Abstract
The circulation of counterfeit or substandard artemisinins (ARTs) in malaria-endemic areas poses a serious threat to the long-term use of these drugs. Here, we validated an indirect competitive enzyme-linked immunosorbent assay (icELISA) for quantification of ARTs and found that 50% of inhibitory concentrations of dihydroartemisinin, artemether, and artesunate were 8.1, 207.0, and 4.7 ng/mL, respectively. We compared the icELISA with high-performance liquid chromatography (HPLC) for quantifying ART and its derivatives in 22 convenience samples of commercial antimalarial drugs. Paired t tests showed a borderline significant difference between the two methods (mean = 0.03, 95% confidence interval [CI] 0.00–0.07, P = 0.074) and the icELISA results were more variable than those of the HPLC analysis (P < 0.001), suggesting that further improvement is needed to enhance the performance of the icELISA. Our results showed that the icELISA has the potential to be improved for quality assurance of ARTs at the point of care in endemic settings.
Introduction
More than 40% of the world's current population live in poverty-stricken areas where malaria, alone or together with acquired immunodeficiency syndrome (AIDS), tuberculosis, and cholera, is a serious public health problem.1,2 According to the World Health Organization (WHO), ∼216 million clinical cases of malaria occurred in 2010 resulting in an estimated 655,000 deaths.3 Among the available public health malaria interventions, chemotherapy remains the predominant tool.4 To combat multidrug resistance in the malaria parasite Plasmodium falciparum, WHO has recommended the use of artemisinin (ART)-based combination therapies (ACTs).5 Currently, a number of ACTs such as artemether (ATM)-lumefantrine, artesunate (ATS)-amodiaquine, ATS-mefloquine, and dihydroartemisinin (DHA)-piperaquine are being used in many malaria-endemic areas.6–8 The ART, first isolated from a Chinese herb Artemisia annua, belongs to sesquiterpene lactone endoperoxides.7,9,10 The use of ART has been superseded by its derivatives such as the water soluble ATS, DHA, and the lipophilic esters ATM and arteether.
Poor quality medicines, including substandard and counterfeit drugs, cause a major loss on public health in resource-poor countries. The WHO has estimated that about 25% of the medicines consumed in developing countries are counterfeit.11 The illicit trade in counterfeit and substandard ARTs is a severe problem for malaria control, because it not only reduces the treatment efficacy and promotes development of resistance, but also may result in life-threatening complications.9 Antimalarial drugs have been reported as a target of counterfeiting in resource-poor areas. The magnitude of this problem is particularly huge in Southeast Asia.12 Newton and others reported that 38% of 104 shop-bought ATS samples from Cambodia, Laos, Myanmar, Thailand, and Vietnam did not contain ATS, whereas in some regions as much as 64% of the drugs contain little ATS.13 Since 1998, an epidemic of multiple types of counterfeit ATS tablets has affected malaria patients in Southeast Asia. As many as 14 physical types of the fake ATS have been found in this region.9,14,15 In addition, some genuine drugs are often substandard,16 compromising their expected therapeutic effect. Another problem associated with substandard antimalarials is expiration and degradation, which require close monitoring. Bate and others17 reported that significant proportions of the antimalarial drugs, including ART derivatives, failed the content and dissolution tests in six most severely malarious regions of Africa. This suggests that counterfeit and substandard antimalarial drugs are a global problem, which may imperil the great stride made towards malaria control in recent years after switching to ACTs. A sensitive, low cost, easy to use diagnostic tool for ART quality control is hence urgently needed.
A number of methods have been developed for the detection of ARTs, including high-performance liquid chromatography (HPLC),18–21 gas chromatography (GC)-flame ionization detection,22 GC-mass spectrometric detection,18,23,24 liquid chromatography–mass spectrometry,25 radioimmunoassay,26 and enzyme-linked immunosorbent assay (ELISA).26–30 The instrumentations and methods used to test the contents of ART are usually expensive and time-consuming, and require rigorous sample preparation, whereas isotope-based assays have potential health hazards. Being rapid, cost-effective, sensitive, simple, and convenient, ELISA has become popular for the detection of botanical chemicals and drugs31; we have previously generated a monoclonal antibody (mAb) 3H2 using ATS-bovine serum albumin conjugate as the immunogen. An indirect competitive ELISA (icELISA) was developed to detect ART in the A. annua samples.31 Here, we have further refined this assay for the quantification of ART and its derivatives. We directly compared the performance of the icELISA with that of the gold standard HPLC method using standards of ART and its derivatives and 22 ART-based antimalarial drugs purchased from the market.
Materials and Methods
Source of antimalarial drugs.
The ART, ATS, DHA, and ATM standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other antimalarial drugs were convenience samples, obtained from clinics, hospitals and private drug stores in Cambodia, China, Ethiopia and Kenya. The drug names, manufacturers, places where drugs were obtained are listed in Table 1.
Table 1.
