Abstract
We determined the prevalence of seven clinically important pathogens that cause sexually transmitted infections (STIs) (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, herpes simplex virus 1 [HSV-1], HSV-2, and Treponema pallidum), by using a multiplex polymerase chain reaction (M-PCR) in samples from Brazilian woman infected with human immunodeficiency virus 1 (HIV-1) and uninfected Brazilian women (controls). The M-PCR assay identified all STIs tested for and surprisingly, occurred association between the control and STIs. This association was probably caused by excellent HIV infection control and regular monitoring in these women established by public health strategies in Brazil to combat HIV/acquired immunodeficiency syndrome. Studies using this M-PCR in different populations may help to better elucidate the roles of STIs in several conditions.
It is estimated that annually more than 340 million new cases of sexually transmitted infections (STIs) occur throughout the world, and the highest incidence is in developing countries. Pelvic inflammatory disease, infertility, ectopic pregnancy, chronic pelvic pain, neonatal morbidity and mortality, and genital cancer all have been assumed to be associated with STIs, although in many cases the exact etiologic agents remain unknown.1 Furthermore, an increasing risk of acquisition of these infections may have a substantial impact on global human immunodeficiency virus 1 (HIV-1) transmission and have the potential to cause serious health risks in HIV-infected persons.2–4 However, few studies in Latin America have examined the reciprocal effect of HIV-1 on susceptibility and prevalence to STIs.
Over the past three decades, diagnostics for STIs depended on traditional methods such as culture, enzyme immunoassay, and fluorescent antibody staining. However, in recent years several molecular methods have become available. These newer methods have advantages because they are more sensitive, enabling detection in symptomatic and asymptomatic patients, including cases of viral infections.5 The multiplex PCR has an additional advantage in screening because it enables simultaneous detection of multiple pathogens, reducing costs and time.6
The purpose of this study was to determine the prevalence of seven clinically important STIs: Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, herpes simplex virus 1 (HSV-1), HSV-2, and Treponema pallidum by using multiplex PCR (M-PCR) in samples from HIV-1-infected Brazilian women and those from HIV1-uninfected Brazilian women.
A total of 556 women were randomly selected for this study: 178 HIV-infected (HIV group) and 378 HIV-uninfected women (control group) registered in the Public Specialized Assistance Service for HIV/acquired immunodeficiency syndrome (AIDS) for detection and control in Maringá/Paraná, Brazil, during April 2011–May 2012. Women spontaneously sought testing for HIV detection. Inclusion criteria were HIV-infected or uninfected women independent of signs and symptoms for STIs determined by a gynecologist; and HIV-infected or uninfected women who had two negative or positive diagnoses for HIV/AIDS by different methods, respectively. Exclusion criteria were pregnancy, age < 18 years, and no history of sexual activity. Data regarding HIV infection and treatment were obtained from Public Specialized Assistance Service medical records. This study was approved by the Committee for Ethics in Research Involving Humans at the State University of Maringá, Paraná, Brazil (no. 085/2011), and each woman involved signed a consent form.
Cervical, endocervical and vaginal samples were collected by using a cytobrush and an Ayre's spatula, transferred to tubes containing 1.0 mL of sterile 0.9% NaCl solution, and stored at −20°C. DNA was extracted by using an AxyPrep™ Body Fluid Viral DNA/RNA Miniprep Kit (Axygen, Union City, CA) according to the manufacturer's instructions. The quality and quantity of purified DNA were measured by spectrophotometry.
We made selected adaptations to a previously designed M-PCR to achieve simultaneous detection of the seven selected STIs.5,6 Primers were characterized by compatible melting temperatures and yielded amplicons with sizes easily separable by agarose gel electrophoresis (Table 1). The optimized protocol consisted of a reaction mixture of 25 μL containing 2.5 mM of each dNTP; 0.6 mM of MgCl2, 25 mM of each primer, 5 μL of the extracted DNA (50 ng of total sample) and 1 unit of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). The M-PCR conditions were 35 amplification cycles of denaturation for 10 minutes at 94°C, annealing for 1 minute at 62°C, extension for 1 minute at 72°C, and final extension for 10 minutes at 72°C (thermal cycler; Applied Biosystems, Foster City, CA). The M-PCR products were separated by electrophoresis on 2.5% agarose gels stained with 1 μg/mL of ethidium bromide. In a few cases, we analyzed products using 8% polyacrylamide gels. Positive controls for all STIs studied were derived from positive clinical samples detected by reference methods, including culture and/or single-target PCR. For validation, single-target PCRs for seven microorganisms for all samples studied and positive controls were also performed by using the same primers as for the M-PCR. The single-target PCR is generally more sensitive than the multiplex PCR and the possibility of cross-reactions, which can occur during M-PCR, are avoided.7 All clinical samples were also tested by using specific primers GH20/ PC04 for the human β-globin gene as an internal control of amplification and DNA integrity under the same conditions of M-PCR or single-target PCR.
