Summary
OPCML, frequently inactivated in ovarian tumors, mediates its anti-tumor effect via binding to the extracellular domains of several important oncogenic receptor tyrosine kinases (RTKs). This, in turn, leads to the down-regulation of RTKs in tumor cells and results in significant inhibition of tumor growth.
Commentary
The use of an endogenous tumor suppressor protein as a therapeutic agent for cancer treatment opens up a new field of targeted therapy. It not only offers an opportunity for personalized therapy, but also permits simultaneous modulation of several oncogenic pathways in cancer cells. Such an approach may decrease the likelihood of pathway redundancy. The article published in this issue of Cancer Discovery by McKie and colleagues suggests that the use of this strategy to treat cancer may not be that distant (1).
OPCML, an opioid-binding protein/cell adhesion molecule, is important for regulation of opioid binding and associated signal transduction (2). It was originally isolated from brain, but has recently been shown to be expressed in other tissues such as stomach, ovaries, oviduct, and uterus (3). While its role in the female reproductive system is currently unknown, the frequent down-regulation of OPCML due to loss of heterozygosity (LOH) or epigenetic inactivation at 11q25 in ovarian tumors triggered the investigation into its role as a tumor suppressor (4). It was reported that tumors with ectopic expression of OPCML had a significant reduction in their growth rate in mice compared to ones lacking OPCML expression; this phenomenon has subsequently been observed in other cancer types, including colon, prostate, breast, and cervix (5-7). Despite these intriguing findings, the mechanism by which this protein mediates its antiproliferative intracellular signaling has not been well understood, especially since OPCML is largely located in an extracellular location and is only linked to the cell membrane via phosphatidylinositol linkage (2). The research conducted by McKie and colleagues provides us with some important clues.
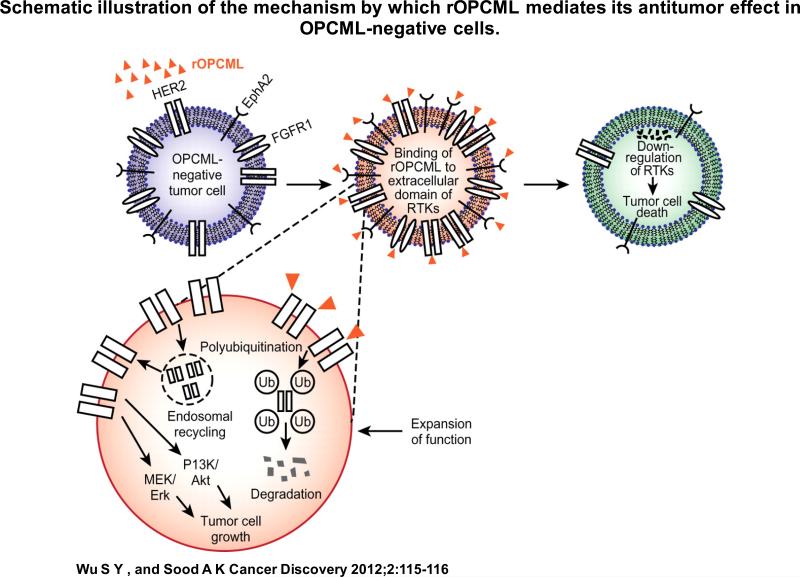
It was shown that OPCML down-regulates a variety of important receptor tyrosine kinases (RTKs), such as EphA2, FGFRs, and HER2, via binding to their extracellular domain in ovarian cancer cells. Using HER2 as a paradigm, the researchers further demonstrated that this protein-protein interaction led to the redistribution of HER2 within the cell membrane, thereby allowing its escape from the canonical clathrin endocytic route. Recycling of HER2 was, therefore, less likely to occur. This, coupled with its subsequent polyubiquitination, resulted in the efficient down-regulation of HER2 (Figure 1). While the exact mechanism of down-regulation of other RTKs by OPCML needs to be further elucidated, this result opens up opportunities for using rOPCML to treat cancer without the need for intracellular delivery, a major hurdle for cancer treatment. To this end, McKie and colleagues have demonstrated approximately 50-80% reduction in total tumor burden following repeated i.p. administration of rOPCML in orthotopic mouse models of ovarian cancer developed using cell lines lacking OPCML. While the effect of rOPCML on EphA2 in these tumors was not demonstrated, a significant reduction of total and phosphorylated HER2 and FGFR1 levels was achieved, which could explain the observed anti-tumor effect.
