Abstract
The canonical AX-CPT task measures two forms of cognitive control: sustained goal-oriented control (“proactive” control) and transient changes in cognitive control following unexpected events (“reactive” control). We modified this task by adding negative and neutral International Affective Picture System (IAPS) pictures to assess the effects of negative emotion on these two forms of cognitive control. Proactive and reactive control styles were assessed based on measures of behavior and electrophysiology, including the N2 event-related potential component and source space activation (Low Resolution Tomography [LORETA]). We found slower reaction-times and greater DLPFC activation for negative relative to neutral stimuli. Additionally, we found that a proactive style of responding was related to less prefrontal activation (interpreted to reflect increased efficiency of processing) during actively maintained previously cued information and that a reactive style of responding was related to less prefrontal activation (interpreted to reflect increased efficiency of processing) during just-in-time environmentally triggered information. This pattern of results was evident in relatively neutral contexts, but in the face of negative emotion, these associations were not found, suggesting potential response style-by-emotion interaction effects on prefrontal neural activation
Keywords: emotion, AX-CPT, reactive control, proactive control, N2, LORETA, prefrontal neural activation
1.0, Introduction
Being able to control one’s behavior, in response to both planned and unexpected events, is critical for socio-emotional functioning, especially when faced with emotionally challenging environments. Cognitive control is a heterogeneous set of psychological processes that can be parsed into unique constructs, including proactive and reactive control. According to the Dual Mechanism of Control (DMC) model, the term “proactive control” refers to psychological processes leading to deployment of planned action patterns derived through actively-maintained contextual information (Braver, Gray, & Burges, 2007). The term “reactive control” refers to psychological processes evoked by stimuli that change action patterns (Braver, et al., 2007). These two processes can be deployed in distinct fashions reflecting different styles of responding. Thus, a more proactive control style of responding leads to better performance in situations that allow for previously planned and strategically executed action strategies, while a more reactive control style of responding leads to better performance in situations that require last minute adjustments to action strategies based on environmental cues. The present study extends the extant literature on these control processes: 1) by comparing the impact of neutral and negative contexts on neural activation underlying events that require proactive and reactive control; and 2) by examining the relations between a person’s relative degree of proactive or reactive control style and underlying neural activation, both in neutral and negative-valence contexts.
Proactive and reactive control mechanisms are generally investigated in the context of one particular task, the AX-CPT, a type of continuous-performance task (CPT; Rosvold, Mirsky, Sarason, Bransome, & Beck, 1956). This task consists of a cue, to which participants have to provide a speeded response, then a delay period, and then a probe, to which participants have to provide a second speeded response. The combined cue and probe information informs the participant on the type of trial being presented and thus the required responses. Proactive control processes are recruited during the cue time period and sustained over the delay time period to actively maintain planned action strategies. Transient reactive control processes, on the other hand, are recruited during the probe time period, either to elicit the primed motor response or to adjust action strategies based on new contextual information. Additionally, the current study uses the Behavior Shift Index (BSI; Braver et al. 2009), a measure generated from task reaction times and error rates, to ascertain a participant’s control style (i.e., more reactive or proactive in nature). This provides a person-specific measure from the task that can be linked to other individual-difference variables, such as personality factors or level of anxiety, which have been linked to styles of cognitive control. Lastly, separate blocks of trials were created, containing either neutral or negative affectively charged pictures. A picture from one of these categories was presented during the delay period, so that neural activation underlying events that illicit proactive and reactive control could be measured in the context of either neutral or negative affective stimuli.
A number of brain regions have been linked with the recruitment of proactive and reactive control processes, including areas of prefrontal cortex. Specifically, recruitment of proactive and reactive control have been associated with activation in the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulate cortex (ACC), and ventromedial prefrontal cortex (VMPFC; Braver et al., 2007; Braver et al., 2009; Krug & Carter, 2012; Nee & Brown, 2012; Paxton, Barch, Racine, & Braver, 2007). However, at present it is unclear if these prefrontal activation patterns for proactive and reactive control change in the context of negative emotion.
Tasks that require other cognitive control processes have yielded prefrontal cortical activation differences depending on the emotional context of the task. For example, Monk et al. (2003) found greater ACC activation to fearful faces than neutral faces during an attention task; Ochsner et al. (2004) found ACC, VLPFC, and DLPFC activation during emotional up-regulation and down-regulation; and lastly, Lamm and Lewis (2010) found elevated VMPFC activation for a negative condition compared to a neutral condition in a motivated go/no-go task. Thus, it may be that prefrontal activation underlying the recruitment of proactive and reactive control processes could also reveal emotion-specific differences.
Prior work also generates specific hypotheses concerning the impact of emotion on neural correlates of cognitive control. Specifically, based on event-related potential (ERP) profiles, van Wouwe, Band, and Ridderinkhof (2010) suggest that reactive control processes, but not proactive control processes, are differentially recruited in unemotional and emotional contexts. This study found decreased (less negative) N2 activation for reactive control in the context of positive affectively charged stimuli compared to neutral stimuli. However, because this study did not include negative emotion, it remains unclear how proactive and reactive control mechanisms are recruited in the context of negative emotion. The present study examined ERP activation underlying proactive and reactive control in the context of both relatively neutral pictures and negative pictures. We examined behavioral performance and N2 activation—an ERP component associated with cognitive control (Folstein & Van Petten, 2008)—for time periods that required proactive and reactive control. We also performed a Low Resolution Tomography (LORETA) analysis to estimate cortical activation. We then exported activation values for four regions of interest (ROIs): the DLPFC, dorsal ACC, VLPFC, and VMPFC. Building on the van Wouwe et al. (2010) results and based on findings from other cognitive control tasks (e.g., Lamm & Lewis, 2010), we predicted greater activation for the negative condition than the neutral condition.
