Introduction
Optical coherence tomography (OCT) is a non-invasive, micrometer scale tomographic imaging modality in widespread clinical use for retinal imaging.1 OCT can be thought of as the optical analog of ultrasound imaging.2 Energy – light in the case of OCT – is delivered into the eye, and the locations of reflectors (cornea, lens, retina, among others) along that path constitute a linear A-scan. Regularly sampling across the eye then generates a series of A-scans that compose the familiar two-dimensional Bscan image or three-dimensional volume.
When viewing B-scan images from commercially available ultrasound and OCT instruments, there is a difference between the represented shape of the posterior segment of the eye from each modality (Fig.1). In ultrasound images, the posterior segment is circular as would be expected for the eye globe. In contrast, the retina typically appears nearly flat in OCT images, even accounting for the smaller field of view.
FIGURE 1. Ultrasound sector scan (left) and magnetic resonance image (MRI) (middle) of the eye, schematic of posterior segment optical coherence tomography (OCT) sector scan (top right), and resultant displayed OCT image (bottom right).
In the ultrasound sector scan image, the posterior eye has a semicircular shape as in the MRI image. Light in posterior segment OCT is also delivered with a sector scan as in ultrasound, but the resultant OCT image is displayed instead in a rectangular fashion with a flattened retina as a result of A-scans being plotted in a parallel format. The true retinal morphology is distorted in this format.
The source of this discrepancy lies in the display of the retina presented by OCT. Both posterior segment OCT and ophthalmic ultrasound are sector scanning technologies: the A-scans originate or pass through a common pivot point and sweep across the curved posterior eye. In ultrasound images, A-scans are presented in an arc (or fan) shape consistent with this scan pattern. In contrast, the A-scans in posterior segment OCT images from all current-generation commercial systems are presented in a parallel format, resulting in a rectangular display inconsistent with the actual scan geometry.
Because of this inconsistency between the display and the actual scanning, the true shape and relative position of ocular structures is incorrectly represented in typical posterior segment OCT. The displayed retina is in effect “distorted”. Figure 2 shows a spectral domain OCT (SDOCT) image of an apparently convex retina from a normal subject. In this extreme case, the displayed retina is non-physiologic, but in reality, the subject has a normal posterior segment. As quantitative morphometric analyses and measures are increasingly applied to OCT image datasets, such display distortions could have increasingly severe consequences on such analyses.
FIGURE 2. Optical coherence tomography image of a clinically normal eye distorted by display artifact.
In this example, the retina appears convex into the eye. However, this is a display artifact due to optics and is not reflective of actual morphology. (This type of imaging artifact is distinct from the dome-shaped maculas described in the literature23 which represent true anatomic findings.)
The purpose of this study was to develop methods to correct the ocular shape in posterior segment OCT images using optical models of the true OCT scan paths. To validate the derived algorithms, the corrected posterior segment OCT representations were compared to a reference non-optical imaging technique (magnetic resonance imaging, MRI). Finally, we characterized the effect of uncorrected and corrected retinal OCT images on current clinical measures of the retina.
Methods
This study was a prospective, single center observational case series. Prospective approval from the Duke University Medical Center Institutional Review Board (IRB) was obtained prior to initiating the study. Informed consent for the research was obtained from each enrolled subject prior to beginning any study activity, and the study was performed in accordance with HIPAA regulations.
Correction of posterior segment optical coherence tomography images
To correct the posterior segment OCT images, the true paths of the A-scans though the eye need to be recovered. Each component A-scan within the OCT image can then be reoriented in angle and space to its correct position within the sector scan.
