Abstract
The mammalian nose is a multi-functional organ with intricate internal structures. The nasal cavity is lined with various epithelia such as olfactory, respiratory, and squamous epithelia which differ markedly in anatomical locations, morphology, and functions. In adult mice, the nose is covered with various skull bones, limiting experimental access to internal structures, especially those in the posterior such as the main olfactory epithelium (MOE). Here we describe an effective method for obtaining almost the entire and intact nasal tissues with preserved anatomical organization. Using surgical tools under a dissecting microscope, we sequentially remove the skull bones surrounding the nasal tissue. This procedure can be performed on both paraformaldehyde-fixed and freshly dissected, skinned mouse heads. The entire deboning procedure takes about 20-30 min, which is significantly shorter than the experimental time required for conventional chemical-based decalcification. In addition, we present an easy method to remove air bubbles trapped between turbinates, which is critical for obtaining intact thin horizontal or coronal or sagittal sections from the nasal tissue preparation. Nasal tissue prepared using our method can be used for whole mount observation of the entire epithelia, as well as morphological, immunocytochemical, RNA in situ hybridization, and physiological studies, especially in studies where region-specific examination and comparison are of interest.
Keywords: Anatomy, Issue 78, Physiology, Surgery, Tissue Engineering, Nose, Olfactory Mucosa, Olfactory Receptor Neurons, Vomeronasal Organ, skull bone removal, nasal cavity, olfactory epithelium, olfactory turbinate, respiratory epithelium, vomeronasal organ, histochemistry, mouse, animal model
Introduction
The mammalian nasal cavity contains various types of tissues and organs that serve distinct functions. The nasal cavity makes up the entry portion of the upper respiratory tract, which allows air travel into and out of the lungs. Inhaled air passes through the nasal cavity where it undergoes temperature and humidity conditioning 1 as well as cleaning or filtering to remove irritating and toxic substances and infectious microorganisms 2. Both treatments are carried out by nasal epithelia and subepithelial tissues, including glands and vessels and are critical for protecting the lower airways and the lungs. In addition to its role in respiration and epithelial defense, the nasal tissue also contains peripheral sensory apparatuses of the olfactory and trigeminal systems, which detect a wide range of chemical substances in the passing air. Depending on which system is activated, sensory detection of chemicals in the nose can elicit either a sense of smell, irritation, or pain 3,4.
The peripheral olfactory system is complex and made up of several anatomically separated olfactory sensory organs within the nasal cavity. Among them, the main olfactory epithelium (MOE) is the largest, which makes up approximately 45-52% of the nasal epithelia in rodents 5 and is located in the posterior region. In the anteroventral region, there is a pair of tubular structures known as the vomeronasal organ 6, which sit along each side of the nasal septum. Two additional small groupings of olfactory sensory neurons, known as the septal organ of Masera 7,8 and the Gruneberg ganglion 9, reside along the ventral septum and the dorsal entry region of the nasal cavity, respectively. These peripheral organs contain neuro-epithelia with distinctive features in morphology, cell marker expression, and physiological function. Together they detect thousands of odor molecules with exquisite sensitivity 10-12.
In addition to the olfactory sensory organs, the nasal cavity also houses other sensory systems. It is known that peptidergic trigeminal nerve fibers are present in the nasal epithelium, especially the respiratory epithelium 13,14. Some of these fibers detect irritating and toxic chemicals and are responsible for initiating protective reflexes such as coughing and sneezing 4,15. Irritating odorous and bitter compounds can also be detected by a recently discovered population of solitary chemosensory cells (SCCs), many of which are innervated by trigeminal nerve fibers 16-19. These SCCs are located in higher density in the entry region of the nasal cavity and vomeronasal entry ducts, hinting that they may also serve a protective function 16-18. Thus, nasal epithelia can differ substantially in function, morphology, and cell composition depending on their anatomical locations.