Finally, we attempted to extend previous neuroimaging studies linking neural processing efficiency with reduced neural activation during cognitive control tasks (Casey et al., 1997; Durston et al., 2006). Here we examined associations between individual differences in response style and brain function, predicting that participants who utilize a more proactive control style of responding would recruit fewer neural resources during the cue and delay periods than individuals utilizing alternative styles. Furthermore, we predicted that participants who exhibit a more reactive control style of responding would reveal less activation during the probe period than individuals utilizing alternative styles. We predicted this pattern of activation for the neutral condition, based on prior results specifically in this context. However, since negative emotions have complex effects on neural activation patterns (Lamm & Lewis, 2010; Lamm, White, McDermott, & Fox, 2012), it is unclear if the previously outlined associations between control style and brain activation would be moderated by the negative condition. More specifically, control style/brain activation associations may show the same pattern of effects in the negative condition compared to the neutral condition but simply at elevated activation levels or the increased more effortful activation for the negative condition may “flood” control style/brain activation associations and thus show few significant effects. Moreover, given prior work documenting associations in cognitive control, effects of negative emotion on brain function, and individual differences in anxiety, we also explored the ways in which individual differences in anxiety related to response style and neural activation.
2.0, Method
2.1, Participants
Thirty-two undergraduate students (age M = 20.19, SD = 4.40, range = 18 – 39 yrs, 14 males) participated in the current study. Participants were recruited through Psychology and Human Development Department undergraduate classes at the University of Maryland. An additional seven participants were excluded due to insufficient artifact free trials. These seven excluded participants did not differ from included participants in demographic factors, such as age, gender, and country of origin. All participants had normal or corrected to normal vision. The current study received IRB approval from the University of Maryland.
2.2, Procedure
Participants were seated in a chair 67 cm from the computer screen and completed the State and Trait anxiety questionnaires of the State-Trait Anxiety Index (STAI). Next, the electrode sensor net (Electrical Geodesic, Inc., Eugene) was applied and the emotional AX-CPT task was administered. Between each block of the task, participants were given the opportunity to stretch and ask questions. After the task was completed, the STAI State Anxiety Questionnaire was administered a second time. Upon completion of the study, participants were given credit to be applied to a psychology class.
2.3, Measures
Spielberger State Trait Anxiety Inventory (STAI; Spielberger, 1983)
The STAI is a reliable and valid measure capturing both state and trait anxiety. In the state measure, participants are asked to respond to 20 items describing how they are feeling ‘right now, at this moment’. The trait measure asks participants to respond to 20 items describing how they ‘generally feel’
Emotional AX-CPT
Images were presented on a 17-in monitor using Eprime Software (Psychology Software Tools, Inc., Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002). Stimuli were shown on a black screen and consisted of negative and neutral photos from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) and single letters presented in either blue (cue) or white (probe). Negative and neutral pictures were 11 cm wide by 8 cm tall and presented in black and white (visual angle was 9.39 degrees). Letters were presented in 60-point size uppercase bold Courier New font. Trials were 3.7 seconds in duration and consisted of the following events (see Figure 1): fixation (200 ms), cue (500 ms), delay (2000 ms), probe (500 ms), and post probe fixation (500 ms). The delay period was comprised of fixation (1000 ms), IAPS picture (500 ms), and fixation (500 ms). Neutral and negative pictures were presented during the delay in two separate blocks, a neutral picture block and a negative picture block, and not randomized by trial, because of potential emotional carry-over effects. A block presenting positive-valence pictures was not included in this task since this would have increased the task length by 300 trials, which was deemed too long.
Figure 1.
Structure of AX-CPT task including both sequence of events and trial types. Figure shows example of negative IAPS pictures. Task design for the neutral condition was identical but showed neutral IAPS pictures.
The task consisted of four trial types: AX, AY, BX, and BY: “A” stands for targeted cues and “X” stands for targeted probes while “B” stands for any nontartet cues and “Y” stands for any nontarget probe. Targets were always “A” and “X” for cues and probes. Nontarget cues and probes were any letter of the alphabet except K, which participants may have confused with X, and only the timing, i.e., if a cue or probe, differentiated how participants should respond to the letter. AX trials were the propensity setting trial type (55% of trials) and required participants to push a 2 after the cue and a 3 after the probe. AY, BX, and BY trial types were presented less frequently (each 15% of trials) and required participants to push the 2 button after both the cue and the probe. Because AX trials were the propensity setting trial type, AY trials required participants to alter their usual action plans from pushing a 3 after the probe to pushing a 2 after the probe. BX trials, on the other hand, required participants to keep-in-mind that a B was presented before the X and not an A. Thus a proactive style of responding, i.e., the use of cue information to prepare for a response, would lead to a stronger bias for a target response after an “A” cue and thus poor performance on “AY” trials. Additionally, a proactive (cue-based) style of responding for non-target “B” trials would lead to better performance on “BX” trials. Alternatively, a reactive control style of responding, i.e., the use of just-in-time probe information to prepare for a response, would lead to better performance on “AY” trials and worse performance on “BX” trials.