We developed two methods to recover the scan path information in this study. The first (termed “numerical”, Fig. 3 left) entailed detailed ray-traced optical modeling of posterior segment SDOCT imaging. Ocular biometric measurements were obtained from the imaged eye using Scheimpflug photography (Pentacam; Oculus; Wetzler, Germany) for corneal radii of curvature and thickness and using partial coherence interferometry (IOLMaster; Carl Zeiss Meditec; Dublin, CA) for anterior chamber depth and axial length. These values are necessary because the cornea and lens serve as the final focusing objectives of the retinal OCT system (Fig. 1 top right). These biometric values were then used to populate and customize a wide-field, schematic model eye3 representing the imaged eye. Using optical design software (ZEMAX SE; RadiantZemax; Redmond, WA), a current generation retinal SDOCT system with two single-axis orthogonal galvanometers and the galvanometer image conjugate at the pupil was modeled. The angular scan range was measured from the actual SDOCT system and entered into the model. A virtual volumetric posterior segment SDOCT sector scan on the individualized schematic model eye was then simulated via ray tracing to recreate the paths taken by each A-scan during imaging. The model was optimized to minimize the spot size at the retinal surface of the model eye across the entire scan range. Based on the imaged model retinal surface data, a second reference surface was created that had equivalent optical path lengths to that of the center (0°,0°) scan configuration to allow remapping of the A-scan depths. This was done because depth in an OCT image represents optical path lengths and not physical distances. Each A-scan was then repositioned according to its modeled path to produce the final corrected posterior segment OCT image.
FIGURE 3. Models of posterior segment optical coherence tomography (OCT) scanning: numerical (left) and analytical (right).
To recover the actual OCT A-scan paths, models were used. The numerical method involves models customized with biometric measurements from the imaged eye. Ray tracing is then undertaken to determine the orientation and position of the A-scans. To reduce the modeling and computational complexity, the analytical method uses a reduced eye model. The retinal scans are assumed to all coincide at the nodal point of the reduced eye model.
To simplify the modeling and computational complexity inherent to the first model, we conceived a second model (termed “analytical”, Fig. 3 right) that assumed all scanning light entering the eye shared a common pivot located at the nodal point of a reduced model eye. For the purposes of this model, all points on the scanned retina were assumed to be equidistant to the nodal point. Hence the physical distance light travelled from the pivot to the retina was equivalent to the distance from the nodal point to the retina – 17.2 mm for Listing’s reduced eye model.4 The system manufacturer’s reported scan width determined the extent of the scan across the retina. This information was then used to reposition each A-scan to its correct path in this simplified model.
Comparison of corrected optical coherence tomography images with a reference technique (magnetic resonance imaging)
To validate the numerical and analytical methods, the corrected posterior segment OCT representations were compared to a reference non-optical imaging technique (magnetic resonance imaging, MRI).
Five patients (4 male, 1 female) from the neuro-ophthalmology clinics of the Duke Eye Center were enrolled into this study. These subjects were included because they had a recent head or orbital MRI but did not have ocular pathology (other than refractive error) on ophthalmic examination. The clinical MRI images were acquired using systems with 1.5 tesla magnetic field strength (GE Healthcare Signa HDxt and Siemens MAGNETOM Avanto) with slice spacing between 3 to 7 mm (mean ± one standard deviation: 4.40 ± 1.52 mm) and an in plane pixel width between 0.3125 to 0.625 mm (0.477 ± 0.147 mm). The mean age of the subjects was 56.38 ± 16.23 years. All eyes were phakic with a mean spherical equivalent refraction of −1.28 ± 2.50 diopters.
Both eyes of each subject were imaged using Scheimpflug photography, partial coherence interferometry, and retinal SDOCT (Bioptigen; Research Triangle Park, NC). The retinal SDOCT volumes consisted of 1024 × 1000 × 100 pixels (depth × A-scans × B-scans) taken at a 20 kHz A-scan rate with a nominal scan width of 6.7 mm per the manufacturer.