Comparison between values measured by icELISA and HPLC in the commercial ART-based drugs
| Drug names (manufacturer) | Lot No. | Sites obtained | Measured content* (mg/mL) | |
|---|---|---|---|---|
| ELISA | HPLC | |||
| DHA-piperaquine phosphate tablets (Chongqing Holley Healthpro Pharmaceutical, Ltd.) | 30211 | Beijing, China | 2.04 ± 0.07 | 2.04 ± 0.03 |
| 10710 | Beijing, China | 1.97 ± 0.19 | 2.03 ± 0.01 | |
| 20807 | Beijing, China | 2.05 ± 0.06 | 2.02 ± 0.00 | |
| ATM soft capsules (Chongqing Holley Healthpro Pharmaceutical, Ltd.) | 20110301 | Beijing, China | 1.93 ± 0.06 | 1.95 ± 0.05 |
| ATM for injection (Kunming Pharma. Corp.) | 10ML02 | Yunnan, China | 2.14 ± 0.07 | 2.21 ± 0.01 |
| 07CM01 | Yunnan, China | 2.12 ± 0.12 | 2.13 ± 0.01 | |
| 20011052.01 | Yunnan, China | 2.08 ± 0.05 | 2.12 ± 0.01 | |
| 20000355.29 | Yunnan, China | 2.08 ± 0.14 | 2.02 ± 0.01 | |
| 99125822 | Yunnan, China | 2.09 ± 0.04 | 2.09 ± 0.01 | |
| ATS tablets (Guilin Pharmaceutical Corp., Ltd) | 40502 | Yunnan, China | 2.12 ± 0.06 | 2.33 ± 0.00 |
| AS100801 | Beijing, China | 2.04 ± 0.05 | 2.12 ± 0.01 | |
| ATS for injection (Kunming Pharma. Corp.) | LA110102 | Beijing, China | 2.09 ± 0.18 | 2.12 ± 0.01 |
| Artefan 20/120 (Ajanta Pharma, Ltd) | P0251C | Kakamega, Kenya | 2.17 ± 0.05 | 2.33 ± 0.02 |
| BNP0501D | Emuhaya, Kenya | 2.17 ± 0.23 | 2.04 ± 0.10 | |
| BNP0031D | Emuhaya, Kenya | 2.16 ± 0.03 | 2.24 ± 0.12 | |
| ATM Injection (Zifam) Artim 80 (Zifam Pinnacle Pty. Ltd.) | AC0030 | Phnom Penh, Cambodia | 2.22 ± 0.10 | 2.28 ± 0.03 |
| Coartem 20/120 (Beijing Novartis Pharma Ltd.) | X1475 | Addis Ababa, Ethiopia | 2.01 ± 0.00 | 2.11 ± 0.02 |
| CO-FALCINUM (CIPLA Ltd.) | B/NK 01885 | Vihiga, Kenya | 2.23 ± 0.21 | 2.18 ± 0.04 |
| B/NK 0C32 | Vihiga, Kenya | 2.16 ± 0.15 | 2.21 ± 0.01 | |
| B/NK 01646 | Vihiga, Kenya | 2.20 ± 0.22 | 2.22 ± 0.12 | |
| N/A† | Vihiga, Kenya | 2.32 ± 0.03 | 2.21 ± 0.01 | |
| B/NK 10489 | Vihiga, Kenya | 2.16 ± 0.19 | 2.22 ± 0.01 | |
Data are means ± SD. Each sample was extracted and analyzed in triplicate. The labeled value of active ingredients (a.i.) was all 2.0.
Lot number is not available.
icELISA = indirect competitive enzyme-linked immunosorbent assay; HPLC = high-performance liquid chromatography; ART = artemisinin; DHA = dihydroartemisinin; ATM = artemether; ATS = artesunate.
Materials and equipment.
The HPLC method has been the most widely used method for quantifying ARTs and was used as the gold standard in this study. The HPLC system consisted of a 600E multisolvent delivery system and a 2487 dual λ absorbance detector (Waters, Milford, MA). Both 0.2- and 0.5-μm syringe filters were purchased from Millipore (Billerica, MA). Ninety-six-well plates were from Corning Costar (Corning, NY). An automated plate washer (Wellwash 4 MK2) and a microplate reader (Multiskan MK3) were from Thermo (Vantaa, Finland). The HPLC-grade acetonitrile, ethyl acetate, and methyl alcohol were purchased from Sinopharm Chemical Reagent (Beijing, China). For ELISA, the buffer solutions included coating buffer (0.05 M carbonate buffer, pH 9.6), phosphate-buffered saline (PBS) (0.1 M phosphate buffer containing 0.9% NaCl, pH 7.5), PBS with 0.1% (v/v) Tween-20 (PBST), PBST containing 0.5% (w/v) gelatin (PBSTG), citrate-phosphate buffer (0.01 M citric acid, and 0.03 M Na2HPO4, pH 5.5), substrate solution (4 μL of 30% H2O2 added to 10 mL of citrate-phosphate buffer containing 2 mg/mL o-phenylenediamine [OPD]), and a stop solution (2 M H2SO4). Goat anti-mouse IgG conjugated with horseradish peroxidase (HRP) and OPD were purchased from Sigma (St. Louis, MO). All other chemicals and organic solvents were of analytical grade.