Table 1.
Nucleotide sequences of amplification primers used in the multiplex polymerase chain reaction*
| Organism and primer | Primer sequence (5′→3′) | Amplicon size (base pairs) |
|---|---|---|
| Chlamydia trachomatis | ||
| Forward | TCTTTTTAAACCTCCGGAACCCACTT | 361 |
| Reverse | GGATGGCATCGCATAGCATTCTTTG | 361 |
| Neisseria gonorrhoeae | ||
| Forward | CGGCAGCATTCAATTTGTTAAAAAGC | 162 |
| Reverse | CGCCATTTTTGTA | 162 |
| Mycoplasma genitalium | ||
| Forward | ACCTTGATGGTCAGCAAAACTTCCTT | 193 |
| Reverse | TGATCTCATTCCAATCAGTA | 193 |
| Trichomonas vaginalis | ||
| Forward | CCAGAAGTGGGCTACACACCATACC | 170 |
| Reverse | AAGGCCGGAAGCAC | 170 |
| HSV-1 | ||
| Forward | CTGTGGTGTTTTTGGCATCAGGTTGT | 123 |
| Reverse | GGAGGAGACGTTG | 123 |
| HSV-2 | ||
| Forward | CATGGGGCGTTTGACCTCTACACAGT | 249 |
| Reverse | GATCGGGATGCT | 249 |
| Treponema pallidum | ||
| Forward | GGAGAAGTTTCACTTCGTGGACTCGC | 291 |
| Reverse | GTCATCACCGTAGTA | 291 |
HSV = herpes simplex virus.
Statistical analysis was performed by using STATISTICA 8.0 software (StatSoft Inc., Tulsa, OK), and all variables were expressed as absolute and relative frequencies. The prevalence rates of STIs in women groups were compared by using a non-parametric Z test. A P value < 0.05 was considered statistically significant.
The median ± SD age of the HIV group was 41.2 ± 10.3 years, which was similar to that of the control group (42.05 ? 13.05 years). Most of the HIV group had been infected by this virus between 2 to 10 years (62.9%), and showed excellent HIV control by: using highly active antiretroviral therapy (HAART) correctly (79.1%), having CD4 > 350 cell/mm3 (73.6%) and a viral load from < minimal limits to 100.000 copies/mL (97.1%).
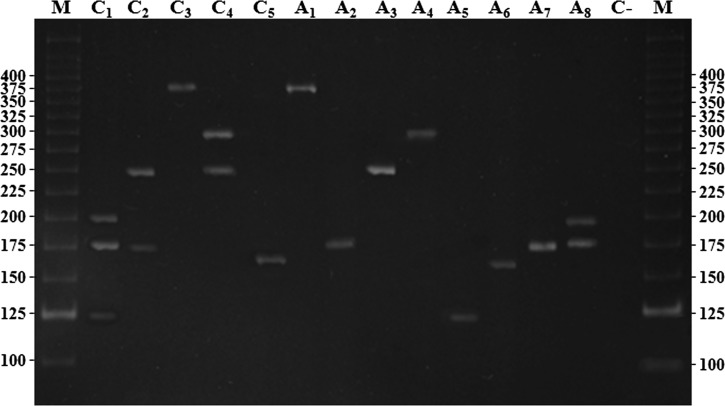
The M-PCR clearly distinguished and correctly identified all seven STIs, whether alone (1 STI) or simultaneously (2–3 STIs), and false-positives results were not detected. Final results, after repeating any tests that showed discrepant results, were accepted as true positives if the single-target PCR result was positive. The overall agreement of M-PCR results with single-target PCR results was 98.82% and the validation parameters were as follows: sensitivity 95.75%, specificity and positive predictive value 100% for both, negative predictive value 98.94% and accuracy 99.4%. Trichomonas vaginalis, HSV-1, C. trachomatis, and T. pallidum showed values of 100% for all parameters. The same results were observed for cases of simultaneous detection of 2 or 3 STIs (Table 2). M-PCR amplification fragments of positive samples after electrophoresis on a 2.5% agarose gel are shown in Figure 1.
