group. For DENV-2-infected animals, moderate increases in IL-2, IL-12, INF-γ, and TNF-α concentrations were evident in some animals. The DENV-3-infected animals had less IL-2 by comparison, and undetectable TNF-α. However, IFN-γ levels appeared to be higher and more sustained in all animals, and MCP-1 levels increased in most animals. Similarly, all DENV-4-infected animals had sustained increases in IFN-γ and MCP-1 levels and two animals had notable increases in IL-8 levels. Increases in cytokines elaborated by activated macrophages (IL-2 and TNF-α) and elevated concentrations of macrophage chemokines (IL-8 and MCP-1) affirm the important role for macrophages in the inflammatory response to DENV infection. Interestingly, the serotype-specific differences in serum cytokines levels further suggest possible virus-associated differences in host inflammatory responses.
Figure 5.
Serum cytokine changes after dengue virus (DENV) infection. Serum samples were analyzed by using nonhuman primate multiplexed microsphere protein-based cytokine assays. Analytes included interleukin (IL)-2, IL-8, IL-10, IL-12, interferon (INF)-γ, monocyte chemotactic protein-1(MCP-1), and tumor necrosis factor (TNF)-α. Serum samples collected from uninfected animals were used as controls. Cytokine concentration (pg/mL) is shown on the y-axis and was calculated using instrument software and linear regression based on observed concentrations from cytokine standards. Day post-infection is shown on the x-axis. Animal numbers for each DENV serotype are indicated in the bottom row of graphs and remain the same for each column.
Disruptions in blood homeostasis during DENV infection.
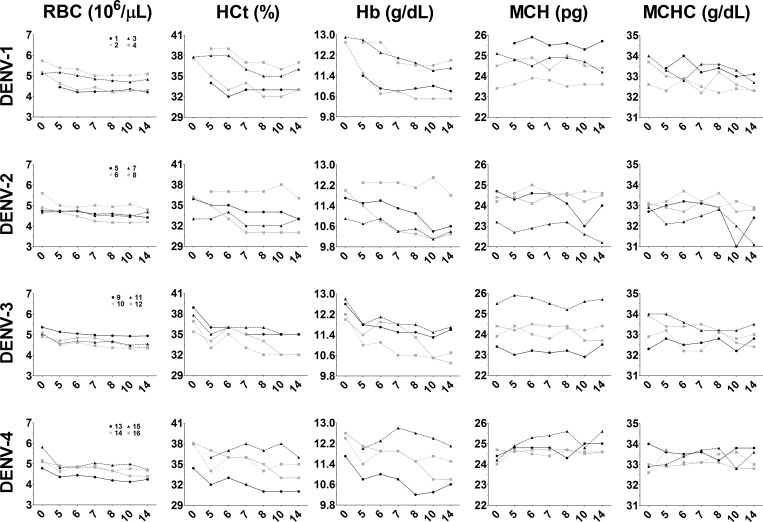
Because hemorrhage and rash can occur after DENV infection and both have been documented occasionally in rhesus macaques, we analyzed changes in RBCs after DENV infection. Absolute RBC counts remained unchanged during the full course of the study (Figure 6). However, decreases were observed in HCt and serum Hb levels beginning at day 5 pi, which persisted in most animals and is consistent with loss of blood. Mean corpuscular hemoglobin and MCHC remained largely unchanged during the entire period of observation, suggesting a normochromic anemia commonly resulting from acute blood loss, such as from repeated blood draws or vascular leakage such as hemorrhage. Decreased MCH and MCHC consistent with hypochromic anemia were observed in two DENV-2-infected animals (#5 and #7) beginning 10 days pi. Hypochromic anemia, often resulting from iron deficiency, in these animals suggests more pronounced disruptions in blood homeostasis.
Figure 6.
Abnormalities in blood hematologic results after dengue virus (DENV) infection. Blood samples collected on days 0 (prebleed), 5, 6, 7, 8, 10, and 14 post-infection were submitted to a veterinary diagnostic service laboratory for analysis. Shown are temporal changes in red blood cell count (RBC), hematocrit (HCt), hemoglobin (Hb), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). Day post-infection is shown on the x-axis. Animal numbers for each DENV serotype are indicated on the left and remain the same for each panel in a row.