After fully automated layer boundary segmentation of the retinal surface and retinal pigment epithelial (RPE) layers,5 both correction algorithms (numerical and analytical) as implemented in MATLAB (MathWorks; Natwick, MA) were used to reorient the imaged A-scans to their modeled paths as described earlier. For ease of comparison and spatial accuracy, we also converted the standard anisotropic SDOCT pixel resolution (axial pixel pitch higher than lateral) to an isotropic pixel resolution (identical axial and lateral pixel pitch) for both the original and corrected images. The radius of curvature of the RPE was calculated as a general quantitative measure of the shape of the posterior eye for the standard uncorrected rectangular SDOCT images and for both types of corrected images.
On the corresponding axial T1 MRI image slabs of the subjects’ eyes, the inner eye wall was manually segmented by a single grader using custom written software, repeated three times. The volumetric mean radius of curvature of the inner eye wall for the three sessions was then calculated and used as the reference measure.
For each subject eye, the MRI derived radius of curvature was compared to the corresponding SDOCT derived radius of curvature. Paired nonparametric Wilcoxon signed rank tests using generalized estimating equations to account for multiple imaged eyes per person were used to identify any significant differences from zero (with α = 0.05) between the SDOCT measures and reference MRI measurements.
Clinical retinal thickness maps from uncorrected and corrected retinal optical coherence tomography images
To determine the effect of correcting the shape of the displayed retina on current clinical measures, retinal thickness maps from the uncorrected and numerically corrected OCT images were compared. For the purposes of this study, retinal thickness was defined as the vertical (parallel to the OCT image z axis) difference between the automatically segmented retinal surface and the RPE.
Because axial motion artifacts between B-scans can affect thickness measurements in the numerically corrected OCT images, volumes with numerical correction only within the B-scan planes were created for this comparison. This mitigated axial motion artifacts by eliminating differences between the original and corrected retinal surfaces in the y (slow) axis direction where axial motion artifacts are grossly apparent between B-scans. Individual B-scans were acquired in 50 msec, so motion artifacts within individual B-scans is limited.
The thickness maps from the uncorrected and numerically corrected retinal OCT images were then subtracted from each other to create difference maps, and the root mean square deviation (RMSD) was calculated to describe the difference between the two thickness maps. The RMSD first takes the differences at each equivalent pixel location between the original thickness map and the one derived from the numerically corrected retinal images. The differences are squared, summed, and divided by the number of pixels to normalize for the number of points. The square root of the result is then taken to return the result back to the original units.