Drugs and sample preparation.
Antimalarial drug tablets were crushed by grinding with a clean mortar, which was washed three times with ∼1.5 mL of acetonitrile. The acetonitrile suspension was transferred into a 15-mL tube, sonicated in a Branson SB5200 ultrasonic oscillation (Danbury, CT) under room temperature for 30 min, followed by centrifugation at 2,080×g for 30 min. The extraction procedure was repeated three times and the supernatants were combined and filtrated through a 0.5-μm syringe filter. The filtrates were collected and stored at 4°C before analysis. For the commercial samples, the sample extracts were diluted into 2 mg/mL with acetonitrile as stock solutions for the icELISA and HPLC assays based on the labeled content of the commercial drugs. Stocks were then diluted using PBSTG to obtain concentrations in the working range of the icELISA.
Optimization of icELISA.
The mAb 3H2 has a high sensitivity and low cross-reactivity to the precursors of ART.31 The optimal concentrations of coating antigen, mAb, and goat anti-mouse IgG-HRP were screened by checkerboard titration. Concentrations of 0.25 mg/mL of coating antigen ATS-ovalbumin (OVA), 0.1 mg/mL of mAb and 0.1 mg/mL of goat anti-mouse IgG-HRP were selected and used throughout this work.
HPLC and icELISA analysis.
We compared these two methods side by side using the same drug preparations. The icELISA was carried out according to the method previously published.31 A microtiter plate was first coated with 100 μL of the ATS-OVA conjugate in coating buffer per well for 3 h at 37°C. After three washes with PBST, 50 μL extracts of drugs and 50 μL mAb 3H2 was added to each well for 30 min at 37°C. After three washes with PBST, 100 μL of goat anti-mouse IgG was added to each well and incubated at 37°C for 0.5 h. After the plate was washed with PBST again, 100 μL of substrate solution with OPD and hydrogen peroxide per well was added. The reaction was stopped by adding 50 μL of 2 M H2SO4. Absorbance was read at 492 nm with the microplate reader. Generally, three replicate samples were run for both the standard curve and unknown samples. For ELISA readings, a standard curve was fitted with the four-parameter sigmoid log-logistic model Y = (A1–A2)/(1 + (X/X0)p) + A2, where A1 and A2 are the minimum and maximum possible values and IC50 = X0. Parameters were estimated by using the maximum likelihood estimation method, and analysis was performed with the Origin 7.5 software (OriginLab, Northampton, MA).
The gold standard HPLC method was used to quantify ART and its derivatives in drugs as described previously.18,23 Briefly, a C18 reverse-phase column (250×4.6 mm, 5-μm particle size; Thermo) was used to separate ART and its derivatives. The mobile phase was 60% aqueous acetonitrile at a flow rate of 1 mL/min. The UV absorption was detected at 210 nm. The injection volume was 20 μL. The HPLC data were recorded and processed using Agilent1200 LC (Agilent Technologies, Santa Clara, CA). All data were collected and analyzed using Waters Millenium software.32
Recovery test for ART-based drug samples.
Commercial drugs usually contain a lot of supplementary materials in addition to the active ingredients. The organic solvent in the sample may also interfere with the icELISA. Sample dilution is a frequently used method to reduce the interference effects on ELISA analysis. Although the high sensitivity of the mAb can afford for up to 200,000-, 400,000-, and 10,000-fold dilutions for the DHA, ATS, and ATM drug samples, respectively, matrix effects on the assay accuracy were evaluated using the spike studies before analysis of drug samples. An amount of 2 mg/mL extracted ART-based drug samples, of which the active ingredient contents were quantified by icELISA, was spiked with corresponding standard substance at 2 and 4 mg, respectively. The extracted ART-based drug samples with no corresponding standard substance added were used as the blank control. The drug samples were added and disposed according to the icELISA procedure as described in the previous section. Three separate samples were taken for each drug sample, and each sample was analyzed in triplicate.
Statistical analysis.
Pearson correlation coefficient was used to measure the correlation between the icELISA and HPLC results by regression adjusted through origin. The paired t test was used to compare the difference between the icELISA and HPLC results, and mean value was used for each drug tested. Outliers (four values exceeding 2×standard deviations) were removed from the statistical analysis. Statistical significance was assessed at P < 0.05 in a two-tailed fashion. Statistical analyses were performed using Excel (Microsoft Corp., Redmond, WA) and JMP 9 software (SAS Institute Inc., Cary, NC).
Results
Optimization of icELISA.