Even within a single and specialized epithelium, there are regional differences. The MOE is one such example. The MOE lines various turbinates, which are complicated and curled structures. Because of them, different regions of the MOE experience different air flow rates, and thus, different diffusion and clearance rates of airborne odor molecules 20. Also, it is known that olfactory sensory neurons (OSNs) expressing a given odor receptor are located in one of four circumvented zones of the MOE 21,22. How this location difference affects an OSN’s response to odorants is largely not known. In addition, some OSN populations exhibit regional preference. Guanylyl cyclase-D (GC-D)-expressing OSNs have zonal distributions favoring the cul-de-sac regions of the ectoturbinates 23,24. More recently, we found a subpopulation of canonical OSNs that expresses transient receptor potential channel M5 (trpM5) and is preferentially located in the lateral and ventral regions 25. These results indicate that MOE is not uniform. However, how these regional differences affect olfactory coding is not understood. This is in part because thorough physiological investigation of the MOE and the nose has been limited by the difficulty of obtaining intact nasal epithelia with preserved anatomical organization using current methods.
The nasal epithelia are predominantly surrounded by the anterior bones of the skull, including the nasal, maxilla, palatine, zygomatic, and ethmoid bones. In adult mice and other rodent models, these bones are hard and difficult to remove without damaging the closely associated nasal tissue, particularly the delicate turbinates. Often, chemical-based decalcification is used to soften bones to allow cryosectioning of nasal tissues for morphological, immunohistochemical, and in situ hybridization studies; however, depending on the age of the animal, the decalcification process can last overnight up to 7 days 24,26-28. This treatment is also limited because it requires tissue be fixative-preserved. Additionally, chemical decalcification can be harsh and affect the immunolabeling of some sensitive antibodies 29,30. For physiological studies, live tissue is required, and thus, these experiments are often conducted on isolated OSNs or MOE slices obtained from neonates whose skull bones are thin and soft 17,31,32. Physiological studies can also utilize whole mount preparations by splitting the head 25,33,34, but usually only the medial surface of the nose is easily accessible, limiting physiological recordings on other areas.
Here, we describe an effective, manual deboning method to prepare intact nasal tissues with preserved original anatomical organization and morphology. We sequentially remove the major bones of the anterior skull under a dissection microscope to expose an almost entirely intact nasal epithelium while keeping the thin turbinate bones intact unless the mice are very old and cryosectioning is needed. We also extend the method to preserve the connection between the nasal tissues and olfactory bulbs, as well as the rest of the brain, thus facilitating simultaneous examination of both peripheral and central circuits. Our method can be used to prepare paraformaldehyde-fixed, as well as fresh, live nasal tissue. Thus, our method is expected to facilitate morphological, immunohistochemical and physiological studies of respiration, olfaction, and nasal damage and illness.
Protocol
1. Mouse Nose Preparation
We used adult C57BL/6 background mice in this study. All animal care and procedures are approved by the Animal Care and Use Committees (IACUC) of University of Maryland, Baltimore County.
1.1 Acquiring the nose from paraformaldahyde-fixed mice
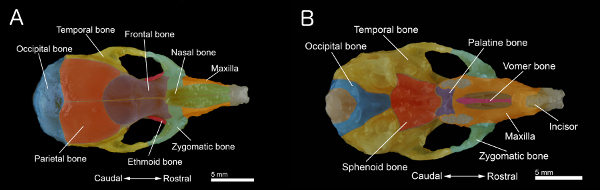 Figure 1. Bones of a mouse skull.A: Dorsal view of the skull. B: Ventral view of the skull with the mandible removed. The skull was prepared from a 40-day old mouse. Individual bones are colored for better visualization. Click here to view larger figure.
Figure 1. Bones of a mouse skull.A: Dorsal view of the skull. B: Ventral view of the skull with the mandible removed. The skull was prepared from a 40-day old mouse. Individual bones are colored for better visualization. Click here to view larger figure.
Perfuse transcardially to fix individual mice following the protocol of Lin, et al., (2008)16. Briefly, mice were deeply anesthetized with tribromoethanol (Avertin 250 μg/g body weight), perfused transcardially with 0.1M phosphate buffer (PB, 30-50 ml), followed by a phosphate buffered fixative containing 3% paraformaldehyde, 19 mM L-lysine monohydrochloride, and 0.23% sodium m-periodate (approximately 35-50 ml). One can also follow the steps in the JoVE article for animal perfusion 35.
Use a pair of scissors to cut off mandible (or the lower jaw) and remove the skin on the head.