Table 2.
Multiplex PCR validation results compared with single PCR for seven pathogens causing clinically important STIs*
| Multiplex PCR validation parameters | General results (%) | Chlamydia trachomatis (%) | Neisseria gonorrhoeae (%) | Mycoplasma genitalium (%) | Trichomonas vaginalis (%) | HSV-1 (%) | HSV-2 (%) | Treponema pallidum (%) | Two or three STIs simultaneously (%) |
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | 95.75 | 100.00 | 91.66 | 87.50 | 100.00 | 100.00 | 90.91 | 100.00 | 100.00 |
| Specificity | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100 | 100.00 | 100.00 |
| PPV | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100 | 100.00 | 100.00 |
| NPV | 98.94 | 100.00 | 97.43 | 97.67 | 100.00 | 100.00 | 97.5 | 100.00 | 100.00 |
| Accuracy | 99.40 | 100.00 | 100.00 | 98.00 | 100.00 | 100.00 | 98.00 | 100.00 | 100.00 |
| Agreement | 98.82 | 100.00 | 89.90 | 88.57 | 100.00 | 100.00 | 90.91 | 100.00 | 100.00 |
PCR = polymerase chain reaction; STIs = sexually transmitted infections; PPV = positive predictive value; NPV = negative predictive value.
Figure 1.
Electrophoretic analysis of the amplified fragments by using a multiplex polymerase chain reaction on an 8% polyacrylamide gel stained with ethidium bromide. Lane C1: control of Mycoplasma genitalium, Trichomonas vaginalis and herpes simplex virus 1 (HSV-1) (193, 170, and 123 bp) triple infection; lane C2: control of HSV-2 and T. vaginalis (249 and 170 bp) double infection; lane C3: control of Chlamydia trachomatis (361 bp); lane C4: control of Treponema pallidum and HSV-2 (291 and 249 bp) double infection; lane C5: control of Neisseria gonorrhoeae (162 bp); lane A1: positive sample of C. trachomatis (361 bp); lane A2: positive sample of T. vaginalis (170 bp); lane A3: positive sample of HSV-2 (249 bp); lane A4: positive sample of T. pallidum (291 bp); lane A5: positive sample of HSV-1 (123 bp); lane A6: positive sample of N. gonorrhoeae (162 bp); lane A7: positive sample of T. vaginalis (170 bp); lane A8: positive sample of M. genitalium and T. vaginalis (193 and 170 bp) double infection; lanes M, molecular mass marker (25 and 100 bp). Values on the left and right sides of the gel are in bp.
The control group showed a higher prevalence of STIs (n = 152, 66.1% of STIs detected) than the HIV group (n = 78) (P = 0.0001). However, there was no difference in the prevalence of each STI between both groups (Table 3). In 556 women, the most frequent STI was T. vaginalis (n = 69) representing 30.0% of STIs detected, followed by C. trachomatis (n = 62, 26.9%), and HSV-2, N. gonorrhoeae, and M. genitalium (n = 25, 10.9% each). For the HIV group, T. vaginalis was the most frequent (n = 20, 25.7% of STIs detected in this group), followed by C. trachomatis (n = 18, 23.1%), and HSV-2 (n = 15, 19.2%). For the control group, T. vaginalis also was the most frequent (n = 49, 32.2% of STIs detected in this group), followed by C. trachomatis (n = 44, 28.9%), N. gonorrhoeae (n = 19, 12.5%), and M. genitalium (n = 17, 11.2%) (Table 3).
Table 3.