Participants completed two practice blocks of 8 trials each in which no pictures were displayed but task performance feedback was provided to ensure task proficiency. Feedback was presented for erroneous cue/probe response patterns or late responding and consisted of a red line, presented for 200 ms. Performance feedback was only provided during the practice block and not during the actual test blocks. Practice blocks were followed by the neutral block and then the negative block. The negative block was always presented after the neutral block to prevent emotional carryover. Each block consisted of 300 unique trials, i.e., each IAPS picture was only presented once. Trial types (AX, AY, BX, or BY) were presented in random order, and equal numbers of each trial type were presented in the neutral and negative conditions.
2.4, EEG data collection and analysis
EEG was recorded using a 64-channel Geodesic Sensor Net and sampled at 250 Hz, using an EGI amplifier and software (Net Station; Electrical Geodesic, Inc., Eugene, OR [data were also processed using Net Station]). Once the impedance values for all EEG channels were reduced to below 50 kΩ, data acquisition was started. During recording, all channels were referenced to Cz and after acquisition, data were re-referenced using an average reference.
Data were filtered using a FIR bandpass filter with a lowpass frequency of 30 Hz and a highpass frequency of .3 Hz. To best capture eye blinks, eye blink artifact thresholds were set to 140µV. Furthermore, signal activation changes across the entire trial of 90 µV were marked as bad and removed after visual inspection. All ERP and source space data were baseline corrected to 200 ms before the stimulus of interest, i.e., cue, picture, or probe. Only data from trials that were correct for both cue and probe events and that had trial counts equal or greater than 10 were analyzed (mean = 28; range = 10–44).
To calculate source space activation, we used a distributed inverse model—which uses the change in activation from one electrode to another (in this case 65 electrodes)—because this type of algorithm estimates activation voxel-by-voxel and sample-by-sample and the user does not have to “fit” any dipoles. We were thereby able to limit the influence of user bias. Specifically, we utilized an algorithm called LORETA, a constraint applied to the minimum-norm solution which minimizes the discrepancy between values of adjacent voxels (to achieve the most realistic model) within the GeoSource interface (Electrical Geodesic, Inc., Eugene, OR; for a review of these constraints and other minimum norm solutions, see Michel, Murray, Lantz, Gonzalez, Spinelli, & Grave de Peralta, 2004). Furthermore, we used a regularization constant (indicating how much noise is modeled) of 10−4. This model, including the amount of regularization, revealed current flow patterns (visual inspection) that matched the grand-averaged scalp topography (collapsing across all conditions to prevent biasing solutions) better than other models or levels (e.g., LORETA: 10−3, 10−5; LAURA: 10−3, 10−5).
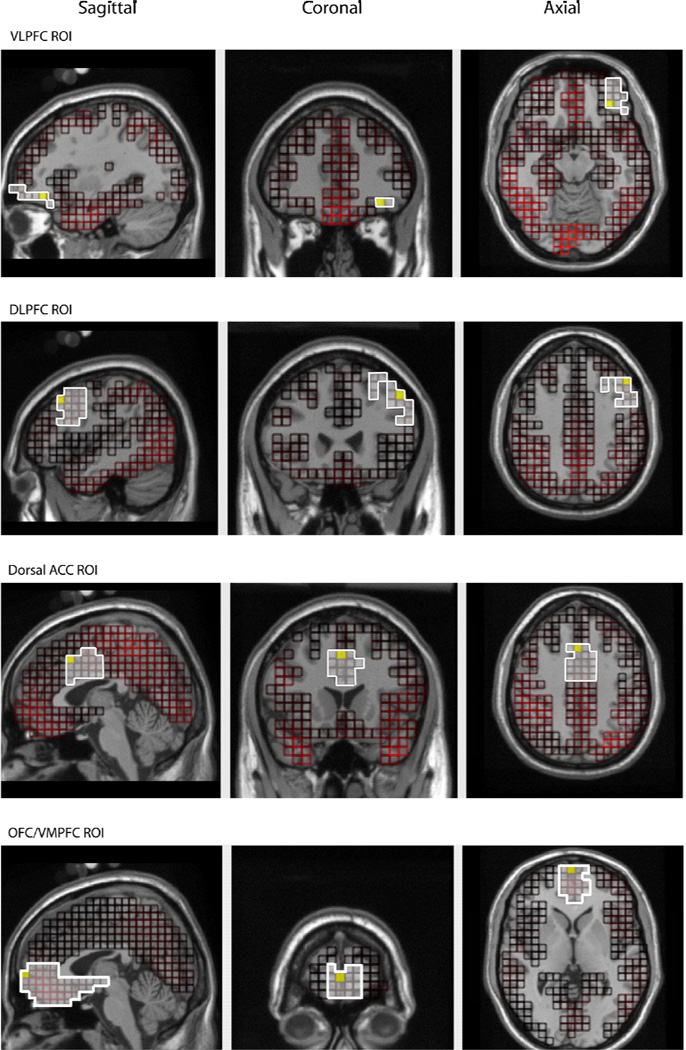
After the inverse model was applied to the entire cortex (2447 voxels), data were exported for morphology-based regions of interest (ROIs), generated using the Montreal Neurological Institute (MNI) average adult MRI. We were interested in four ROIs: dorsal ACC, DLPFC, VLPFC, and VMPFC (see Figure 2). Source waveform amplitudes (nA) for all voxels within an ROI were extracted and the voxel that showed the most activation for each ROI was analyzed.