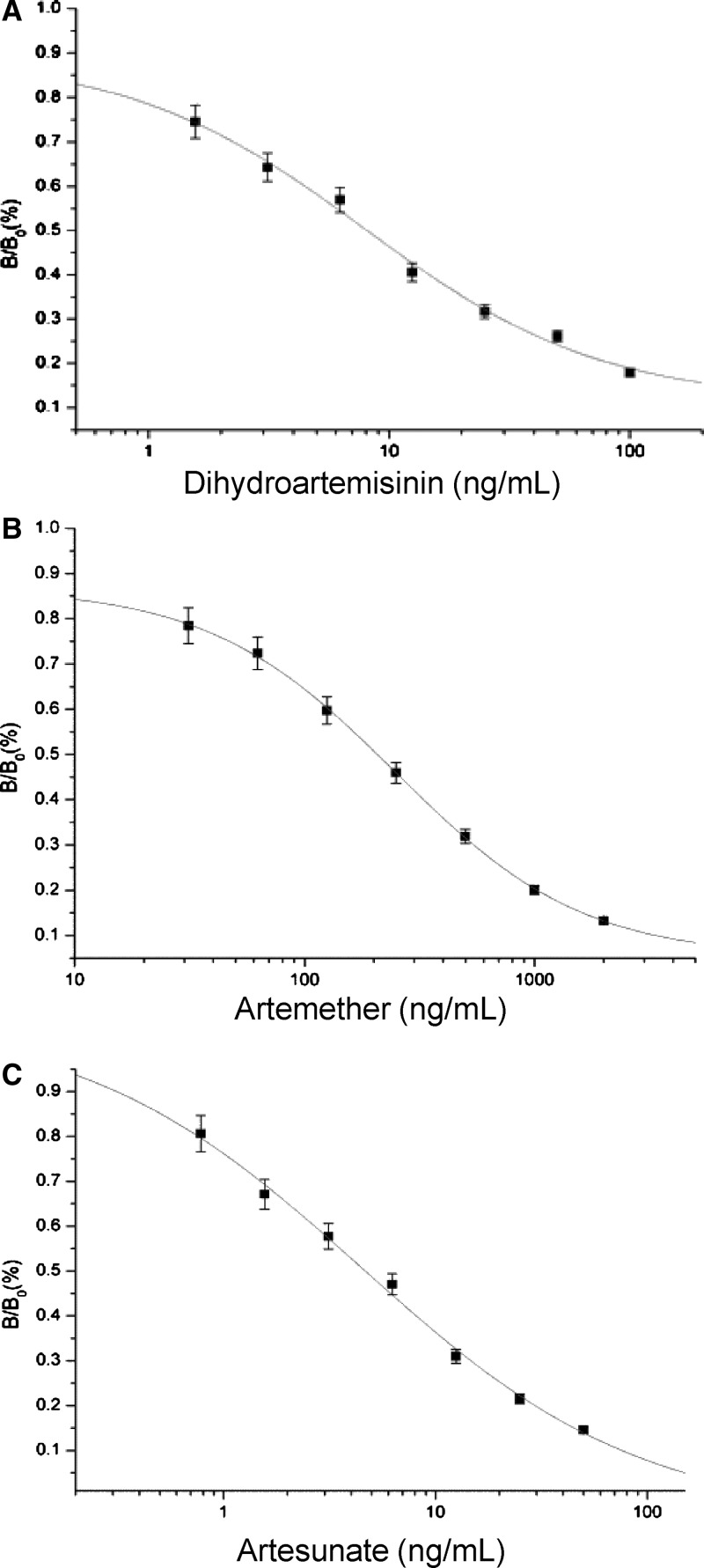
Under the optimized conditions mentioned previously, the IC50 values of the icELISA and working range of the calibration curve, based on 10–90% of inhibition of binding of mAb 3H2 to DHA, ATS, and ATM, were 8.10 and 1.56–100 ng/mL, 4.70 and 0.78–50 ng/mL, 207.20 and 31.25–2000 ng/mL, respectively. The limit of detection, defined as the lowest measurable concentration of target ingredients that could be distinguishable from zero concentration ±3 SD was 10.77, 0.12, and 87.42 ng/mL for DHA, ATS, and ATM, respectively (Figure 1).
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) analysis of artemisinin (ART) active ingredient in drugs. Each value represents the mean of three replicates. (A) Standard inhibition curve of dihydroartemisinin (DHA) in the indirect competitive ELISA (icELISA) format. IC50 = 8.09, R2 = 0.99. (B) Standard inhibition curve of artemether (ATM) in the icELISA format. IC50 = 207.20, R2 > 0.99. (C) Standard inhibition curve of artesunate (ATS) in the icELISA format. IC50 = 4.66, R2 > 0.99.
Matrix interference.
Using three drug samples spiked with standard drugs, we determined whether the matrices of the drug formulations interfere with the assay. As shown in Table 2, regardless of the drug formulations, the ART compounds had excellent recovery rates, suggesting that the crude extracts containing the drug matrix did not have noticeable influences on the icELISA results at the minimum dilution conditions used (> 10,000-fold).
Table 2.