Agents causing STIs detected by multiplex polymerase chain reaction*
| Agent | Total STDs detected, no. (%)† | STDs in HIV group, no. (%)† | STDs in control group, no. (%)† | P |
|---|---|---|---|---|
| Trichomonas vaginalis | 69 (30.0) | 20 (25.7) | 49 (32.2) | 0.6067 |
| Chlamydia trachomatis | 62 (26.9) | 18 (23.1) | 44 (28.9) | 0.0562 |
| HSV-2 | 25 (10.9) | 15 (19.2) | 10 (6.6) | 0.3852 |
| Neisseria gonorrhoeae | 25 (10.9) | 6 (7.7) | 19 (12.5) | 0.7495 |
| Mycoplasma genitalium | 25 (10.9) | 8 (10.2) | 17 (11.2) | 0.9290 |
| Treponema pallidum | 16 (6.9) | 8 (10.3) | 8 (5.3) | 0.7148 |
| HSV-1 | 8 (3.5) | 3 (3.8) | 5 (3.3) | 0.9714 |
| Total | 230 (100.0) | 78 (33.9) | 152 (66.1) | 0.0001‡ |
A total of 556 women were studied (HIV group = 178, control group = 378). STI = sexually transmitted infection; STD = sexually transmitted disease; HIV = human immunodeficiency virus; HSV = herpes simplex virus.
In single or multiple infections.
P < 0.05 was considered significant, by Z test.
We detected STIs in 51 (28.7%) of 178 in the HIV group and 130 (34.4%) of 378 in the control group. One STI was detected in 45 (88.2%) of 51 in the HIV group and 107 (83.3%) of 130 in the control group (P = 0.27). For two simultaneous STIs detected, 6 (11.8%) of 51 were positive in the HIV group 21 (16.1%) of 130 were positive in the control group (P = 0.19). A positive result for 3 simultaneous STIs was found only in 2 (1.5%) of 130 in the control group. The most common STIs found in the HIV group were HSV-2 and C. trachomatis (4 of 16, 25.0%) followed by T. vaginalis and C. trachomatis (n = 3 of 16, 18.7%). For the control group, the most common double STIs found were T. vaginalis and C. trachomatis (5 of 21, 23.8%) followed by T. vaginalis and HSV-2 and T. vaginalis and T. pallidum (2/21, 9.5% each).
To our knowledge, this is the first study to detect simultaneous STIs using a M-PCR in HIV-1-infected women compared with HIV-1-uninfected controls in Brazil and Latin America. This method enabled detection of seven STIs, many of which are difficult to identify by using other methods. This advantage simplifies the workflow, enabling performance of these assays in routine diagnostic laboratories with basic molecular biology facilities.8 Further investigations applying M-PCR in different populations may help to better understand the roles of STIs in several genital conditions.
Surprisingly, we found an association between the control group and the STIs studied. Brazil has implemented aggressive public health strategies to combat HIV/AIDS, including free distribution of regular physician monitoring of infected patients.9 The HIV-infected women in this study had excellent control of HIV infection, which together with regular physician monitoring are likely the main reasons for the lower STI prevalence when compared with the control group.
A positive or negative association between HIV and STIs has been found in studies comparing results obtained by different methods, such as serologic analysis, presence of one or more agents by other highly sensitive molecular techniques, serologic status, and culture.10,11 Because of these methodological differences, it is difficult to compare results from different studies. However, STIs and HIV infection facilitate each transmission of each other and have similar epidemiologic determinants.12 Our results show that the control of HIV is an essential component in the control of other STIs.
Overall, this study provides important information about the prevalence of seven STIs in HIV-infected Brazilian women. However, we recognize that these women should be further investigated for additional STIs.
Footnotes
Financial support: This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, AUX-PE-PRODOC 2571/2010, Brazilian Government.
Disclosure: None of the authors have any commercial or other conflicts of interest.