Figure 1.
Schematic illustration of the mechanism by which rOPCML mediates its anti-tumor effect in OPCML-negative cells.
While these results demonstrate the potential of developing therapeutic strategies based on OPCML and open up another potential avenue for treatment of ovarian cancer, several important issues must be considered before its use can be realized in a clinical setting. For instance, it would be important to study the biodistribution of rOPCML and its ability to target tumors following both intravenous and intraperitoneal administration, given the potential involvement of OPCML in several other tumor types. In silico high throughput identification of other potential receptors that OPCML could bind to would also be of importance for its future clinical development. The common issues concerning the use of recombinant proteins in humans, such as stability or potential immunotoxicity, will also need to be addressed (8). Importantly, the combinational effect of rOPCML and current standard therapy in OPCML-negative tumors remains to be seen. Since rOPCML was able to down-regulate p-Akt and p-Erk both in vitro and in vivo, rOPCML treatment may enhance killing of cancer cells in conjunction with chemotherapy. An increase in OPCML expression in tumors is also likely to occur following platinum-based therapy in the subgroup of patients whose tumors possess unmethylated OPCML promoter and yet have low OPCML expression (5). Synergistic antitumor effect between chemotherapy and rOPCML is therefore plausible and warrants further investigation. Other rational approaches for combinational therapy can also be made based on the detailed mechanistic study presented in this report. The use of bevacizumab in conjunction with rOPCML, for example, may be appropriate as OPCML does not appear to target VEGF receptors.
Despite the need to further evaluate the feasibility of using rOPCML to treat ovarian cancer, the study performed by McKie and colleagues provides important insights into the mechanism by which OPCML exerts its tumor suppressive phenotype. Given the ability of OPCML to modulate several important RTKs in ovarian cancer cells via binding to their extracellular domains, the strategy of using rOPCML to treat cancer seems promising. It is foreseeable that patients who have tumors with low expression of OPCML will benefit the most from rOPCML treatment. However, several questions remain: will all patients with low OPCML expression respond to rOPCML therapy? What are the other signaling pathways that could govern the therapeutic response to rOPCML? Does it have a direct impact on tumor microenvironment? Answers to these questions, coupled with an improved understanding of both the biological and clinical effects of this tumor suppressor protein, could lead to benefit for ovarian cancer patients from OPCML-based therapy.
References
- 1.McKie AB, Vaughan S, Zanini E, Okon IS, Louis L, de Sousa C, et al. The OPCML tumor suppressor functions as a cell surface repressor-adaptor, negatively regulating receptor tyrosine kinases in epithelial ovarian cancer. Cancer Discov. 2012 doi: 10.1158/2159-8290.CD-11-0256. XXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wick M, Fan G, Loh H. Expression of OBCAM-related cDNA clones in Cos 1 cells: evidence for a phosphatidylinositol linkage to the cell membrane. Brain Res Mol Brain Res. 1996;36:322–8. doi: 10.1016/0169-328x(95)00258-t. [DOI] [PubMed] [Google Scholar]
- 3.Fleming J, McQuillan H, Millier M, Sellar G. Expression of ovarian tumour suppressor OPCML in the female CD-1 mouse reproductive tract. Reproduction. 2009;137:721–6. doi: 10.1530/REP-08-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellar G, Watt K, Rabiasz G, Stronach E, Li L, Miller E, et al. OPCML at 11q25 is epigenetically inactivated and has tumor-suppressor function in epithelial ovarian cancer. Nat Genet. 2003;34:337–43. doi: 10.1038/ng1183. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Ying Y, van Hasselt AN, Ng KM, Yu J, Zhang Q, et al. OPCML is a broad tumor suppressor for multiple carcinomas and lymphomas with frequently epigenetic inactivation. PLoS One. 2008;3:e2990. doi: 10.1371/journal.pone.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed J, Dunn J, du Plessis D, Shaw E, Reeves P, Gee A, et al. Expression of cellular adhesion molecule ‘OPCML’ is down-regulated in gliomas and other brain tumours. Neuropathol Appl Neurobiol. 2007;33:77–85. doi: 10.1111/j.1365-2990.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 7.Ye F, Zhang S, Xie X, Lu W. OPCML gene promoter methylation and gene expression in tumor and stroma cells of invasive cervical carcinoma. Cancer Invest. 2008;26:569–74. doi: 10.1080/07357900701837044. [DOI] [PubMed] [Google Scholar]
- 8.Almeida A, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–90. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]