Figure 2.
Morphology-based regions of interest (ROIs) generated using the Montreal Neurological Institute (MNI) average adult MRI.
2.5, Data Analyses
All data (brain and behavior) with values greater than + or − 2 standard deviations were corrected to +/−2 SD, thus eliminating outliers. A prior t-tests were conducted to test for sex differences. No sex differences were found for reaction times, error rates, scalp N2 activation, and some source-space analyses. However, the raw source-space activation (without subtraction controlling for practice effect) revealed some sex differences. Therefore Sex was entered as a covariate to these analyses only (regression analyses examining BSI/brain activation effects).
A prior correlation analyses were conducted to test for age effects. No age effects were found for any of the measures (i.e., reaction times, error rates, scalp N2 activation, and source-space activation), thus age was not entered as a covariate.
Reaction time
Only trials with correct cue and probe responses were included in the reaction time (RT) data analyses. A 2 (Condition: neutral, negative) by 4 (Trial Type: AX, AY, BX, BY) repeated-measures ANOVA was conducted on cue RTs revealing a main effect of condition, F(1, 31) = 81.00, p < .001, η2 = .72, with the negative condition revealing faster RTs than the neutral condition. Since the negative condition was presented after the neutral condition, and prior literature suggests that RTs slow in the face of negative emotion (e.g., De Houwer & Tibboel, 2010), these results were interpreted to reflect a practice effect. Thus, subsequent condition differences (ANOVA analyses) were analyzed controlling for this practice effect. More specifically, to control for practice effects, condition specific cue RTs, averaged across all trial types, were subtracted from each probe trial type (i.e., AX, AY, BX, and BY). See Table 1 for means and SDs for all behavioral and brain data without correcting for the practice effects.
Table 1.
Raw (without controlling for practice effects) means and SDs for behavioral and brain data
| AX | AY | BX | BY | |
|---|---|---|---|---|
| Cue RT | ||||
| Neutral | 395.10(64.81) | 394.68(63.07) | 455.10(73.02) | 457.97(69.46) |
| Negative | 331.45(50.95) | 332.34(57.43) | 367.76(68.75) | 371.95(65.52) |
| Probe RT | ||||
| Neutral | 384.18(56.87) | 510.58(69.79) | 337.40(98.67) | 341.02(78.65) |
| Negative | 357.66(57.26) | 475.40(64.65) | 307.57(87.16) | 319.63(81.92) |
| Cue & Probe Error | ||||
| Neutral | .08(.05) | .19(.10) | .18(.13) | .11(.08) |
| Negative | .10(.09) | .21(.14) | .16(.11) | .09(.08) |
| Cue N2 | ||||
| Neutral | −4.64(2.02) | −5.23(2.02) | −5.79(2.42) | −5.71(2.38) |
| Negative | −4.37(1.50) | −5.46(2.42) | −6.26(3.21) | −6.34(2.95) |
| Probe N2 | ||||
| Neutral | −4.72(2.33) | −6.95(2.33) | −4.72(2.11) | −5.15(2.28) |
| Negative | −4.15(1.87) | −6.67(3.06) | −4.64(1.90) | −4.64(2.60) |
| Cue DLPFC | ||||
| Neutral | .15(.06) | .28(.14) | .25(.09) | .22(.07) |
| Negative | .18(.08) | .30(.15) | .30(.12) | .30(.11) |
| Cue dorsal ACC | ||||
| Neutral | .11(.05) | .19(.10) | .18(.06) | .16(.06) |
| Negative | .12(.06) | .21(.10) | .20(.09) | .18(.08) |
| Cue VLPFC | ||||
| Neutral | .42(.18) | .75(.29) | .73(.32) | .64(.22) |
| Negative | .48(.23) | .66(.29) | .80(.48) | .72(.34) |
| Cue VMPFC | ||||
| Neutral | .79(.49) | 1.40(.80) | 1.37(.58) | 1.17(.56) |
| Negative | .88(.60) | 1.17(.69) | 1.29(.84) | 1.20(.73) |
| Delay DLPFC | ||||
| Neutral | .24(.08) | .42(.14) | .40(.22) | .36(.12) |
| Negative | .31(.12) | .44(.16) | .52(.20) | .45(.16) |
| Delay dorsal ACC | ||||
| Neutral | .16(.05) | .24(.06) | .22(.08) | .25(.10) |
| Negative | .20(.06) | .27(.09) | .28(.10) | .27(.10) |
| Delay VLPFC | ||||
| Neutral | .33(.10) | .53(.16) | .55(.25) | .50(.16) |
| Negative | .37(.16) | .57(.21) | .54(.18) | .54(.16) |
| Delay VMPFC | ||||
| Neutral | .58(.23) | .91(.36) | .98(.36) | .93(.36) |
| Negative | .65(.34) | .94(.39) | .97(.44) | .91(.35) |
| Probe DLPFC | ||||
| Neutral | .26(.10) | .35(.10) | .36(.12) | .32(.11) |
| Negative | .30(.13) | .44(.14) | .36(.12) | .38(.14) |
| Probe dorsal ACC | ||||
| Neutral | .20(.08) | .29(.11) | .25(.09) | .26(.11) |
| Negative | .23(.11) | .30(.11) | .26(.11) | .26(.11) |
| Probe VLPFC | ||||
| Neutral | .35(.13) | .44(.15) | .45(.17) | .40(.17) |
| Negative | .37(.17) | .50(.16) | .44(.13) | .44(.14) |
| Probe VMPFC | ||||
| Neutral | .52(.20) | .70(.25) | .61(.23) | .55(.22) |
| Negative | .55(.20) | .73(.25) | .61(.18) | .59(.23) |
Error Rate
Performance accuracy data consisted of cue and probe accuracy scores, i.e., only if both the cue and probe were accurate was the trial considered accurate and included in subsequent analyses. Error rate was calculated as 1-performance accuracy.