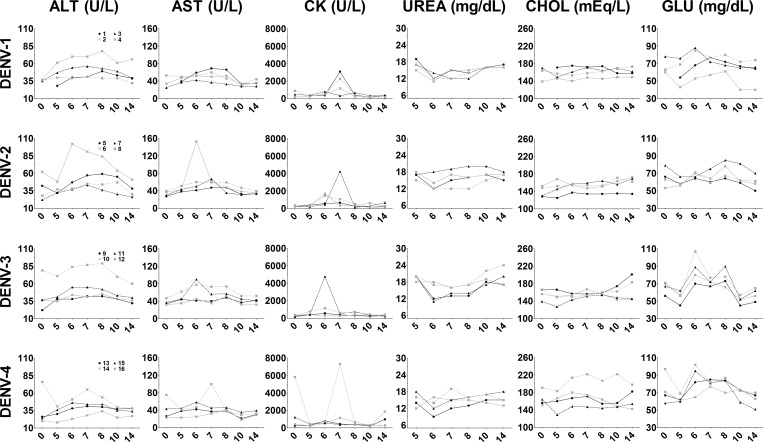
Serum hematologic analysis showed mild deregulation of blood biochemical results during infection coincident with observed cellular effects and viremia. Most animals had increased levels of serum ALT and/or AST during days 5–10 pi, which suggested tissue damage, particularly in liver and cardiac tissues (Figure 7). Creatine kinase, which is released after damage to cardiac and striated muscle, was also significantly increased among various animals during days 5–8 pi (Figure 7). Increased serum ALT, AST, and CK levels may suggest damage to cardiac muscle after DENV infection. These changes were noted in all groups of animals and were not specific for virus isolate or serotype. Similarly, during the same period post-infection, lowered serum urea levels were evident on day 6 in most animals, minor deregulation of serum cholesterol occurred in DENV-3- and DENV-4-infected animals, and increased serum glucose levels were noted in all animals over the 14-day sample period (Figure 7). Abnormal levels of AST, ALT. CK, UREA, CHOL, and GLU among most inoculated animals suggest the common occurrence of liver damage during DENV infection. Other serum proteins remained relatively unchanged during the observation period after infection, except for serum globulin, for which marked decreases were observed in all but one animal (Supplemental Figure 2).
Figure 7.
Fluctuations in serum biochemical results after dengue virus (DENV) infection. Serum samples were collected on days 0 (prebleed), 5, 6, 7, 8, 10, and 14 post-infection and submitted to a veterinary diagnostic service laboratory for analysis. Shown are temporal changes in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), urea (UREA), cholesterol (CHOL), and glucose (GLU). Day post-infection is shown on the x-axis. Animal numbers for each DENV serotype are indicated in the panel of graphs on the left and remain the same across rows.
Total CO2, a measure of the blood bicarbonate level, decreased in all animals (with the exception of #10) 5–6 days after DENV infection (Supplemental Figure 3). Decreased blood bicarbonate concentration correlates with mild blood acidosis and may suggest impairment of proper kidney function. Mild individual variations were observed in serum electrolyte levels (Supplemental Figure 3), which is suggestive of disruptions in blood biochemistry consistent with kidney impairment.
Activation of coagulation pathway during DENV infection.
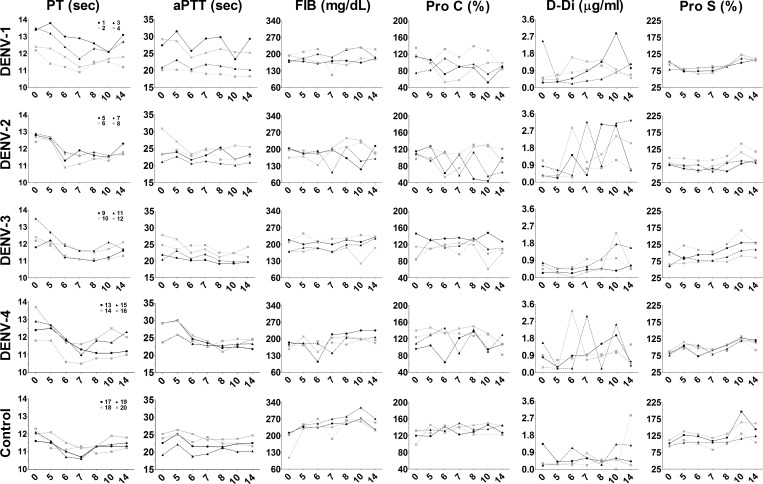
Uncontrolled activation of blood coagulation pathways during dengue hemorrhagic fever (DHF) can result in disseminated intravascular coagulation and hemorrhage.30 Although these changes are observed most commonly during re-infection, changes in blood biochemical results and circulating WBC populations suggest disruption of blood homeostasis and acute liver injury, which could exacerbate host coagulopathies.