Authors' addresses: Raquel P. Souza, André L. P. de Abreu, Fabrícia Gimenes, and Marcia E. L. Consolaro, Departamento de Análises Clínicas e Biomedicina, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020900, Maringá, Paraná, Brazil, E-mails: raquelpantarotto@hotmail.com.br, andreluelsdorf@gmail.com, fabricia.gimenes@gmail.com, and melconsolaro@gmail.com. Érika C. Ferreira, Departamento de Estatística, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020900, Maringá, Paraná, Brazil, E-mail: erikacris84@hotmail.com. Sheila C. Rocha-Brischiliari, Maria D. de B. Carvalho, and Sandra M. Pelloso, Departamento de Enfermagem, Universidade Estadual de Maringá, Av. Colombo, 5790, 87020900, Maringá, Paraná, Brazil, E-mails: sheilarocha.enfermeira@hotmail.com, mdbcarvalho@gmail.com, and smpelloso@gmail.com. Marcelo G. Bonini, College of Medicine, Department of Pharmacology, University of Illinois at Chicago, Chicago, IL, E-mail: mbonini@uic.edu.
References
- 1.World Health Organization . World Health Organization Global Strategy for the Prevention and Control of Sexually Transmitted Infections. Geneva: World Health Organization; 2007. pp. 2006–2015. [Google Scholar]
- 2.Simms I, Warburton F, Westrom L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sex Transm Infect. 2003;79:491–494. doi: 10.1136/sti.79.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gore-Felton C, Vosvick M, Bendel T, Koopman C, Das B, Israelski D, Herrera M, Litzenberg K, Spiegel D. Correlates of sexually transmitted disease infection among adults living with HIV. Int J STD AIDS. 2003;14:539–546. doi: 10.1258/095646203767869156. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Pol B, Kwok C, Pierre-Louis B, Rinaldi A, Salata RA, Chen PL, van de Wijgert J, Mmiro F, Mugerwa R, Chipato T, Morrison CS. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 5.Sankuntaw N, Sukprasert S, Engchanil C, Kaewkes W, Chantratita W, Pairoj V, Lulitanond V. Single tube multiplex real-time PCR for the rapid detection of herpes virus infections of the central nervous system. Mol Cell Probes. 2011;25:114–120. doi: 10.1016/j.mcp.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Muvunyi CM, Dhont N, Verhelst R, Crucitti T, Reijans M, Mulders B, Simons G, Temmerman M, Claeys G, Padalko E. Evaluation of a new multiplex polymerase chain reaction assay STD finder for the simultaneous detection of 7 sexually transmitted disease pathogens. Diagn Microbiol Infect Dis. 2011;71:29–37. doi: 10.1016/j.diagmicrobio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.McIver CJ, Rismanto N, Smith C, Naing ZW, Rayner B, Lusk MJ, Konecny P, White PA, Rawlinson WD. Multiplex PCR testing detection of higher-than-expected rates of cervical mycoplasma, ureaplasma, and Trichomonas and viral agent infection in sexually Australian women. J Clin Microbiol. 2009;47:1358–1363. doi: 10.1128/JCM.01873-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKechnie ML, Hillman R, Couldwell D, Kong F, Freedman E, Wang H, Gilbert GL. Simultaneous identification of 14 genital microorganisms in urine by use of a multiplex PCR-based reverse line blot assay. J Clin Microbiol. 2009;47:1871–1877. doi: 10.1128/JCM.00120-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quint KD, de Koning MN, Geraets DT, Quint WG, Pirog EC. Comprehensive analysis of human papillomavirus and Chlamydia trachomatis in-situ and invasive cervical adenocarcinoma. Gynecol Oncol. 2009;114:390–394. doi: 10.1016/j.ygyno.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira PR, Vitória MA, Barcarolo J. Antiretroviral treatment in resource-poor settings: the Brazilian experience. AIDS. 2004;18((Suppl 3)):S5–S7. doi: 10.1097/00002030-200406003-00002. [DOI] [PubMed] [Google Scholar]
- 11.van de Wijgert JH, Morrison CS, Brown J, Kwok C, Van Der Pol B, Chipato T, Byamugisha JK, Padian N, Salata RA. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis. 2009;36:357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 12.Diaz N, Dessì D, Dessole S, Fiori PL, Rappelli P. Rapid detection of coinfections by Trichomonas vaginalis, Mycoplasma hominis and Ureaplasma urealyticum by a new multiplex polymerase chain reaction. Diagn Microbiol Infect Dis. 2010;67:30–36. doi: 10.1016/j.diagmicrobio.2009.12.022. [DOI] [PubMed] [Google Scholar]