To examine differences between the central and more peripheral regions of the volume, the thickness maps were also compared for two regions: a central 3 mm zone and a peripheral zone outside of the 3 mm zone.
Results
Correction of posterior segment optical coherence tomography images
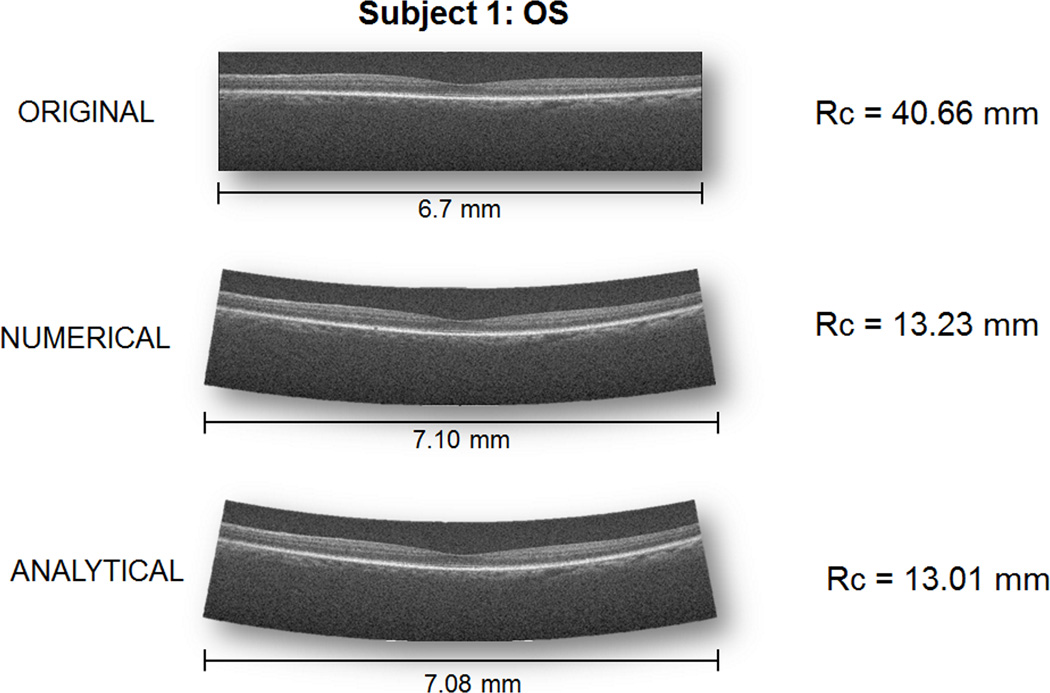
Figure 4 shows a representative standard uncorrected retinal SDOCT image and retinal images corrected using the numerical and analytical models. The uncorrected retina is visually flatter than either of the corrected images as is reflected in the radius of curvature measures. Table 1 shows the curvature values for all the eyes from all modalities in this study. For comparison, reported values of retinal curvature for anatomically accurate schematic eye models in the literature range from 11.06 mm to 14.1 mm with a mean of 12.05 mm.6
FIGURE 4. Representative standard uncorrected retinal spectral domain optical coherenct tomography (OCT) image and images corrected using models of the scan paths from Subject 1 OS.
The posterior segment in the standard image is much flatter than published values for the normal eye. Once the display is corrected based on optical principles, the curvature of the eye is within physiologic values. These images are displayed with 1:1 isotropic pixels rather than having the vertical dimension stretched as is typical in OCT.
Table 1.
Radius of curvature of posterior eye in millimeters for OCT images (original and corrected with numerical and analytical techniques) and for a reference technique (MRI).
| Subject/Eye | Original | Numerical (Ray Traced) |
Analytical (Reduced Eye) |
MRI |
|---|---|---|---|---|
| 1/OD | 69.30 | 14.92 | 16.02 | 12.04 |
| 1/OS | 40.66 | 13.23 | 13.01 | 11.46 |
| 2/OD | 17.54 | 10.01 | 8.37 | 12.24 |
| 2/OS | 24.15 | 11.18 | 8.74 | 12.97 |
| 3/OD | 98.31 | 15.37 | 21.04 | 12.42 |
| 3/OS | 128.95 | 14.62 | 21.22 | 12.92 |
| 4/OD | 31.55 | 10.94 | 11.13 | 10.61 |
| 4/OS | 51.38 | 12.58 | 12.25 | 11.02 |
| 5/OD | 52.51 | 15.40 | 14.12 | 12.30 |
| 5/OS | 134.13 | 17.81 | 13.83 | 12.32 |
Comparison of corrected optical coherence images with a reference technique (magnetic resonance imaging)
Figure 5 shows the mean difference in radius of curvature from the retinal SDOCT images as compared to the radius of curvature measured from MRI images. The mean difference between the uncorrected SDOCT retina and MRI derived radius of curvature was 52.8 ± 41.8 mm; this difference was statistically significant with a p value less than 0.001. The mean difference between the numerically corrected SDOCT retina and the corresponding MRI radius of curvature was 1.6 ± 2.3 mm with p = 0.091. The mean difference between the retina from SDOCT corrected with the analytical model and MRI radius of curvature was 1.9 ± 4.3 mm with p = 0.278.
FIGURE 5. Mean paired difference between radius of curvature of the retinal pigment epithelium in optical coherence tomography (OCT) and corresponding inner eye curvature in magnetic resonance imaging (MRI).
Across all eyes, the posterior segments represented in the uncorrected images were significantly flatter than in MRI. With correction, the posterior segment in OCT approaches that found in MRI.