Behavioral Shift Index (BSI)
Based on the works of Braver and colleagues (Braver, Paxton, Locke, and Barch, 2009; supporting information) a Behavioral Shift Index was calculated (AY−BX)/(AY+BX) for both RTs and error rates to show control style (i.e., more proactive or reactive control style). Since the RT and error rate BSI scales were highly correlated (neutral: r = .80, p = .003; negative: r = .46, p = .008) all scores were standardized and a composite measures was generated by averaging the RT and error rate PSI values, separately for the neutral and negative conditions. This procedure yielded one neutral and one negative BSI composite scale, which were used for all further BSI analyses. More positive values indicate a proactive style of responding while less positive values indicate a more reactive style of responding. Neutral and Negative condition BSI scores were highly correlated, r = .73, p < .001.
Electrophysiological analyses
All N2 and source-space analyses were based on trials with correct cue and probe responses. Scalp N2 activation was exported for 8 mediofrontal electrodes: four midline electrodes (8, 6 [Fz], 4 [FCz], and VREF [Cz]) as well as 4 flanking electrodes (9, 7, 3, 54; two on each side) for the time period of the N2 (230–600 ms after stimulus onset). Because of individual differences in peak N2 activation across electrodes, each participant’s greatest (most negative) activation was analyzed. Even though proactive control is viewed as a protracted period of control (Braver, et al., 2007), a longer time range was not used for N2 analyses because the latency range of the N2 traditionally does not extend beyond this time period. For the source-space analyses however, neural activation underlying the recruitment of proactive control processes was measured as mean activation across time for two longer time periods: First, early, cue-based proactive control starting 230 ms after cue onset and continuing to 1000 ms after cue onset, and second, late, delay-based proactive control starting at picture onset time and continuing to 1000 ms after picture onset time. We measured neural activation underlying proactive control by subtracting the average activation of all trial types (i.e. AX, AY, BX, and BY) from BX activation for cue and delay time periods, thus controlling for potential practice effects (See Tables 1 for means and SDs for uncorrected data). Because reactive control is a brief stimulus-oriented change in control mechanism (Braver, et al., 2007), we captured it by measuring peak ROI activation 230–600 ms after probe onset, the time period marked by the N2. To control for potential practice effects, we subtracted the average activation of all trial types (i.e., AX, AY, BX, and BY) from AY trials for probe activation (See Tables 1 for means and SDs for uncorrected data). To assess the quality of the LORETA model, correlations were conducted between each ROI (DLPFC, dorsal ACC, VLPFC, and VMPFC) and scalp N2 activation. Results revealed that each ROI significantly correlated with scalp N2 activation (p < .05). Lastly, due to violations of sphericity, all ANOVA models were conducted with the Greenhouse Geisser correction.
3.0, Results
3.1, Emotion Check Analysis
To determine if the task altered participants’ emotional state, the STAI state anxiety measure was administered before and after the emotional AX-CPT task. A t-test was conducted between the before and after state measurements, t(31) = −3.11, p = .004 (before: mean = 35.44, SD = 11.28; after: mean = 43.09, SD = 12.47), revealing greater state anxiety after the task than before, suggesting that viewing affectively charged pictures increased participant’s levels of state anxiety.
3.2, Behavioral Results
Reaction time
A 2 (Condition: neutral, negative) by 4 (Trial Type: AX, AY, BX, BY) repeated-measures ANOVA was conducted on probe RTs revealing a main effect of condition, F(1, 31) = 27.87, p < .001, η2 = .47, with the negative condition revealing slower RTs than the neutral condition. We also found a main effect of trial type, F(3, 93) = 132.23, p < .001, η2 = .81 (see Figure 3). Contrasts revealed slower RTs for the AY trial type than the AX, BX, and BY trial types (all at p < .001). Additionally, contrasts also revealed slower RTs for the AX trial type than the BX and BY trial types (all at p < .001). These reaction time results provide additional evidence of emotional interference during the negative condition.
Figure 3.
left: probe reaction times. Right: combined cue and probe error rate (accurate only if both cue and probe response were correct.
Error rate
A 2 (Condition: neutral, negative) by 4 (Trial Type: AX, AY, BX, BY) repeated-measures ANOVA was conducted on error rate, revealing a main effect of trial type, F(3, 93) = 20.63, p < .001, η2 = .40. Contrasts revealed higher error rates for the AY trial type than the AX and BY trial types (all at p < .001). Additionally, the BX trial type revealed higher error rates than the AX and BY trial types (all at p < .001; see Figure 3). Thus, the AY and BX trial types revealed higher error rates and the AY trial type also showed slower RTs.