To determine if coagulation deregulation was present in DENV-infected animals, automated diagnostic laboratory equipment was used to measure plasma concentration of specific coagulation factors, as well as specific component activity times. The DENV-infected animals did not exhibit abnormal blood clotting capacity because increased PT, aPTT, or TT were not evident with the exception of animal #1 where an increase in aPTT was documented (Figure 8 and Supplemental Figure 4). Plasma fibrinogen, which is cleaved to produce fibrin and mediates clot formation, was decreased in several infected animals. Marked decreases in Pro C, which contributes to regulating clot formation, vascular permeability, and inflammation were noted in most infected animals 5–10 days pi (Figure 8). Surprisingly, although viral RNA was detected in all animals inoculated with DENV-3, abnormal plasma concentration of FIB and Pro C were seen only in animal #10. Highly increased D-Di, a degradation product of fibrin, was found in nearly all infected animals, particularly among animals inoculated with DENV-2 or DENV-4 (Figure 8). These data suggest modulated activation of the systemic coagulation pathway among inoculated animals coincident with viremia and other abnormalities in blood homeostasis.
Figure 8.
Activation of the blood coagulation pathway after dengue virus (DENV) infection. Plasma was collected on days 0 (prebleed), 5, 6, 7, 8, 10, and 14 post-infection. Each sample was loaded on an automated STA Stago compact analyzer for measuring blood coagulation factors as described in the Materials and Methods. Shown are the temporal measures of prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer (D-Di), fibrinogen (FIB), protein C (PROC), and protein S (PROS) as indicated. Plasma samples collected from uninfected animals were used as controls. Day post-infection is shown on the x-axis. Animal numbers for each DENV serotype are indicated in the panel of graphs on the left and remain the same across rows.
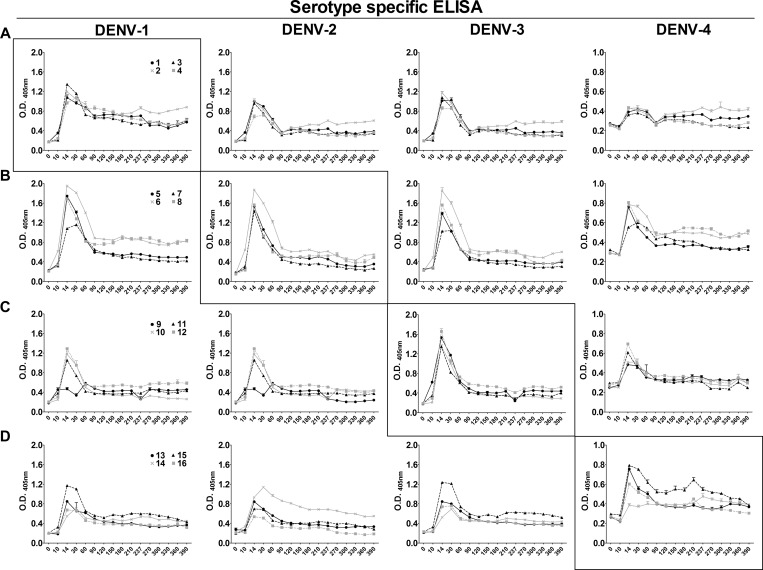
Durability of host IgG after DENV infection.
Serologic assays were performed to survey homotypic and heterotypic IgG responses for 390 days after DENV infection. Rapid and pronounced expansion of DENV-specific serum IgG occurred among all animals during days 10–14 pi (Figure 9). DENV-specific serum IgG remained increased for months and decreased to equilibrium levels by 90 days pi. The relative magnitude and antibody persistence of homotypic DENV responses were comparable for each DENV serotype (Figure 9, boxed panels). For each group of infected animals, heterotypic antibody responses were only slightly reduced as compared with homotypic responses, which suggests a high level of shared antigenic structure between each of the DENVs used in the study (Figure 9). Notably, DENV-2 infection led to the highest amounts of heterotypic antibody with almost no difference in homotypic and heterotypic antibody profiles. Interestingly, the DENV-1 and DENV-4 homotypic antibodies appear to persist at higher levels than DENV-2 and DENV-3 homotypic antibodies. Furthermore, pan-reactive heterotypic antibodies persisted through day 390, although levels were significantly lower than heterotypic antibody levels present in the first three months after infection.