Sample matrix effects on ART derivatives using mAb 3H2
| Sample | ART content* (mg/mL) | ART ± SD† (mg/mL) | Mean recovery (%, N = 3) | |
|---|---|---|---|---|
| Fortified detected | ||||
| DHA- piperaquine phosphate tablets (030211) | 2.00 | 0.00 | 2.05 ± 0.03 | – |
| 2.00 | 2.00 | 4.09 ± 0.04 | 102.0% | |
| 2.00 | 4.00 | 6.21 ± 0.14 | 104.0% | |
| ATM for Injection (10ML02) | 2.00 | 0.00 | 1.93 ± 0.09 | – |
| 2.00 | 2.00 | 4.02 ± 0.05 | 104.5% | |
| 2.00 | 4.00 | 6.09 ± 0.05 | 104.0% | |
| ATS tablets (040502) | 2.00 | 0.00 | 2.08 ± 0.06 | – |
| 2.00 | 2.00 | 4.13 ± 0.04 | 102.5% | |
| 2.00 | 4.00 | 6.28 ± 0.05 | 105.0% | |
Contents are means theoretical value by extracted and diluted.
Data are means ± SD of three determinations.
ART = artemisinin; DHA = dihydroartemisinin; ATM = artemether; ATS = artesunate.
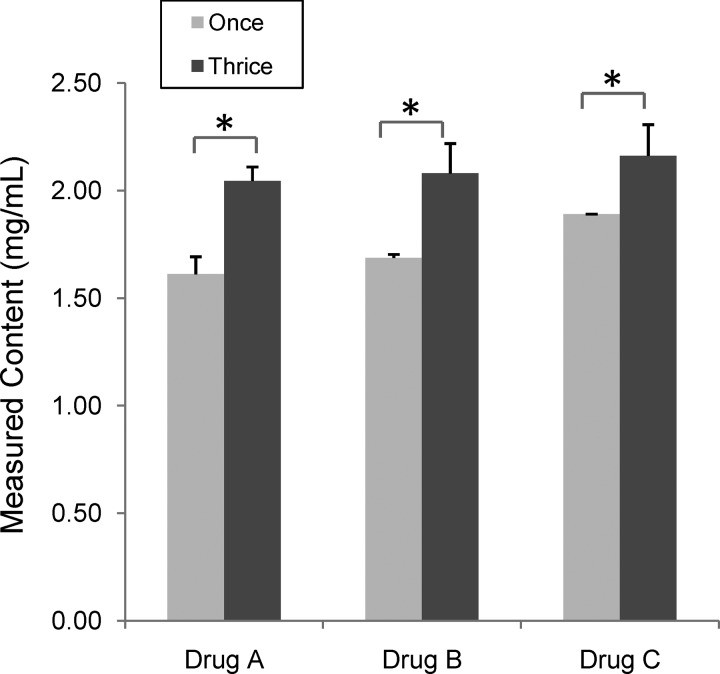
We then tested whether multiple extractions of the samples could significantly improve the recovery rates of the ARTs. We tested three commercial drug formulations (A: DHA-piperaquine phosphate tablets, B: ATM for injection, and C: Co-Falcinum) and found that extraction of the samples three times would increase the amount of recovered drug contents by 14–27% as measured by icELISA (Figure 2).
Figure 2.
Comparison of drug content detected by indirect competitive enzyme-linked immunosorbent assay (icELISA) between two extraction protocols (one versus three). (A) Dihydroartemisinin (DHA) and piperaquine phosphate tablets (Lot no. 030211); (B) artemether (ATM) for injection (Lot no.20000355.29); (C) CO-FALCINUM (Lot no. B/NK01885). An asterisk indicates significant difference in measured artemisinin (ART) family drug contents between the two extraction protocols (P < 0.05, t test).
Analysis of standard ART-based drugs with HPLC.
We further evaluated the conditions of HPLC for quantification of standard ART drugs.32,33 The concentrations of standard compounds were used at 1, 2, and 4 mg/mL. The retention times of DHA α-epimer, DHA β-epimer, ATM, and ATS were 5.8, 8.1, 20.5, and 7.1 min, respectively (Figure 3), consistent with previous reports.32,33 The peak intensities of different concentrations of standard compounds were used to make a working plot analysis of samples with an R2 of 1.00 (y = 0.64× + 79.71), 0.99 (y = 0.76×+ 58.23), and 0.98 (y = 0.84×+ 459.04) for DHA, ATM, and ATS, respectively.
Figure 3.
High-performance liquid chromatography (HPLC) chromatograms of the reference active ingredients and some commercial drugs. (A) Dihydroartemisinin (DHA) standard [α-epimer (1) and β-epimer (2)]; (B) artemether (ATM) standard; (C) artesunate (ATS) standard; (D) ATM for injection (Lot. No. 10ML02); (E) ATS tablet (Lot. No. AS100801).
Analysis of commercial ART-based drug samples.
To evaluate the reliability and accuracy of the icELISA for quantitation of ART drugs, we directly compared the icELISA with the gold standard HPLC using 22 commercial ART-based drugs from convenience samples (Table 1). The two methods showed an average difference of 0.011 mg/mL with a confidence interval of −0.037–0.058. The paired t test on the average content of each of the 22 drug samples showed that there was a borderline significant difference between the HPLC and icELISA methods (t = 1.87, degrees of freedom (d.f.) = 22, two-tail P = 0.074). The minimum detectable error of the paired t test was 0.055 mg/mL with 90% power and significance level of 5%. Comparison of SD of the average ELISA and HPLC results indicated a larger variation in ELISA results than that in HPLC results (0.114 versus 0.028, paired t = 4.71, d.f. = 22, P < 0.0001).