3.3, Electrophysiological Results
N2 Results
A 2 (Condition: neutral, negative) by 2 (Event: probe AY, cue BX) repeated-measures ANOVA was conducted on N2 activation. Results revealed a main effect of Event, F(1, 31) = 15.27, p < .001, η2 = .33., with the probe event showing greater (more negative) activation than the cue event (see Figure 4). No condition differences were found.
Figure 4.
Brain activation differences across conditions (neutral vs. negative) and events (cue, delay, and probe). Image A: N2 scalp activation. Image B: ERP waveforms for correct trials. Image C: dorsolateral prefrontal cortex (DLPFC) activation. Image D: dorsal anterior cingulate (ACC) activation. Image E: ventromedial prefrontal cortex (VMPFC) activation. Image F: ventrolateral prefrontal cortex (VLPFC) activation. For images A and B, more activation is down. For images C, D, E, and F, more activation is up.
Source-Space Results
A 2 (Condition: neutral, negative) by 3 (Event: probe AY, cue BX, delay BX) repeated-measures ANOVA was conducted separately for each ROI (DLPFC, dorsal ACC, VLPFC, and VMPFC). For the DLPFC analysis, results revealed a main effect of Condition, F(1, 31) = 10.70, p = .003, η2 = .26 (see Figure 4), with the negative condition showing greater activation than the neutral condition. For the dorsal ACC analysis, results revealed a trend level main effect of Event, F(2, 62) = 3.12, p = .06, η2 = .09. Contrasts revealed greater probe activation than both cue (p = .06, trend level) and delay (p = .05) activation. For the VLPFC analysis, results revealed a main effect of Event, F(2, 62) = 3.41, p = .05, η2 = .10. Contrasts revealed greater cue activation than both probe (p = .06, trend level) and delay (p = .04) activation. No main effects or interactions were found for VMPFC.
Additionally, linear regression analyses were conducted to examine the relationship between the BSI composite and source-space activation for each ROI (DLPFC, dorsal ACC, VLPFC, and VMPFC), Condition (neutral, negative), and Event (probe, cue, and delay). For the probe trials, results revealed a positive association between the BSI composite and DLPFC (β = .04, t = 2.12, p = .04) and VMPFC (β = .10, t = 1.92, p = .06, trend level) activation for the neutral condition, and between BSI composite and VMPFC (β = .11, t = 2.15, p = .04) activation for the negative condition. Secondary regression analyses were conducted to determine if neutral condition effects were greater than negative condition effects or the reverse. Results revealed that the association between the BSI composite and probe DLPFC activation for the neutral condition was still significant after controlling for negative condition activation, β = .04, t = 2.44, p = .02, suggesting this effect was specific to the neutral condition. Secondary analyses for VMPFC were non-significant, indicating that the association between BSI and probe VMPFC activation was not unique to either neutral or negative trials.
Furthermore, results revealed negative associations between the BSI composite and cue activation for the DLPFC, β = −.04, t = −2.22, p = .03, VMPFC, β = −.35, t = −3.23, p = .003, and VLPFC, β = −.17, t = −2.77, p = .01, but only for the neutral condition and not the negative condition. Secondary regression analyses were conducted to determine if neutral condition effects were greater than negative condition effects or the reverse. Results revealed that the association between the BSI composite and cue DLPFC, β = −.04, t = −2.01, p = .05, VMPFC, β = −.33, t = −3.16, p = .004, and VLPFC, β = −.14, t = −2.54, p = .02, activation for the neutral condition was still significant after controlling for negative condition activation, again suggesting this effect was specific to the neutral condition.
Results also revealed a negative association between BSI composite and delay activation for the VLPFC, β = −.12, t = −2.58, p = .02, and VMPFC, β = −.13, t = −1.78, p = .09 (trend level), however, again only for the neutral condition. Secondary regression analyses were conducted to determine if neutral condition effects were greater than negative condition effects or the reverse. Results revealed that the association between the BSI composite and delay VLPFC activation for the neutral condition was still significant after controlling for negative condition activation, β = − .11, t = −2.38, p = .02, again suggesting this effect was specific to the neutral condition.
Lastly, a moderation analysis was conducted to ascertain if increases in state anxiety from before to after the task moderated the relation between BSI and brain activation for the negative condition. Results revealed no significant effects.
4.0, Discussion
The present study examined behavioral performance and prefrontal cortical activation underlying events that illicit proactive and reactive control using an AX-CPT task with neutral and negative charged pictures. Cortical activation was captured by analyzing N2 activation and source space activation (LORETA: specifically DLPFC, dorsal ACC, VLPFC, and VMPFC). Results from the current study address two broad research questions: 1) does neural activation underlying events that require proactive and reactive control processes differ in the context of negative vs. neutral affectively-charged pictures; and 2) is a person’s control style (i.e., more proactive or reactive in nature) associated with neural activation, either more generally or specifically in either neutral or negative emotional contexts.