Figure 9.
Rhesus macaque serum IgG responses after primary dengue virus (DENV) infection. Serum samples from days 0 (Pre) 5, 6, 7, 8, 10, 14, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 360, and 390 were diluted 1:50 and assayed in triplicate for IgG by using capture enzyme-linked immunosorbent assay (ELISA) reagents from Venture Technologies. The optical density (O.D.) was determined by measuring the absorbance at 405 nm, and the mean and SEM for each sample is shown on the y-axis. Day post-infection is shown on the x-axis. Boxed panels show serologic results for the homotypic DENV antibody responses. Animal numbers for each DENV serotype are indicated in the panel of graphs on the left and remain the same across rows. A, DENV-1-infected animals; B, DENV-2-infected animals; C, DENV-3-infected animals; D, DENV-4-infected animals.
To assess antibody function at later time points after infection, host neutralizing antibody responses were measured by FRNT. Serum samples collected monthly from day 120 through day 390 were tested against each of the four DENV serotypes, and neutralizing antibody titer corresponding to the FRNT50 was determined. All animals had significant homotypic neutralizing antibody titer on day 120, although titers varied between animals and serotypes (Table 2) . Serum neutralizing activity for animals inoculated with DENV-3 were highly serotype specific; there was almost no detectable cross-reactive antibody titer, whereas animals infected with the remaining DENV serotypes had varying levels of heterotypic neutralizing antibodies that persisted for different lengths of time. At 120 days pi, homotypic neutralizing antibody titers exceeded 1:100 for all animals and, in some, were greater than 1:7,000 (Table 2). Animals infected with DENV-1 or DENV-2 exhibited weak cross-neutralizing activity against DENV serotypes -1, −2, and -3 (Table 2). Although DENV-3 did not elicit detectable cross-reactive neutralizing antibodies, DENV-4 elicited notable cross-reactive antibodies to DENV-3. The neutralizing antibody response after infection with DENV-1, DENV-3, and DENV-4 was highly durable with little or no loss in FRNT50 titer during days 120–390 pi (Table 2). However, neutralizing antibody responses in animals infected with DENV-2 continued to decrease over the course of the study, decreasing more than a log in titer in two animals during days 120–390 pi (Table 2). The serum IgG profiles and neutralization titers demonstrate that non-neutralizing, pan-reactive, and serotype-specific neutralizing antibodies persist for over a year in DENV-infected animals. Cross-reactive neutralizing antibodies also persisted in half of the DENV-1-, DENV-2-, and DENV-4 infected animals.
Table 2.