Comparison of retinal thickness maps from uncorrected and corrected images
Figure 6 shows representative retinal thickness maps derived from uncorrected and numerically corrected SDOCT images of the retina. The overall RMSD between the thickness maps from uncorrected and numerically corrected maps ranged from 2.32 to 8.57 µm with a mean RMSD of 5.61 µm. The RMSD centrally (within the 3 mm diameter central zone) ranged from 0.82 to 4.25 µm with a mean of 2.54 µm while the peripheral RMSD (outside of the central 3mm area) ranged from 2.50 to 9.14 µm with a mean of 6.02 µm.
FIGURE 6. Representative retinal thickness maps from original (left: top and bottom) and numerically corrected (right: top and bottom) spectral domain optical coherence tomography images from subject 1 OS.
The top images are the three-dimensional retinal surfaces. Of note, features in the original three-dimensional surfaces such as the foveal pit or the rectangular depression due to motion are retained after display correction for the true scan paths. Retention of the jagged y-axis motion artifacts, however, can inflate differences in thickness maps derived from the corrected retinal surfaces. To mitigate this, the bottom right thickness map incorporates display correction only in the relatively motionless x-z (within B-scan) plane. The thickness scale bars are given in micrometers.
Discussion
In biomedical imaging, there is an implicit expectation that the image to some degree represents a faithful reproduction of the imaged structure. Such an assumption underlies the rationale for making quantitative measurements on imaged structures as surrogates for measurements on the actual structure itself. Posterior segment OCT images as currently displayed, however, do not faithfully represent reality because the component A-scans as displayed do not match their physical paths through the eye. This misrepresentation affects images of the posterior segment of the eye and can affect measurements on the displayed structures.
Using optical modeling of the imaged eyes in this study, we recreated the OCT scan paths of the individual A-scans which allowed us to reposition the A-scans to their expected orientation and to correct the OCT display. Using the radius of curvature as a broad measure of ocular shape, we found that the shape of the eye in the corrected SDOCT images was much closer to the reference technique (MRI) and to published values for posterior segment curvature than the uncorrected images.
The ocular radii of curvature were also far less variable in the corrected images than in the uncorrected images. This is not because the correction algorithms simply forced the imaged retina onto a standard spherical surface. Our algorithms are optically based, and any captured features such as the foveal pit or even jagged motion artifacts (Fig. 6) are retained in the corrected image. The decreased variability in the corrected images, then, merely reflects the small variability in ocular curvature in this population of normal eyes.
Correcting ocular shape benefits clinical applications such as retinal layer segmentation algorithms5 and intrasurgical OCT7–9. Current state of the art automated segmentation algorithms utilize sophisticated algorithms to reduce the errors that arise from unexpected retinal shapes.10 By removing display variability due to optics, the range of possibilities that segmentation algorithms must consider is reduced to only anatomic variation. This could increase the efficiency and accuracy of automatic segmentation algorithms. For real-time intrasurgical OCT, it would seem useful for a vitreo-retinal surgeon to have the actual curved anatomy displayed rather than an artificially flattened representation of the anatomy.
The shape of the eye is also clinically important in correlating myopic progression with pathology.11–12 These clinical studies are currently only possible with MRI imaging and in some studies, the subjects have been as young as 6 months. OCT has been used successfully in preterm infants13 and is more logistically feasible for clinical ophthalmic settings than MRI. Without correction of the displayed retina, though, OCT data cannot be used for correlations to pathology where the absolute shape of the eye is necessary.
Currently, the predominant quantitative analysis performed in retinal OCT is through the macular thickness map. Overall, we found that retinal thickness as defined in the methods changed only a small amount between the original and corrected retinal surfaces. For context, the overall mean RMSD of almost 6 µm seen in this study represents only 3% of an assumed 200 µm normal retinal thickness. Further, the overall difference is predominantly due to the peripheral region. There are more points outside of the 3 mm central area in our rectangular scans with a greater RMSD peripherally (6 µm) than centrally (2.54 µm). The central retinal thickness, then, is largely unaffected by the correction of ocular shape in retinal OCT.