Separate the entire head from the rest of the body.
Remove the palate. Also, clean and remove the remaining connective tissue and muscle on the surface of the skull to obtain the specimen shown in Figures 1A and 1B.
Under a dissection microscope, remove the skull bone covering the brain and olfactory bulbs. Trim off excess tissue and bones. Note, for the extended tissue preparation in which the brain and nose remain connected, only the skull bones are removed. For immunohistochemical experiments, the tissue was post-fixed for 1.5 hr and transferred to 0.1M phosphate buffered saline (PBS) with 25% sucrose overnight. Nasal tissue should be kept humidified throughout the dissection by dipping it in the buffered sucrose solution several times.
1.2 Acquiring the nose from freshly euthanized mice
Individual mice were transferred to a clean cage and exposed to CO2 gas, which was followed by cervical dislocation 5 min after the final breath. To reduce the blood in the nose tissue, use a pair of scissors to open the chest and cut the heart to allow blood to drain.
Repeat steps 1.1.2 to 1.1.5, except the post-fixation and cryoprotection with 25% sucrose. The specimen should be kept humidified and maintained with Tyrode’s saline containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 Na pyruvate, 10 D-glucose, and 10 N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid buffer (HEPES, adjusted at pH 7.4). Alternatively, fold a piece of Kimwipes, soak it with Tyrode’s solution, and place beneath the specimen while dissecting and occasionally dip the specimen in the Tyrode's solution or drop some solution onto the tissue to keep it humidified to maintain the viability of the cells and tissues.
2. Incisor, Anterior Vomer and Maxilla Bone Removal
Start from a ventral viewpoint. Locate the vomer bone and break the vomer bone along its length using a rongeur or forceps with teeth to break the ventral most part of the bone.
Using serrated forceps, remove the broken segments of the vomer bone by gently plying the bone fragments away from the vomeronasal organ (VNO). Note, for mice less than a month old, or if only interested in the MOE, these two steps can be skipped.
Hold the head firmly with forceps. Use the rongeur to break the front portion of both incisors and the jointed region of anterior maxilla between the two incisors and the vomer bone.
Break the ventral portion of the right maxilla at the region just anterior to the zygomatic arch up to the level of the dorsal zygomatic plate.
Flip over the nose for a dorsal viewpoint. Use the rongeur to break the right maxilla anterior of the dorsal zygomatic plate. The entire right maxilla anterior of the zygomatic plate should be loose. Use the fine forceps to gently separate any nasal tissue underlying the maxilla, and then gently lift away the bone fragment.
Repeat steps 2.4 and 2.5 to loosen the left incisor and left anterior maxilla and remove them after the nasal bone has been removed.
3. Nasal Bone and Dorsal Zygomatic Plate Removal
Use the serrated forceps or the rongeur to remove the remainder of the frontal bones just caudal to the nasal bones. After removing this piece of bone, the caudal part of the nasal bone can be gripped using forceps.
Use the rongeur to break the anterior portion of the zygomatic arch, which is connected to the zygomatic plate.
Take the fine forceps at the lateral edge of the dorsal end of the zygomatic plate and gently flip the bone and remove it. If the bone is not loose, use the rongeur to clamp it gently to loosen it for removal.
Use the fine forceps or a razor blade to loosen the medial suture between the right and left nasal bones.
Use the serrated forceps to grip the caudal end of the right nasal bone. Gently move the bone from side to side to separate it from underlying tissue. It is useful to move the forceps along the caudal third of the nasal bone for moving the bone side to side. As the nasal bone separates, slowly lift the bone from the caudal end.
While the nasal bone is slightly lifted, tilt the bone laterally to reveal a lateral outcropping of the bone that is lined with thin respiratory epithelial tissue. Use fine forceps to gently release this tissue from the nasal bone. Continue to lift the nasal bone. When the bone is completely separated from underlying tissue, use scissors to cut off the nasal bone at the rostral end.
Repeat steps 3.1, 3.5 and 3.6 for the removal of left nasal bone.
Repeat steps 3.2 and 3.3 for the left side of the nose.
4. Lateral Zygomatic Plate Removal
Remove the zygomatic plate one side at a time. Either zygomatic plate can be removed first.