Longevity of serum neutralizing antibody titer after DENV infection*
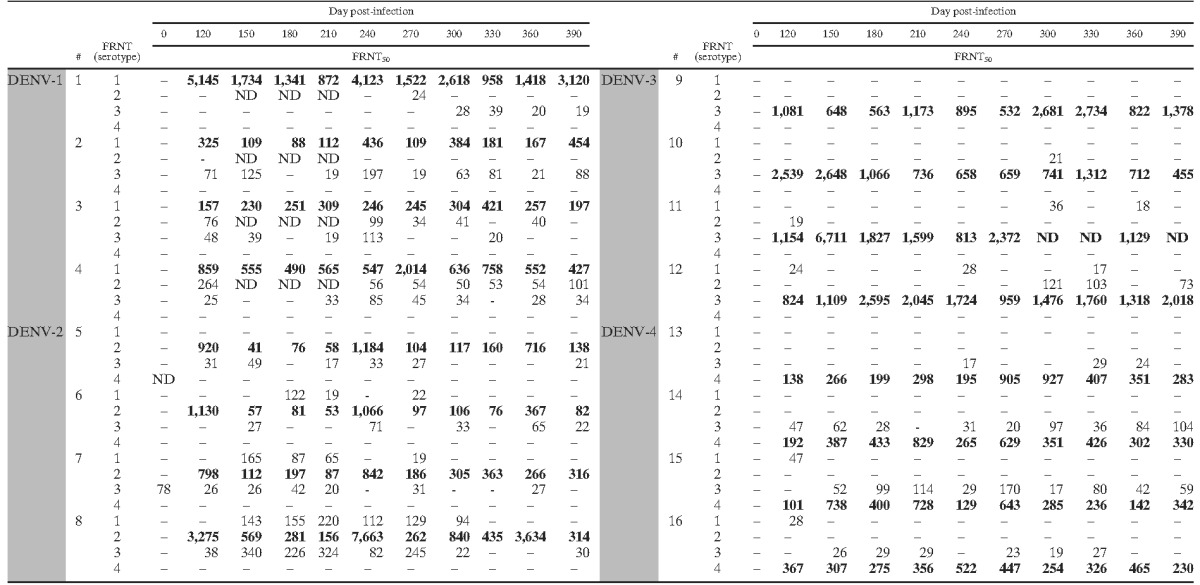
# = animal number; FRNT = focus reduction and neutralization test; FRNT50 = reciprocal serum dilution at which 50% foci reduction occurred; – = FRNT50 titer ≤ 1:16; ND = not determined. Homotypic results are shown in bold. Shading indicates challenge virus.
Discussion
Although acute dengue fever and DHF have not been reproduced in NHPs previously, experimental infection of rhesus macaques can be used to examine virus replication and host responses, which may shed light on mechanisms that underlie fulminant illness. Infection of rhesus macaques with a lower dose of DENV did not result in clinical or behavioral changes nor rash and hemorrhage, as seen in previous reports with higher doses of DENV. Infection of animals with a lower dose of DENV more closely resembles a natural infection and the changes in blood homeostasis and host immune responses would likely better represent primary human DENV infection, particularly sub-clinical infection.
Infection of peripheral blood leukocytes and abnormalities in WBC sub-populations during DENV infection is widely documented.31,32 Dengue virus infection led to leukopenia and neutropenia, consistent with prior reports,33–35 but also led to increases in blood monocytes. In addition, increases in serum cytokines IL-2, TNF-α, and chemokines IL-8 and MCP-1 indicated an inflammatory response during DENV infection and suggest systemic activation of macrophages. Cytokine responses varied across serotype and may indicate differences in the host inflammatory response associated with virus isolate or serotype. Potentially these differences could contribute to inflammation and disease pathogenesis during primary and subsequent DENV infections.
Coincident with temporal changes in WBC populations, abnormal blood biochemical results were detected, which suggested tissue injury in multiple organs. Although not pathognomonic, serum ALT, AST, and CK levels may be increased after damage to cardiac muscle and could be indicative of cardiac injury during acute DENV infection. Furthermore, blood acidosis and fluctuation in blood electrolytes are symptoms that have been associated with impaired renal activity. Cardiac and acute kidney injury have been documented after DENV infection.36–40 However, biochemical changes were seen after primary DENV infection in the absence of DHF/dengue shock syndrome (DSS). Acute injury of the heart and kidneys likely results from indirect effects of DENV infection, including tissue inflammation, rhabdomyolysis, changes in blood biochemical parameters, and systemic activation of the coagulation pathway.
Infection of liver tissues and DENV-mediated liver damage has been reported extensively.41–43 Not surprisingly, we found abnormal levels of serum UREA, CHOL, GLU, AST, ALT, and CK in most infected animals. These irregularities in blood biochemical parameters are often suggestive of impairment and/or damage of liver tissue. Furthermore, liver disease impairs biosynthesis of coagulation factors such as fibrinogen and is a common underlying cause of coagulation dysfunctions.44,45 Impairment of liver function may contribute to decreasing serum fibrinogen levels, as we observed in this study, during activation of the coagulation pathway. In addition, activation of coagulation and disruption of blood biochemical parameters may have systemic effects impacting organ health in the heart and kidneys. Further study is required to clearly define comparative organ pathologies in the host during infection with the different DENV serotypes.