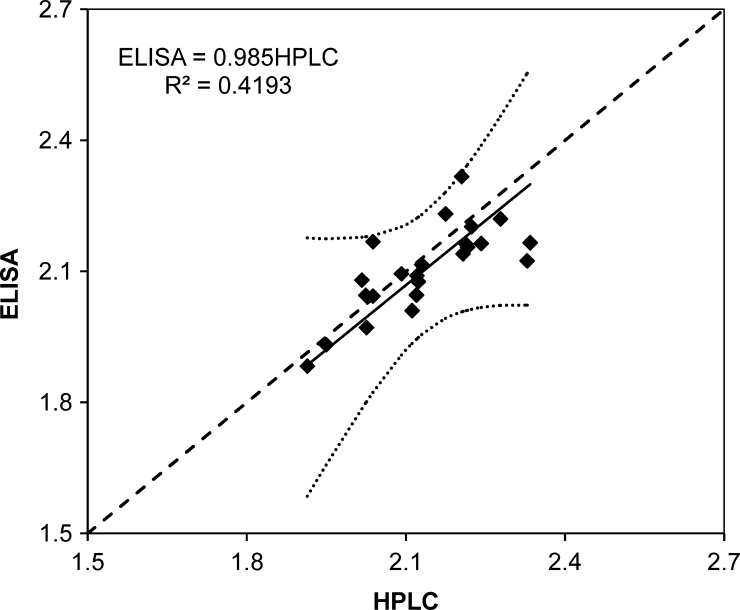
There was a high degree of correlation between the icELISA and HPLC results (Pearson R = 0.64, d.f. = 22, P < 0.001) and the observed statistical power of the regression was 97% with a type one error of 5%. Regression analysis showed that the overall difference in measured contents between the two methods was < 2% (HPLC = 0.985 icELISA) and differences between measured contents and predicted values are all within the 95% confidence interval (Figure 4). Together, this study provided validation of the icELISA for accurate quantitation of ARTs in antimalarial drugs. We also want to mention that although this study was not intended to determine the quality of the drugs, we found that the concentrations of the target compound measured by the two assays were close to those indicated on the labels, albeit the determined drug contents tended to be slightly higher than the labeled contents.
Figure 4.
Measured contents (mg/mL) by high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA). Solid line represents the linear regression result, dotted lines are the 95% confidence interval of the predictions, and dashed line represents the perfect fit (ELISA = HPLC).
Discussion
Poor quality medicines, both substandard and counterfeit, constitute a major burden on the public health in resource-poor countries. The use of such drugs not only severely jeopardizes the health of patients but also thwarts control efforts. Extensive investigations documented such epidemics of counterfeit ART drugs in Southeast Asia,15,34,35 and there is clear evidence showing that such threats have also emerged in other continents.14 In resource-poor countries, other neglected tropical diseases suffer similar fate, and a recent report of poor-quality generic drug for the treatment of visceral leishmaniasis in the national elimination program of Bangladesh is another vivid example.36 Although these examples stress the requirement for strict quality assurance by the government regulatory authorities, the development of simple and rapid methods to assess drug quality convenient methods for quality control at the field sites are desperately needed. Based on our success of generating specific antibodies for ART and its derivatives, we developed an icELISA for accurate measuring of ART drug contents.
Here, we further validated the icELISA method using both standard and 22 commercial ART drugs sampled from various hospitals and pharmacies. The contents of ARTs in these drugs determined by icELISA and the gold standard HPLC method showed a borderline significant difference (P = 0.0074). In particular, the variation of the icELISA results was significantly higher than that of the HPLC method (P < 0.001), suggesting that performance of the icELISA needs to be improved. In addition, we want to acknowledge that the convenience samples represented a disparate collection of pills, and some were from known sources of good-quality drugs. Therefore, testing of the method using samples of counterfeit and substandard drugs may be needed for further validation purpose.
Commercial drugs contain matrix materials that might interfere with the assay. We showed that the icELISA method was highly sensitive for ARTs, which allows the samples to be highly diluted. This could eliminate the potential interference from the matrices of the commercial drugs. With all drug formulations tested, we did not detect significant interference of the matrices with either method. Furthermore, the use of chromatographically pure acetonitrile for the sample extraction may enhance assay tolerance against matrix interference. In addition, sample extraction may be repeated to increase ART recovery rates.