From a ventral viewpoint, break the maxilla inferior to the zygomatic arch until break reaches the zygomatic arch.
From a dorsal viewpoint, gently grab the zygomatic arch and lift forward and lateral. If the zygomatic plate is still attached to any tissue, take the fine forceps and gently sever the connections between the tissue and the bone.
Repeat for the zygomatic plate on the other side of the nose.
5. Orbit Bone Removal
From a ventral viewpoint, break the palantine bone between the molars of the nose.
Use the rongeur to break the 3 molars and the maxilla on each side of the nose.
Break and remove any remaining thick pieces of bone ventral and posterior to the turbinates on each side of the nose.
6. Ethmoid Bone Removal
Break any portion of the ethmoid bone protruding caudal to the turbinates. This is necessary to avoid the loss of turbinate tissue when removing thin pieces of the ethmoid bone covering the turbinates.
For the right side of the nose, place the fine forceps at the anterior edge of the ethmoid bone and gently remove it. If a portion of the bone remains, repeat this procedure until all the thin bone covering the turbinates has been removed. The turbinate bones do not need to be removed in most of the preparations. In aged mice, the cribriform plate becomes brittle. If cryosectioning of the nasal tissue is needed, remove small pieces of the plate with fine forceps to reduce the potential damage caused by the bone.
Repeat step 6.2) for the left side of the nose.
Remove any remaining bone fragments prior to sectioning. Note: in animals more than a year-old, the posterodorsal region of the septum bone is somewhat thick and hard. One can remove this portion using fine forceps. Insert the tip of the forceps into both side of the bone to separate the dorsal portion of the septum bone and the lining epithelial tissue. Use one pair of forceps to grab the bone and hold the specimen. Use another pair of forceps to break the upper bony portion from the lower cartilaginous portion of the septum and gently remove it.
7. Nose Preparation for Cryosectioning
Set up the aspirator vacuum pump.
Place the nose in an embedding mold. Submerge the nose in OCT media.
Use a vacuum to remove air bubbles trapped within the nose tissue. This process takes up to 5 min.
After removing air bubbles, set the tissue in the desired orientation.
Freeze the OCT and tissue in the mold using dry ice. The embedded tissue can then be cryosectioned immediately or stored at -80 °C for future use.
Representative Results
Using this method, we can reliably obtain almost entirely intact nasal tissue. Figure 2A shows an image of adult nasal specimen from a paraformaldehyde-fixed head. In this specimen, all four sub-olfactory sensory organs, including the MOE, septal organ, the Gruneberg ganglion, and VNO, are intact. Also, the respiratory epithelia and subepithelial tissues, such as glands and vessels, are preserved. We have successfully used this method in a number of studies in which we investigated morphology, distribution, nerve innervation, and cell marker expression in various specialized sensory cell populations 16,17,36-38.
Figure 2B shows a specimen with the brain, olfactory bulbs, and nose together. This extended preparation is especially useful in studies that require nerve connection between the peripheral and central olfactory systems. We prepared this specimen by removing the skull bones surrounding the brain prior to the removal of facial bones. Our extended method allows investigators to conduct experiments on both peripheral and central olfactory system in a single preparation.
The nasal tissue shown in Figure 2 can be used for whole-mount observation, as well as for preparation of tissue sections. Figure 3A show a horizontal section cut through both the MOE and respiratory regions. Figure 3B shows a coronal section cut through the middle of the MOE. We stained the sections with neutral red for a better view of various types of epithelial and submucosal structures at different anatomical locations. These results demonstrate that our technique preserves even the delicate MOE turbinate structure and anatomical organization of the nose, which enable comprehensive and comparative investigation of nasal morphological and functional changes under normal and pathological conditions.
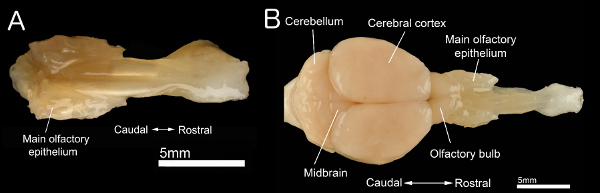 Figure 2. Isolated nasal and brain tissue.A: Dorsal view of intact nose tissue dissected using our deboning method. B: Dorsal view of a nose and brain with neural connections between the central and peripheral olfactory systems intact. Click here to view larger figure.