All viruses studied were capable of producing irregularities in hematologic results/coagulation and were able to stimulate cellular responses. Under normal circumstances, inflammation and blood coagulation must be tightly controlled and severe disease during DENV infection most likely result from deregulation of these host processes. However, the exact molecular mechanisms mediating perturbations of blood homeostasis remain largely unknown because of lack of proper animal models for DENV. Moreover, the decreased capacity of host systems to maintain blood homeostasis because of liver damage may also contribute to disease pathogenesis. Other DENV isolates may differ in their ability to disrupt host systems or result in higher viremia, thereby influencing the potential for severe disease. Improved animal models that demonstrate clinical symptoms of dengue fever, DHF, and DSS are needed to faithfully correlate disease mechanisms with the magnitude of disruption in hematologic parameters, circulating WBC populations, inflammation, blood biochemical parameters, and activation of blood coagulation pathways.
Because DENV-specific antibodies mediate immunity and development of severe disease, an understanding of the durability and specificity of primary antibody responses will be essential for determining the pathogenic mechanisms involved in severe dengue disease. In the current study, DENV infection resulted in robust pan-reactive humoral responses among all inoculated animals regardless of serotype. These antibody responses persisted to the end of the study and included serotype-specific highly neutralizing antibodies, as well as cross-reactive non-neutralizing antibodies. Our data suggest that after day 120, the host may become susceptible to heterotypic infection as pan-reactive neutralizing titers decrease and may have an increased prolonged risk of developing severe disease because of persistence of non-neutralizing antibodies and antibody-dependent enhancement.
The extensive data sets generated in the current study represent a comprehensive analysis of primary DENV infection in rhesus macaques. Importantly, serum samples collected during this study have been deposited in BEI Resources Repository, giving DENV researchers direct access to new well characterized serotype-specific serologic tools. In the future, comparing outcomes of primary and secondary heterotypic DENV infection will be useful for further elucidating host response mechanisms that might be involved in pathogenesis, which could identify early markers of severe DSS/DHF disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the Laboratory Animal Science Center of Boston University Medical Center for their exceptional dedication, support and care of the animals over the length of the study; Jose Osorio for independently verifying rhesus macaque flavivirus serologic data before challenge; Steve Whitehead for providing DENV isolates used in this study and for guidance in developing various assays; Aravinda de Silva for providing protocols and guidance to optimize FRNT immunostaining assays; Cristina Cassetti for facilitating successful completion of the project; Carlos Hillson for exceptional support in specimen processing and FRNT staining; and the National Emerging Infectious Diseases Laboratories Specimen Processing and Immunology cores for supplying reagents and time to perform the coagulation and multiplexed cytokine assays.
Footnotes
Financial support: This study was supported in part by National Institutes of Health contract HSN272201000007I-HHSN27200001 to Katharine N. Bossart.
Authors' addresses: Andrew C. Hickey, Jacob A. Koster, Claudia M. Thalmann, and Katharine N. Bossart, Department of Microbiology, National Emerging Infectious Diseases Laboratories Institute, Boston University, Boston, MA, Emails: drewh44@bu.edu, jkoster@bu.edu, thalmann@bu.edu, and kbossart@bu.edu. Kathy Hardcastle, Rocky Mountain Veterinary Branch, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rocky Mountain Laboratories, Hamilton, MT, E-mail: Kath.hardcastle@nih.gov. Phaik-Hooi Tio, Venture Technologies Sdn Bhd, INFORMM Building, Universiti Sains Malaysia, Minden, Penang, Malaysia, E-mail: phtio@yahoo.com. Mary J. Cardosa, Sentinext Therapeutics Sdn Bhd, 55 Jalan Sultan Ahmad Shah, Georgetown, Penang, Malaysia, E-mail: jc@sentinext.com.