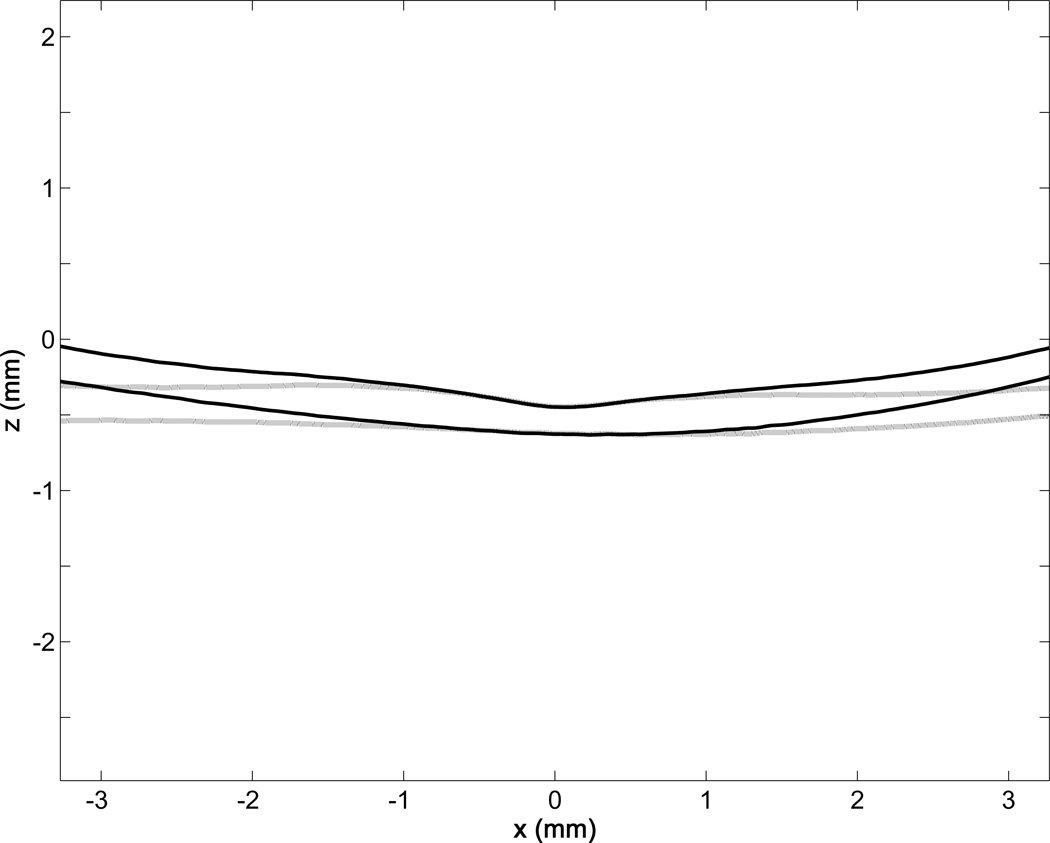
The source of the increased peripheral versus central deviation is seen in Figure 7. Even with correction, the central A-scans remain fairly vertical as they would be in the uncorrected rectangular display which has vertical A-scans throughout. The discrepancy between the sector scanning and the rectangular display becomes more pronounced farther from the center. For current systems, small features confined to the center of the image should be less affected by this optical artifact than features more peripheral in the scan. As wide-field diagnostics enter clinical practice, though, this effect should be considered when performing quantitative analyses on emerging wide-field OCT systems.14
FIGURE 7. Central profile of retinal inner limiting membrane and retinal pigment epithelial surfaces from Subject 1 OS showing retinal optical coherence tomography image correction affects the periphery more than the center.
Because central A-scans are effectively vertical even after correction, the central region of the corrected profile (black lines) will not deviate substantially from the original profiles (gray lines). However, as the A-scans move peripherally, the effects of sector scanning become more pronounced on the imaged structure.