Figure 2. Isolated nasal and brain tissue.A: Dorsal view of intact nose tissue dissected using our deboning method. B: Dorsal view of a nose and brain with neural connections between the central and peripheral olfactory systems intact. Click here to view larger figure.
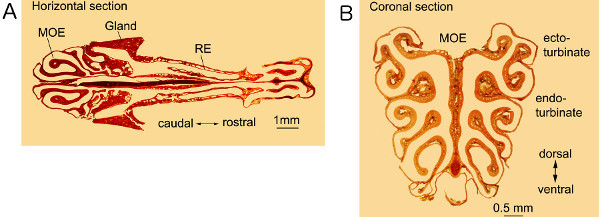 Figure 3. Sections of nasal tissues.A: A horizontal section showing olfactory and respiratory epithelia as well as other nasal structures. B: Coronal section through the turbinates of the MOE in the posterior of the nose. Both sections were 14 μm-thick and stained with neutral red. MOE: Main olfactory epithelium. RE: Respiratory epithelium. Click here to view larger figure.
Figure 3. Sections of nasal tissues.A: A horizontal section showing olfactory and respiratory epithelia as well as other nasal structures. B: Coronal section through the turbinates of the MOE in the posterior of the nose. Both sections were 14 μm-thick and stained with neutral red. MOE: Main olfactory epithelium. RE: Respiratory epithelium. Click here to view larger figure.
Discussion
Here, we demonstrated a step-by-step procedure for isolating intact olfactory and respiratory tissue from the mouse nose by sequentially removing the surrounding bones while sparing the tissue below. We show that careful bone removal can preserve even the most delicate tissues in their entirety. We also share insight into possible modifications of this technique, in which we isolate both the brain and nose tissue together to preserve the nerve connection. This new method provides a means for isolating whole olfactory and respiratory tissues in a single specimen for further processing in immunohistochemical, RNA in situ hybridization, and physiological experiments.
Advantages of Bone Removal
Before our method, decalcifying agents were typically used to soften bones for tissue sectioning and immunohistochemistry. However, depending on the size of the bone and the age of the animals, decalcification can be a lengthy process requiring tissue incubation in bone decalcifiers for hours or even several days29,30. The agents commonly used also contain acids, which may have effects on the tissue that can alter or impede immunolabeling 29,30. To combat this, some researchers use ethylenediaminetetraacetic acid (EDTA) solution to sequester calcium ions from the bone 24,26-28. This method of decalcification also requires days. The dissection shown here can be performed in about 20-30 min and does not require the application of any chemical solutions that may alter the stainability of tissue.
Our method can also be used to obtain intact nasal tissue from freshly dissected noses, providing an advantage for obtaining live tissue for physiological recordings. For example, nose slices are commonly used for Ca2+ imaging of OSNs and supporting cells in the olfactory epithelium 31,32. These slices have to be prepared from neonatal mice before the bones surrounding the tissues hardened. However, neonatal specimens are not identical to adults because the olfactory epithelium continues development and maturation postnatally. Using our method, researchers can obtain intact olfactory tissue from mice older than neonatal ages. This presents a significant advantage, especially in studies of age-dependent changes.
Modifications and Troubleshooting
We showed one effective sequence to remove surrounding bones. However, there are other sequences of bone removal to obtain intact nasal tissues, and the step by step procedure may be modified depending on the individual needs of the researcher and the tissues of most interest. For example, if the vomeronasal organ is needed, the vomer bone should be removed early in the procedure because there are more bone-covered areas to grasp to hold the tissue in the desired place during removal. By adapting the procedure to each application, tissues of interest are more likely to remain intact for further processing. The nasal bones also tend to be difficult to remove, as the tissue below clings to the bones and will often tear as the bones are lifted away. In addition to wiggling the nasal bones as shown in the video, small forceps may be gently inserted between the nasal bone and the underlying tissue to help move the tissue down and away from the bones during removal.