References
- 1.Gubler DJ. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. pp. 1153–1252. [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 5.Shepard DS, Undurraga EA, Lees RS, Halasa Y, Lum LC, Ng CW. Use of multiple data sources to estimate the economic cost of dengue illness in Malaysia. Am J Trop Med Hyg. 2012;87:796–805. doi: 10.4269/ajtmh.2012.12-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstead SB, Palumbo NE. Studies on the immunization of monkeys against dengue. II. Protection following inoculation of combinations of viruses. Am J Trop Med Hyg. 1973;22:375–381. doi: 10.4269/ajtmh.1973.22.375. [DOI] [PubMed] [Google Scholar]
- 7.Peiris JS, Dittus WP, Ratnayake CB. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J Med Primatol. 1993;22:240–245. [PubMed] [Google Scholar]
- 8.Rodhain F. The role of monkeys in the biology of dengue and yellow fever. Comp Immunol Microbiol Infect Dis. 1991;14:9–19. doi: 10.1016/0147-9571(91)90036-d. [DOI] [PubMed] [Google Scholar]
- 9.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973;128:7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis. 1973;128:15–22. doi: 10.1093/infdis/128.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Onlamoon N, Noisakran S, Hsiao HM, Duncan A, Villinger F, Ansari AA, Perng GC. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115:1823–1834. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassetti MC, Durbin A, Harris E, Rico-Hesse R, Roehrig J, Rothman A, Whitehead S, Natarajan R, Laughlin C. Report of an NIAID workshop on dengue animal models. Vaccine. 2009;28:4229–4234. doi: 10.1016/j.vaccine.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, Chen L, Hayes C, Dong JY, Porter K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 15.Blaney JE, Jr, Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005;79:5516–5528. doi: 10.1128/JVI.79.9.5516-5528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardo L, Izquierdo A, Prado I, Rosario D, Alvarez M, Santana E, Castro J, Martínez R, Rodríguez R, Morier L, Guillén G, Guzmán MG. Primary and secondary infections of Macaca fascicularis monkeys with Asian and American genotypes of dengue virus 2. Clin Vaccine Immunol. 2008;15:439–446. doi: 10.1128/CVI.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freire MS, Marchevsky RS, Almeida LF, Yamamura AM, Caride EC, Brindeiro PA, Motta MC, Nogueira RM, Kubelka CF, Bonaldo MC, Galler R. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Mem Inst Oswaldo Cruz. 2007;102:203–208. doi: 10.1590/s0074-02762007005000011. [DOI] [PubMed] [Google Scholar]
- 18.Kochel TJ, Watts DM, Gozalo AS, Ewing DF, Porter KR, Russell1 KL. Cross-Serotype neutralization of dengue virus in Aotus nancymae monkeys. J Infect Dis. 2005;191:1000–1004. doi: 10.1086/427511. [DOI] [PubMed] [Google Scholar]
- 19.Martín J, Hermida L, Castro J, Lazo L, Martínez R, Gil L, Romero Y, Puente P, Zaragoza S, Cosme K, Guzmán MG, Cardosa J, Guillén G. Viremia and antibody response in green monkeys (Chlorocebus aethiops sabaeus) infected with dengue virus type 2: a potential model for vaccine testing. Microbiol Immunol. 2009;53:216–223. doi: 10.1111/j.1348-0421.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 20.Martın J, Hermida L, Castro J, Romero Y, Cardosa J, Guillen G. Viremia and the magnitude of the immune response upon infection of green monkeys with dengue virus type 2 are strain-dependent. Curr Microbiol. 2009;59:579–583. doi: 10.1007/s00284-009-9488-6. [DOI] [PubMed] [Google Scholar]
- 21.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. Efficacy of a live attenuated tetravalent candidate dengue vaccine in naïve and previously infected macaques. Vaccine. 2007;25:5409–5416. doi: 10.1016/j.vaccine.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 22.Koraka P, Benton S, van Amerongen G, Stittelaar KJ, Osterhaus AD. Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect. 2007;9:940–946. doi: 10.1016/j.micinf.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson M, Johnes S, Li L, Heath A, Barrett A. Effect of genomic variation in the challenge virus on the neutralization titres of recipients of inactivated JE vaccines–report of a collaborative study on PRNT50 assays for Japanese encephalitis virus (JE) antibodies. Biologicals. 2008;36:111–116. doi: 10.1016/j.biologicals.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Eighth edition. Washington, DC: National Academies of Science Press; 2011. [Google Scholar]