A potential use of the icELISA method is for quantification of ARTs in commercial ACT drug formulations, which contain other partner antimalarial drugs. In our tested samples, the partner drugs did not interfere with the assay, suggesting the icELISA method is specific to detect ARTs in the antimalarial drugs. Although the cross-reactivity of mAb 3H2 with ATS, DHA, and ATM prevents differential detection of ART and its derivatives in the same samples, it does not constitute a major problem for our purpose of using the icELISA for quality assurance of ART drugs because all ART drugs contain a single target analyte of ART or its derivatives. Further applications of the icELISA under a variety of field settings are needed to validate its value for quality control of ART drugs. At this point, there is no intent for commercialization of the icELISA, and collaborations with colleagues on further testing of the icELISA are encouraged.
ACKNOWLEDGMENTS
Footnotes
Financial support: This work was supported by the National Institutes of Allergy and Infectious Diseases (NIAID), National Institute of Health (NIH) (U19AI089672).
Authors' addresses: Min Wang, Beijing Key Laboratory of Plant Resources Research and Development, College of Science, Beijing Technology and Business University, Beijing, China, E-mail: wangm@th.btbu.edu.cn. Yongliang Cui, China Agricultural University, College of Agronomy and Biotechnology, Beijing, China, E-mail: cuiyongliang@yahoo.cn. Goufa Zhou and Giuyun Yan, UC-Irvine, Public Health, Irvine, CA, E-mails: zhoug@uci.edu and guiyuny@uci.edu. Liwang Cui, Department of Entomology, Pennsylvania State University, University Park, PA, E-mail: luc2@psu.edu. Baomin Wang, College of Agronomy and Biotechnology, China Agricultural University, Beijing, China, E-mail: wbaomin@263.net.
References
- 1.Bates IF, Gruber J, Lalloo D, Medina Lara A, Squire SB, Theobald S, Thomson R, Tolhurst R. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4:267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- 2.Vitoria M, Granich R, Gilks CF, Gunneberg C, Hosseini M, Were W, Raviglione M, De Cock KM. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. Am J Clin Pathol. 2009;131:844–848. doi: 10.1309/AJCP5XHDB1PNAEYT. [DOI] [PubMed] [Google Scholar]
- 3.WHO World Malaria Report. 2010. http://www.who.int/malaria/world_malaria_report_2010/en/ Available at. Accessed September 25, 2013.
- 4.Bassel GW, Holdsworth MJ, Provart NJ. Seed bioinformatics. Methods Mol Biol. 2011;773:403–419. doi: 10.1007/978-1-61779-231-1_23. [DOI] [PubMed] [Google Scholar]
- 5.WHO The Use of Antimalaria Drugs: Report of An Informal Consultation. 2001. http://rbm.who.int/cmc_upload/0/000/014/923/am_toc.htm/ (WHO/CDS/RBM/2001.33) Available at. Accessed September 25, 2013.
- 6.Schlitzer M. Antimalarial drugs—what is in use and what is in the pipeline. Arch Pharm Chem Life Sci. 2008;341:149–163. doi: 10.1002/ardp.200700184. [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner JO, Jomaa H, Schlitzer M. New antimalaria drugs. Angew Chem Int Ed Engl. 2003;42:5274–5293. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]
- 9.Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJ, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NP, Greenwood BM, Nosten F, White NJ. Manslaughter by fake artesunate in Asia–will Africa be next? PLoS Med. 2006;3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJ, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NP, Greenwood BM, Nosten F, White NJ. Manslaughter by fake artesunate in Asia–will Africa be next? PLoS Med. 2006;3:752–755. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Counterfeit Medicines. Geneva: World Health Organization; 2006. Fact Sheet No 275. [Google Scholar]
- 12.Newton PN, Dondorp A, Green M, Mayxay M, White NJ. Counterfeit artesunate antimalarials in Southeast Asia. Lancet. 2003;362:169. doi: 10.1016/S0140-6736(03)13872-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pea N. Fake artesunate in Southeast Asia. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 14.Newton PN, Green MD, Fernandez F. Counterfeit artemisinin derivatives and Africa: update from authors. PLoS Med. 2007;4:e139. doi: 10.1371/journal.pmed.0040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton PN, Fernandez FM, Plancon A, Mildenhall DC, Green MD, Ziyong L, Christophel EM, Phanouvong S, Howells S, McIntosh E, Laurin P, Blum N, Hampton CY, Faure K, Nyadong L, Soong CW, Santoso B, Zhiguang W, Newton J, Palmer K. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keoluangkhot V, Green MD, Nyadong L, Fernandez FM, Mayxay M, Newton PN. Impaired clinical response in a patient with uncomplicated falciparum malaria who received poor-quality and underdosed intramuscular artemether. Am J Trop Med Hyg. 2008;78:552–555. [PMC free article] [PubMed] [Google Scholar]