Ocular magnification from axial length or anterior segment refractive error is known to cause changes in measured retinal layer thicknesses.15–19 The source of this error is that the OCT system assumes a scan pattern on a normal eye with a standard, expected refractive error or axial length. Because the OCT system must image through the eye, deviations from the expected normal eye can affect the size of the scan causing more central or more peripheral areas to be incorporated in the scan. The correction algorithms described in our work are related but different from these magnification errors. Although published methods exist to correct for image magnification,20–21 these methods do not correct the shape of the image. A flattened retina remains flattened after magnification is corrected; a flattened retina, however, is restored to its actual curved shape after correction for its true scan paths. Because our correction technique is based on both the optics of the system and the eye, our algorithms simultaneously correct both magnification and shape (Fig. 4). This allows for improved intersubject comparisons by eliminating variability due to imaging artifacts and ensures that any observed variability is due to actual structural changes.
An important source of variability in OCT is patient motion during scanning. Because OCT collects A-scans over time to create the image volume, patient motion during a scanning session can cause artifacts such as the jagged retinal surface and the rectangular depression artifacts seen in Figure 6. Motion artifacts were not removed in this study, as our algorithms sought only to correct optical and display artifacts. We mitigated larger motion artifacts in our retinal thickness analyses by examining differences only in the plane of the B-scan which should be less affected by motion due to collection speed. Directly addressing motion with techniques such as faster acquisitions or appropriate registration techniques would likely improve the accuracy of the original source volume and hence the resultant corrected retinal volume.
Another important consideration is that the model eyes in our correction algorithms assumed a symmetric, on axis system in which the visual axis and optical axis coincide in a rotationally symmetric model. This assumption was made to simplify the modeling and to allow for focusing to occur along the optical axis. The visual axis and the optical axis of the eye are known to not be coincident.22 Though currently difficult to measure precisely, the position of the eye relative to the OCT system axes could be incorporated into future, more rigorous implementations to improve the correction. As a first order correction, however, the current implementation significantly reduces the display artifact, and derived quantitative measures of retinal shape were much closer to reference values than uncorrected images.
In this study, we have addressed the correction of the shape of the eye in posterior segment OCT images and shown that the correction reduces the displayed difference between OCT and another clinical imaging technique -- MRI – which was used as our reference technique. Correction of this display error had a limited effect on current retinal thickness maps, but the effect was seen more towards the periphery. Retinal OCT shape correction should be a consideration, if not pre-requisition, in the development of more sophisticated image-based morphological measurements.
Acknowledgments
Funding/Support: This study was funded in part by grants from the National Institutes of Health (EY021522, EY020001, EY005722) and an unrestricted grant from Research to Prevent Blindness to the Duke Eye Center.
Other Acknowledgements: The authors would like to thank Dr. Sandra S. Stinnett of the Duke University Department of Biostatistics and Bioinformatics for her assistance with the statistical analyses in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Financial disclosures: In addition to his academic responsibilities, Dr. Izatt serves as Chairman and Chief Scientific Officer of Bioptigen Inc., a SDOCT device manufacturer. Dr. Izatt has stock interests with Bioptigen. He also has university patents that have been licensed to Carl Zeiss Meditec, St. Jude Medical, and Bioptigen. Drs. Kuo, McNabb, Izatt, and Toth are authors on intellectual property related to OCT imaging (but not on the content of this manuscript) that have been licensed to Bioptigen. Drs. Chiu, Toth, and Farsiu are authors on a provisional patent describing their segmentation algorithms. Dr. Toth receives grant support from Genentech, Bioptigen, and Physical Sciences and royalties from Alcon. Dr. Farsiu has a subcontracting relationship with the Duke Reading Center. Dr. El-Dairi has a consulting relationship with Prana pharmaceuticals.