The dissection also varies slightly depending on the age of the mice. In young mice, the bones tend to be softer, while the bones of older mice are more brittle and strongly fused to neighboring bones in some regions. Soft bone is easier to separate and to remove in small pieces than harder bone. This difference can be easily overcome by using forceps to score or scrape at connections between bones to aid in their separation and clean removal. In older animals, the posterodorsal region of septum is somewhat thick and hard and should be removed prior to cryosectioning. The bony portion of the septum can be removed as described in procedure 6.4. Although there are small differences in the dissection for older and younger mice, age is not a limiting factor. In our studies, we have successfully prepared intact nasal tissues from mice ranging from 14 days to two years old.
There is no significant difference between the dissecting steps used for fixative-fixed nose and for fresh nose although the fresh tissue is softer and requires more careful manipulation. In both preparations, keeping the tissue humidified during the dissection is important. It is not necessary to perform the fresh tissue dissection in buffered saline, however, periodically dipping the tissue in Tyrode’s solution or dripping the solution onto the tissue while dissecting is critical as it will keep the tissue humidified and nutrient supplied to maintain its viability.
The procedure presented here can be further modified to include bone removal that leaves both the brain and nose intact in a single specimen (Figure 2B). This modification spares the nerve connections between the brain and nose that pass through the cribriform plate. More technical skill and time are required for this dissection than the nose alone. However, preserving the connections from the periphery to the brain allows for more diverse applications of this method.
Limitations
We have used this method to prepare nasal tissues for a number of studies, including immunohistochemical labeling and mRNA in situ hybridization analysis, and have successfully labeled many proteins in signaling pathways, stained cell markers in various cell populations, and examined morphological features and cell distribution through the nasal cavity16,17,36-39. We did not experience limitations in cryosectioning and immunolabeling. For studies that require cryosectioning of the extended preparation that keeps the brain and nasal tissue connected in a single specimen, we recommend to use mice younger than 4 months since in older mice the brittle cribriform plate may create tissue damage during sectioning. However, for fresh tissue intended for electrophysiological recordings, the medial tissues between turbinates are not readily accessible in the whole mount preparation. Removal of small pieces of tissue or splitting in discrete regions may overcome the problem. Because conventional electro-olfactogram is done on the medial surface of endo-turbinates 25,33,34, our tissue preparation may allow recordings from other regions with little manipulation.
Applications beyond Immunohistochemistry
Many applications of the procedure exist for those who have mastered the technique. Possible applications include zonal studies on olfactory and respiratory tissues which, using other methods, have been difficult to obtain intact and in their original anatomical configuration. Applications also exist for electrophysiology by allowing simultaneous multiple-recordings on whole, fresh tissues. Some of these applications, which were difficult to perform using existing techniques, are made possible by our deboning method. Further, this protocol can also be adapted to biochemical, genomic, and proteomic studies that require consistent sample collection at various regions for critical comparison.
In summary, we have developed a procedure to rapidly and reliably access olfactory and respiratory tissues in the mouse nose by removing the surrounding bones. This method has significant advantages over commonly used techniques such as decalcification. Further, our method requires no chemical treatment; therefore, tissues are not limited for use in immunohistochemistry experiments, but also can be applicable for in situ hybridization and physiological studies. Thus, our method is a faster, more direct way to prepare the mouse nose for further processing. We expect our method will facilitate olfactory and respiratory studies in nasal regions.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by research grants (NIH/NIDCD 009269, 012831 and ARRA administrative supplement NIH grants) to Weihong Lin. We especially thank Mr. Tim Ford at UMBC for his technical assistance in videotaping and processing. We also wish to thank Dr. Daphne Blumberg, Ms. Chere Petty at UMBC and Mr. Nicholas McCollum from Olympus America Inc. for their equipment assistance in videotaping.