4.1, Neutral vs. Negative Contexts
Results revealed greater DLPFC activation for the negative condition than the neutral condition, across cue, delay, and probe events. These results are in line with other studies that used N2 activation to measure cognitive control in the context of emotion. For example, a number of studies have found increased (more negative) no-go N2 activation (e.g., Lewis, Lamm, Segalowitz, Steiben, & Zelazo, 2006) and increased source-space activation underlying the N2 (Lamm, Granic, Zelazo, & Lewis, 2011; Lamm & Lewis, 2010) for a negative condition (loss of points) than a neutral condition during a go/no-go task. Additionally, van Wouwe et al. (2009) found less N2 activation after a positive video clip compared to after a neutral video clip during an AX-CPT task. Lastly, Lamm, White, McDermott, and Fox (2012) found greater DLPFC activation for a negative condition (scary animal pictures) compared to a neutral condition (non-scary animal pictures) using a go/no-go task. Together, this pattern of results suggests that effectively applying cognitive control in the context of negative emotion may require additional prefrontal resources, particularly in DLPFC, than when applying cognitive control in relatively unemotional contexts.
As outlined above, van Wouwe et al. (2009) showed less activation for the N2 (scalp) after a positive video clip vs. a neutral video clip. In the current study, we did not find N2 (scalp) activation differences between our negative and neutral conditions but we did find predicted condition differences for source-space data, i.e., greater activation during the negative condition compared to the neutral condition. It is not clear why the current study and the van Wouwe et al. (2009) study showed different patterns of results, though the marked differences in the emotional manipulation represents one possible explanation, i.e., differences in stimulus valence (positive vs. negative charged stimuli) or stimulus salience (still IAPS pictures vs. video clips). Since scalp activation is the sum of all cortical activation projecting to that electrode at that moment in time, the cross study differences also could arise from variable cortical projects. More specifically, in the current study, multiple cortical regions, including regions not directly related to cognitive control, may have projected to the mediofrontal area, thus essentially “watering down” the signal (see Lamm & Lewis, 2010 for more information).
4.2, Behavior Shift Index (BSI) Composite
A number of neuroimaging studies have shown decreased neural activation associated with more efficient neural processing (Casey et al., 1997; Durston et al., 2006). The results of these studies, viewed in the context of the Dual Mechanism of Control (DMC) model (Braver, Gray, & Burgess, 2007; Braver, Paxton, Locke, & Barch, 2009), suggest that efficient recruitment of proactive control processes might be associated with less cue-based PFC activation and that recruitment of efficient reactive control processes might be associated with probe-based PFC activation. As a result, we predicted differential patterns of activation for participants who used more proactive vs. reactive styles of responding. More specifically, we predicted that participants who used a more proactive style of responding (using the BSI composite measure) would show less PFC activation during cue and delay periods. We predicted this negative association because individuals who routinely use a proactive style of responding should have practice keeping action plans in mind (active maintenance) during the cue and delay time periods. Therefore, we interpret this proactive-style-related decrease in activation to reflect more efficient processing. Our results supported our prediction, indicating that participants with a more proactive style of responding did indeed show less cue and delay period activation for the DLPFC, VLPFC, and VMPFC for neutral trials.
Similarly, we predicted less PFC activation (interpreted as more efficient processing) for individuals with a more reactive style of responding during the probe time period, a time period that requires “just-in-time” changes in action strategies based on environmental information. Again, our results supported our predictions; participants with a reactive style in responding showed less DLPFC and VMPFC activation during the probe time period for neutral trials.
Our hypotheses for the negative condition were less clear. Given the complex nature of associations between prefrontal activation and cognitive control across neutral and negative contexts (e.g., Lamm & Lewis, 2010; Lamm, White, McDermott, & Fox, 2012), clear predictions could not be generated concerning the nature of associations between BSI and PFC activation. Our results suggest person-specific effects from negatively-valenced stimuli may attenuate associations between BSI and PFC activation. Most of our results that were evident during the neutral condition were no longer evident during the negative condition. Additionally, most of the effects during the neutral condition, when reanalyzed controlling for the negative condition, were still significant, indicating that these associations between the BSI and PFC activation were unique to the neutral condition. Furthermore, the one BSI/PFC activation association evident for the negative condition did not survive controlling for neutral activation, indicating that is was not unique to the negative condition. These results, in conjunction with the fact that response styles (BSI index) did not change substantially between the neutral and negative conditions (highly correlated), suggest variability in emotion-induced recruitment of neural processes beyond a simple additive model. In the future, replication of this study including groups of participants prescreened for level of emotionality might allow for a thorough investigation of potential emotion-by-response style interactions.
4.3, Cue, Delay, Probe Event Effects
According to the DMC model, prefrontal neural activation underlying events that require proactive control and reactive control processes include lateral PFC, with activation underlying proactive control processes being sustained while activation underlying reactive control processes being more transient (Braver, Gray, and Burgess, 2007; Braver, Paxton, Locke, & Barch, 2009). Additionally, the DMC model suggests that neural activation underlying reactive control processes also includes dorsal ACC activation, in a conflict monitoring capacity. In other words, when conflict between the originally planned action strategy and the new environmentally cued information occurs (i.e., specifically the probe AY time period), the dorsal ACC becomes involved.
Our results support the DMC model. Firstly, for dorsal ACC, we found greater activation for the probe time period (probe AY) than both the cue and delay time periods. This result supports the notion of elevated dorsal ACC activation underlying the heightened conflict occurring during the probe AY time period, a time period that requires reactive control processing.