Contributions of Authors: Design of the study (ANK, MAE, CAT, JAI); Conduct of the study (ANK, RPM, SJC, MAE, SF); Drafting and revising the manuscript (ANK, RPM, SJC, MAE, SF, CAT, JAI); Final approval ((ANK, RPM, SJC, MAE, SF, CAT, JAI).
References
- 1.Swanson EA, Huang D. Ophthalmic Optical Coherence Tomography Market: Past, Present, & Future. [Accessed May 4, 2012];Optical Coherence Tomography News. 2009 Available at http://www.octnews.org/articles/1027616/ophthalmic-optical-coherence-tomography-market-pas/.
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goncharov AV, Dainty C. Wide-field schematic eye models with gradient-index lens. J Opt Soc Am A. 2007;24(8):2157–2174. doi: 10.1364/josaa.24.002157. [DOI] [PubMed] [Google Scholar]
- 4.Katz M. The human eye as an optical system. In: Duane TD, editor. Clinical ophthalmology. Philadelphia: Harper & Row; 1979. pp. 1–52. [Google Scholar]
- 5.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atchison DA, Smith G. Optics of the human eye. Boston: Butterworth-Heinemann; 2002. Monochromatic aberrations of schematic eyes; pp. 160–173. [Google Scholar]
- 7.Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett. 2010;35(20):3315–3317. doi: 10.1364/OL.35.003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 9.Ray R, Barañano DE, Fortun JA, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118(11):2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Chiu SJ, Izatt JA, O'Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2012;53(1):53–61. doi: 10.1167/iovs.11-7640. [DOI] [PubMed] [Google Scholar]
- 11.Lim LS, Yang X, Gazzard G, et al. Variations in eye volume, surface area, and shape with refractive error in young children by magnetic resonance imaging analysis. Invest Ophthalmol Vis Sci. 2011;52(12):8878–8883. doi: 10.1167/iovs.11-7269. [DOI] [PubMed] [Google Scholar]
- 12.Moriyama M, Ohno-Matsui K, Hayashi K, et al. Topographic analyses of shape of eyes with pathologic myopia by high-resolution three-dimensional magnetic resonance imaging. Ophthalmology. 2011;118(8):1626–1637. doi: 10.1016/j.ophtha.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein T, Wieser W, Eigenwillig CM, Biedermann BR, Huber R. Megahertz OCT for ultrawide-field retinal imaging with a 1050 nm Fourier domain mode-locked laser. Opt Express. 2011;19(4):3044–3062. doi: 10.1364/OE.19.003044. [DOI] [PubMed] [Google Scholar]
- 15.Leung CK, Cheng AC, Chong KK, et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2007;48(7):3178–3183. doi: 10.1167/iovs.06-1315. [DOI] [PubMed] [Google Scholar]
- 16.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007;114(6):1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SH, Hong SW, Im SK, Lee SH, Ahn MD. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(8):4075–4083. doi: 10.1167/iovs.09-4737. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kim NR, Kim H, et al. Negative refraction power causes underestimation of peripapillary retinal nerve fibre layer thickness in spectral-domain optical coherence tomography. Br J Ophthalmol. 2011;95(9):1284–1289. doi: 10.1136/bjo.2010.186536. [DOI] [PubMed] [Google Scholar]
- 19.Savini G, Barboni P, Parisi V, Carbonelli M. The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectraldomain OCT. Br J Ophthalmol. 2012;96(1):57–61. doi: 10.1136/bjo.2010.196782. [DOI] [PubMed] [Google Scholar]
- 20.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232(6):361–367. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 21.Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52(1):625–634. doi: 10.1167/iovs.10-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atchison DA, Smith G. Optics of the human eye. Boston: Butterworth-Heinemann; 2002. Axes of the eye; pp. 30–38. [Google Scholar]
- 23.Gaucher D, Erginay A, Lecleire-Collet A, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol. 2008;145(5):909–914. doi: 10.1016/j.ajo.2008.01.012. [DOI] [PubMed] [Google Scholar]