- 25.Bossart KN, Rockx B, Feldmann F, Brining D, Scott D, LaCasse R, Geisbert JB, Feng YR, Chan YP, Hickey AC, Broder CC, Feldmann H, Geisbert TW. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci Transl Med. 2012;4:146ra107. doi: 10.1126/scitranslmed.3004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JI, Shin JS, Lee JE, Jung WY, Lee G, Kim MS, Park CG, Kim SJ. Reference values of hematology, chemistry, electrolytes, blood gas, coagulation time, and urinalysis in the Chinese rhesus macaques (Macaca mulatta) Xenotransplantation. 2012;19:244–248. doi: 10.1111/j.1399-3089.2012.00713.x. [DOI] [PubMed] [Google Scholar]
- 27.Messer WB, Yount B, Hacker KE, Donaldson EF, Huynh JP, de Silva AM, Baric RS. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl Trop Dis. 2012;6:e1486. doi: 10.1371/journal.pntd.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 29.Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, Mattapallil JJ, Geisbert JB, Bossart KN, Broder CC. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE. 2010;5:e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang T, Cardosa MJ, Guzman MG. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol Cell Biol. 2007;85:43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- 31.Halstead SB, Marchette NJ, Sung Chow JS, Lolekha S. Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med. 1976;151:136–139. doi: 10.3181/00379727-151-39160. [DOI] [PubMed] [Google Scholar]
- 32.Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328–1340. doi: 10.1111/j.1365-3156.2008.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deparis X, Murgue B, Roche C, Cassar O, Chungue E. Changing clinical and biological manifestations of dengue during the dengue-2 epidemic in French Polynesia in 1996/97: description and analysis in a prospective study. Trop Med Int Health. 1998;3:859–865. doi: 10.1046/j.1365-3156.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilder-Smith A, Earnest A, Paton NI. Use of simple laboratory features to distinguish the early stage of severe acute respiratory syndrome from dengue fever. Clin Infect Dis. 2004;39:1818–1823. doi: 10.1086/426029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBride WJ, Mullner H, LaBrooy JT, Wronski I. The 1993 dengue 2 epidemic in Charters Towers, North Queensland: clinical features and public health impact. Epidemiol Infect. 1998;121:151–156. doi: 10.1017/s0950268898001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee IK, Lee WH, Liu JW, Yang KD. Acute myocarditis in dengue hemorrhagic fever: a case report and review of cardiac complications in dengue-affected patients. Int J Infect Dis. 2010;14:e919–e922. doi: 10.1016/j.ijid.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Marques N, Gan VC, Leo YS. Dengue myocarditis in Singapore: two case reports. Infection. 2013;41:709–714. doi: 10.1007/s15010-012-0392-9. [DOI] [PubMed] [Google Scholar]
- 38.Lima EQ, Gorayeb FS, Zanon JR, Nogueira ML, Ramalho HJ, Burdmann EA. Dengue haemorrhagic fever-induced acute kidney injury without hypotension, haemolysis or rhabdomyolysis. Nephrol Dial Transplant. 2007;22:3322–3326. doi: 10.1093/ndt/gfm431. [DOI] [PubMed] [Google Scholar]
- 39.Lima EQ, Nogueira ML. Viral hemorrhagic fever-induced acute kidney injury. Semin Nephrol. 2008;28:409–415. doi: 10.1016/j.semnephrol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Mehra N, Patel A, Abraham G, Reddy YN. Acute kidney injury in dengue fever using Acute Kidney Injury Network criteria: incidence and risk factors. Trop Doct. 2012;42:160–162. doi: 10.1258/td.2012.120023. [DOI] [PubMed] [Google Scholar]
- 41.de Macedo FC, Nicol AF, Cooper LD, Yearsley M, Pires AR, Nuovo GJ. Histologic, viral, and molecular correlates of dengue fever infection of the liver using highly sensitive immunohistochemistry. Diagn Mol Pathol. 2006;15:223–228. doi: 10.1097/01.pdm.0000213462.60645.cd. [DOI] [PubMed] [Google Scholar]
- 42.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 43.Tristao-Sa R, Kubelka CF, Zandonade E, Zagne SM, Rocha Nde S, Zagne LO, Araujo NF, Amin B, Fazoli F, Souza LJ, Cruz OG, Cunha RV, Nascimento D, Froes IB, Nogueira RM. Clinical and hepatic evaluation in adult dengue patients: a prospective two-month cohort study. Rev Soc Bras Med Trop. 2012;45:675–681. doi: 10.1590/s0037-86822012000600004. [DOI] [PubMed] [Google Scholar]
- 44.Mammen EF. Coagulation abnormalities in liver disease. Hematol Oncol Clin North Am. 1992;6:1247–1257. [PubMed] [Google Scholar]
- 45.Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83–96. doi: 10.1055/s-2002-23205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.