Secondly, we found no differences between cue, delay, and probe time periods in DLPFC activation, which is in line with the DMC model assertion that DLPFC fosters both sustained activation underlying proactive control and transient activation underlying reactive control. Additionally, these results in conjunction with the fact that our cue and delay activation was computed as mean activation for lengthy periods of time (cue: 230–1000 ms after cue onset; delay: 0–1000 ms after picture onset; i.e., sustained activation) while our probe activation was calculated as maximum activation during a brief period of time (between 230 and 600 ms after probe onset; i.e., transient activation) adds further support for the DMC model, and its notion of flexible DLPFC activation.
Lastly, our VLPFC results showed greater activation for the cue time period than both the delay and probe time periods. These results are in line with the DMC model (Braver, Gray, & Burgess, 2007; Burgess & Braver, 2004) and studies investigating cognitive interference (e.g., Jonides & Nee, 2006). Specifically, anticipated interference during the cue time period has been associated with elevated VLPFC activation (Burgess & Braver, 2004).
4.4, Limitations
There are limitations to the current study. First, the use of source-space analyses allowed us to ask region specific questions which scalp ERPs do not. However, activation patterns are estimated effects and therefore should be interpreted with caution.
Second, the current study presented participants with neutral and negative affectively charged pictures. Because this study did not include positive affectively charged pictures, it is not clear if our condition effects are due to valence or arousal. Van Wouwe et al. (2010) found decreased magnitude of activation for the N2 (less negative activation) for a positive condition compared to a neutral condition, for reactive control, using a similar AX-CPT task that presented positive affectively charged video clips. Since the van Wouwe et al. (2010) study found less N2 activation for positive affectively charged stimuli and we found more N2 activation for negative affectively charged stimuli, it is likely that our prefrontal condition effects are due to the valence of the stimuli and not just due to arousal. However, future research should examine this issue within one task.
4.5, Conclusions
The current study found emotion-related increases in response times and DLPFC activation. Additionally, we found that a proactive style of responding was related to more efficient neural processing during actively maintained previously cued information and that a reactive style of responding was related to more efficient neural processing during just-in-time environmentally triggered information, in line with the Dual Mechanisms of Control model (e.g., Braver et al., 2007). This pattern of results was evident in relatively neutral contexts, but in the face of negative emotion, these associations were not found, suggesting potential response style-by-emotion interaction effects on prefrontal neural activation.
Highlights.
-
-
AX-CPT paradigm was administered with neutral and negative IAPS pictures
-
-
N2 (ERP) and LORETA (source space) activation was measured
-
-
Behavior shift index (BSI), measure of response style, was computed
-
-
A proactive style of responding related to less PFC activation while planning action strategies
-
-
A reactive style of responding related to less PFC activation while adjusting action strategies
Acknowledgements
The project described was supported by Grant Number P50MH078105 to Megan R. Gunnar from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors also thank Anna Kresse for her dedication in collecting this data in a timely manner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson M, Ochsner K, Kuhl B, Cooper J, Robertson E, Gabrieli S, et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Dockree PM, Aimola L, Robertson IH, Robertson Attenuation of spatial attentional asymmetries with poor sustained attention. NeuroReport. 2004;15(6):1065–1069. doi: 10.1097/00001756-200404290-00027. [DOI] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, et al. Brain regions underlying response inhibition and interference monitoring and suppression. European Journal of Neuroscience. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom L, Fissell K, Carter C, Cohen J. Conflict monitoring versus selection-for-action in anterior cingulated cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse J, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Carter C, Braver T, Barch D, Botvinick M, Noll D, Cohen J. Anterior Cingulate Cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol M-J, van der Wee NJA, et al. Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010;49:963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Science. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Archives of General Psychiatry. 2011;68:654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Rothbart MK. Developing a model for adult temperament. Journal of Research in Personality. 2007;41:868–888. [Google Scholar]
- Folstein JR, van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable Executive Functions in the Dynamic Control of Behavior: Inhibition, Error Detection, and Correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MGH. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Sniedt FLF, Kemner C. Source localization of the Nogo-N2: A developmental study. Clinical Neurophysiology. 2007;118:1069–1077. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Krug MK, Carter CS. Proactive and reactive control during emotional interference and its relationship to trait anxiety. Brain Research. 2012;1481:13–36. doi: 10.1016/j.brainres.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville, FL: University of Florida; 2008. International affective picture system(IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. [Google Scholar]
- Lamm C, Granic I, Zelazo PD, Lewis MD. Magnitude and chronometry of neural mechanisms of emotion regulation in subtypes of aggressive children. Brain and Cognition. doi: 10.1016/j.bandc.2011.06.008. in press. [DOI] [PubMed] [Google Scholar]
- Lamm C, Lewis MD. Developmental change in the neurophysiological correlates of self-regulation in high-and low-emotion conditions. Developmental Neuropschology. 2010;35(2):156–176. doi: 10.1080/87565640903526512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, White LK, Martin McDermott J, Fox NA. Neural activation underlying inhibitory control in the context of neutral and affectively charged pictures in children. Brain and Cognition. doi: 10.1016/j.bandc.2012.02.013. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave d Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Reviews Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh, PA: Psychology Software Tools; 2002. [Google Scholar]
- Sohn M-H, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc. Natl Acad. Sci. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- van Wouwe NC, Band GPH, Ridderinkhof KR. Positive affect modulates flexibility and evaluative control. Journal of Cognitive Neuroscience. 2010;23:524–539. doi: 10.1162/jocn.2009.21380. [DOI] [PubMed] [Google Scholar]