- 17.Bate R, Coticelli P, Tren R, Attaran A. Antimalarial drug quality in the most severely malarious parts of Africa—a six country study. PLoS ONE. 2008;3:e2132. doi: 10.1371/journal.pone.0002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woerdenbag HJ, Salomons MC, Visser JF, Hendriks H, Malingre TM. Analysis of artemisinin and related sesquiterpenes from Artemisia annua L. by combined gas chromatography/mass spectrometry. Phytochem Anal. 1991;2:215–219. [Google Scholar]
- 19.Acton NK, Rollman IJ. Reductive electrochemical HPLC assay for artemisinin (Qinghaosu) Planta Med. 1985;51:445–446. doi: 10.1055/s-2007-969545. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Zeng M. Studies on the analysis of qinghaosu by high-pressure liquid chromatograph and spectrometry (HPLC) Planta Med. 1985;51:233–237. [Google Scholar]
- 21.Singh AV, Husain A. Evaluation of Artemisia annua strains for higher artemisinin production. Planta Med. 1988;51:233–237. doi: 10.1055/s-2006-962515. [DOI] [PubMed] [Google Scholar]
- 22.Peng CA, Wood AJ. Direct analysis of artemisinin from Artemisia annua L. using high-performance liquid chromatography with evaporative light scattering detector, and gas chromatography with flame ionization detector. J Chromatogr A. 2006;1133:254–258. doi: 10.1016/j.chroma.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Banthorpe DV. Two unexpected coumarin derivatives from tissue cultures of compositae species. Phytochemistry. 1989;28:3003–3007. [Google Scholar]
- 24.Woerdenbag HJ, Salomons MC, Hendriks H, Pras N, Malingre TM. Volatile constituents of Artemisia annua L. (Asteraceae) Flav Frag. 1993;8:131–137. [Google Scholar]
- 25.Wang MP, Wu Q, Simon JE. Analysis of artemisinin in Artemisia annua L. by LC-MS with selected ion monitoring. J Agric Food Chem. 2005;53:7010–7013. doi: 10.1021/jf051061p. [DOI] [PubMed] [Google Scholar]
- 26.Song ZY, Liang XT, Liu CX, Yi MG. Radio immunoassay of qinghaosu and artesunate. Acta Pharmacol Sin. 1985;20:610–614. [PubMed] [Google Scholar]
- 27.Jaziri MD, Vanhaelen M, Homes J, Yoshimatsu K, Shimomura K. Immunodetection of artemisinin in Artemisia annua cultivated in hydroponic conditions. Phytochemistry. 1993;33:821–826. [Google Scholar]
- 28.Ferreira JF. Immunoquantitative analysis of artemisinin from Artemisia annua using polyclonal antibodies. Phytochemistry. 1996;41:97–104. doi: 10.1016/0031-9422(95)00542-0. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka HP, De-Eknamkul W, Matangkasombut O, Shoyama Y. Preparation of a novel monoclonal antibody against the antimalaria drugs, artemisinin and artesunate. Planta Med. 2007;73:1127–1132. doi: 10.1055/s-2007-981560. [DOI] [PubMed] [Google Scholar]
- 30.Eggelte TA, van Agtmael M, Vuong TD, van Boxtel CJ. The development of an immunoassay for the detection of artemisinin compounds in urine. Am J Trop Med Hyg. 1999;61:449–456. doi: 10.4269/ajtmh.1999.61.449. [DOI] [PubMed] [Google Scholar]
- 31.He SP, Li G, Tan WM, Nan TG, Wang BM, Li ZH, Li QX. Development of a sensitive monoclonal antibody-based enzyme-linked immunosorbent assay for the antimalaria active ingredient artemisinin in the Chinese herb Artemisinin annua L. Anal Bioanal Chem. 2009;393:1297–1303. doi: 10.1007/s00216-008-2527-5. [DOI] [PubMed] [Google Scholar]
- 32.Wu JW, Shen WW, Zhang SQ. HPLC with post-column derivatization for simultaneous determination of dihydroartemisinin and piperaquine. Chin Remedies Clinics. 2009;9:178–182. [Google Scholar]
- 33.Cabri WD, Simone P, Di Iorio M, Di Mattia M, Gasparrini F, Giorgi F, Mazzanti A, Pierini M, Quaglia M, Villani C. Stereolability of dihydroartemisinin, an antimalarial drug: a comprehensive kinetic investigation. Part 2. J Org Chem. 2011;76:4831–4840. doi: 10.1021/jo102392p. [DOI] [PubMed] [Google Scholar]
- 34.Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, Chotivanich K, Mayxay M, Looareesuwan S, Farrar J, Nosten F, White NJ. Fake artesunate in Southeast Asia. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 35.Dondorp AM, Newton PN, Mayxay M, Van Damme W, Smithuis FM, Yeung S, Petit A, Lynam AJ, Johnson A, Hien TT, McGready R, Farrar JJ, Looareesuwan S, Day NP, Green MD, White NJ. Fake antimalarials in Southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Trop Med Int Health. 2004;9:1241–1246. doi: 10.1111/j.1365-3156.2004.01342.x. [DOI] [PubMed] [Google Scholar]
- 36.Dorlo TP, Eggelte TA, Schoone GJ, de Vries PJ, Beijnen JH. A poor-quality generic drug for the treatment of visceral leishmaniasis: a case report and appeal. PLoS Negl Trop Dis. 2012;6:e1544. doi: 10.1371/journal.pntd.0001544. [DOI] [PMC free article] [PubMed] [Google Scholar]