References
- Naclerio RM, Pinto J, Assanasen P, Baroody FM. Observations on the ability of the nose to warm and humidify inspired air. Rhinology. 2007;45:102–111. [PubMed] [Google Scholar]
- Bjermer L. The nose as an air conditioner for the lower airways. Allergy. 1999;54(57):26–30. doi: 10.1111/j.1398-9995.1999.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Bryant B, Silver WL. Chemisthesis: The common chemical sense. 2nd. Wiley-Liss; 2000. [Google Scholar]
- Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. J. Anat. 1982;135:83–88. [PMC free article] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu. Rev. Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Rodolfo-Masera T. Su l'esquoestizenza di un particulare organo olfacttivo nel setto nasale della cavia e di altri roditori. Arch. Ital. Anat. Embryol. 1943;48:157–212. [Google Scholar]
- Levai O, Strotmann J. Projection pattern of nerve fibers from the septal organ: DiI-tracing studies with transgenic OMP mice. Histochemistry and Cell biology. 2003;120:483–492. doi: 10.1007/s00418-003-0594-4. [DOI] [PubMed] [Google Scholar]
- Storan MJ, Key B. Septal organ of Gruneberg is part of the olfactory system. J. Comp. Neurol. 2006;494:834–844. doi: 10.1002/cne.20858. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm. Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol. Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 2009;71:115–140. doi: 10.1146/annurev.physiol.70.113006.100608. [DOI] [PubMed] [Google Scholar]
- Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J. Comp. Neurol. 1990;294:293–305. doi: 10.1002/cne.902940212. [DOI] [PubMed] [Google Scholar]
- Papka RE, Matulionis DH. Association of substance-P-immunoreactive nerves with the murine olfactory mucosa. Cell Tissue Res. 1983;230:517–525. doi: 10.1007/BF00216198. [DOI] [PubMed] [Google Scholar]
- Baraniuk JN, Kim D. Nasonasal reflexes, the nasal cycle, and sneeze. Curr. Allergy Asthma Rep. 2007;7:105–111. doi: 10.1007/s11882-007-0007-1. [DOI] [PubMed] [Google Scholar]
- Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J. Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- Ogura T, et al. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J. Neurophysiol. 2011;106:1274–1287. doi: 10.1152/jn.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J. Neurophysiol. 2008;99:2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chemical Senses. 2006;31:107–118. doi: 10.1093/chemse/bjj008. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Fulle HJ, et al. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juilfs DM, et al. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. The Journal of Neuroscience: The Official journal of the Society for Neuroscience. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Omura M, Mombaerts P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J. Neurocyt. 2004;33:657–669. doi: 10.1007/s11068-005-3334-y. [DOI] [PubMed] [Google Scholar]
- Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chemical Senses. 2009;34:695–703. doi: 10.1093/chemse/bjp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 2011;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JB, Mason GI. Influence of decalcifying agents on immunoreactivity of formalin-fixed, paraffin-embedded tissue. Histochem J. 1984;16:771–787. doi: 10.1007/BF01095281. [DOI] [PubMed] [Google Scholar]
- Athanasou NA, Quinn J, Heryet A, Woods CG, McGee JO. Effect of decalcification agents on immunoreactivity of cellular antigens. J. Clin. Pathol. 1987;40:874–878. doi: 10.1136/jcp.40.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehr M, et al. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. The Journal of Neuroscience: the official journal of the Society for Neuroscience. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Chen WR, Shepherd GM. Electrophysiological characterization of rat and mouse olfactory receptor neurons from an intact epithelial preparation. J. Neurosci. Methods. 1999;92:31–40. doi: 10.1016/s0165-0270(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Cygnar KD, Stephan AB, Zhao H. Analyzing responses of mouse olfactory sensory neurons using the air-phase electroolfactogram recording. J. Vis. Exp. 2010. p. e1850. [DOI] [PMC free article] [PubMed]
- Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012. p. e3564. [DOI] [PMC free article] [PubMed]
- Lin W, Margolskee R, Donnert G, Hell SW, Restrepo D. Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2471–2476. doi: 10.1073/pnas.0610201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ezekwe EA, Zhao Z, Liman ER, Restrepo D. TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci. 2008;9:114. doi: 10.1186/1471-2202-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. Chemoreception regulates chemical access to mouse vomeronasal organ: role of solitary chemosensory cells. PLoS One. 2010;5:e11924. doi: 10.1371/journal.pone.0011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan A, Feijoo AA, Mehta ST, Nimarko AF, Lin W. Expression profile of G-protein βγ subunit gene transcripts in the mouse olfactory sensory epithelia. Frontiers in Cellular Neuroscience. 2013;7:84. doi: 10.3389/fncel.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]


