Abstract
Cholesteryl ester transfer protein (CETP) shuttles lipids between lipoproteins, culminating in cholesteryl ester delivery to liver and increased secretion of cholesterol as bile. Since gut bile acids promote insulin sensitivity, we aimed to define if CETP improves insulin sensitivity with high-fat feeding. CETP and nontransgenic mice of both sexes became obese. Female but not male CETP mice had increased ileal bile acid levels versus nontransgenic littermates. CETP expression protected female mice from insulin resistance but had a minimal effect in males. In liver, female CETP mice showed activation of bile acid-sensitive pathways including Erk1/2 phosphorylation and Fxr and Shp gene expression. In muscle, CETP females showed increased glycolysis, increased mRNA for Dio2, and increased Akt phosphorylation, known effects of bile acid signaling. These results suggest that CETP can ameliorate insulin resistance associated with obesity in female mice, an effect that correlates with increased gut bile acids and known bile-signaling pathways.
Keywords: Insulin resistance, Obesity, Cholesterol, Bile, Glucose, Sex-differences
1. Introduction
Insulin resistance associated with obesity leads to metabolic syndrome, diabetes, and increased risk of developing coronary heart disease (CHD). These risk factors are reduced by weight loss; however, weight reduction regimens rarely result in long-term maintenance of reduced body weight [1], [2]. Activation of pathways that improve insulin sensitivity would be an attractive alternative to weight loss as a way to prevent complications of obesity. Bile acids secreted into the gut have recently been linked to insulin sensitivity by activating signaling pathways that improve liver and muscle glucose metabolism [3], [4], [5]. Activation of pathways that promote bile production and secretion may improve insulin sensitivity in the setting of obesity. Cholesteryl ester transfer protein (CETP) is a lipid transfer protein that promotes the exchange of lipids between lipoproteins, ultimately resulting in delivery of cholesterol esters to the liver, where they are converted into bile acids [6], [7]. Activation of CETP, therefore, may contribute to improved insulin sensitivity with obesity.
In addition to their role in digestion, bile acids secreted into the gut activate signaling pathways that improve peripheral glucose metabolism. Oral treatment of obese humans with the bile acid tauroursodeoxycholic acid improves liver and muscle insulin sensitivity [5]. Similarly, treatment with bile acid sequestrants, which increase the bile acid content in the gut, improves glucose metabolism, increases energy expenditure, and improves parameters of type-2 diabetes in both mice and humans [8], [9], [10]. Gut bile acids improve insulin sensitivity by upregulating multiple pathways that signal from the gut to liver and peripheral tissues. Gut bile acids promote the production of the enterokine fibroblast growth factor 15 (FGF15), which signals to the liver through the FGF4 receptor (FGF4R), leading to activation of Extracellular Related Kinase 1/2 (Erk1/2), increased glycogen storage, and reduced gluconeogenesis [4], [11], [12]. Additionally, gut bile acids activate the bile sensitive g-protein coupled bile acid receptor (GPBAR1, also known as TGR5), which contributes to improved glucose metabolism [9]. A small amount of bile acids enter the systemic circulation where they can activate GPBAR1 in adipose and muscle tissue [13], [14]. GPBAR1 activity causes activation of type-2 thyroid deiodinase (Dio2), which leads to an increase in energy expenditure and glucose oxidation by mitochondria [3], [14]. Since bile acids are produced from cholesterol in the liver, mechanisms that increase cholesterol delivery to the liver might increase gut bile acid levels and thus promote insulin sensitivity.
CETP has been shown to increase reverse cholesterol transport in mice [15], [16]. Since mice do not naturally express CETP, transgenic mice expressing CETP have been studied [17], [18]. Because of its lipid transfer capacity, CETP alters lipid uptake and metabolism by tissues. CETP has the net effect of increasing delivery of CE to the liver by promoting the movement of CE from HDL to VLDL or LDL, which can be taken up by the liver through LDLR and VLDLR [6], [7], [15], [19]. CETP also facilitates uptake of CE from serum macrophages into HDL, which is then delivered to the liver by scavenger receptor B1 (SR-B1) [16], [20]. CE delivered to the liver is converted into bile acids, which are stored in the gallbladder and then secreted into the intestine to facilitate absorption of lipophilic nutrients. Taken together, these results suggest that increased cholesterol delivery to the liver mediated by CETP might increase bile acids in the gut and their related signaling to promote insulin sensitivity in liver and muscle. To test this hypothesis, we assessed insulin sensitivity in high-fat diet fed male and female CETP transgenic mice and their non-transgenic littermates. We observed increased gut bile acids in female but not male CETP expressing mice. This increase in bile acid levels corresponded with improved insulin sensitivity and increased bile acid related signaling in liver and muscle in CETP-expressing female mice. Overall, we show that CETP expression protects female mice from the metabolic complications of obesity.
2. Materials and methods
2.1. Animals and diets
CETP transgenic mice on a C57BL/6 background were purchased from Jackson Laboratories (C57BL/6-Tg(CETP)1Pnu/J, Stock number: 001929). This strain expresses a simian CETP gene under control of a constitutive promoter [17], [18]. All mice were maintained on a standard chow diet until placement on a sucrose-free high-fat diet (HFD) with carbohydrate content comprised of cornstarch (60% fat, Research Diets D08060104). Mice were 12–14 weeks old at onset of diet because we find more robust weight gain in adult mice. Mice were fed HFD for 4 weeks. All procedures were performed in accordance with National Institutes of Health Guidelines for the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at Vanderbilt University.
2.2. Surgical catheterization
Five to seven days prior to the clamp study, mice received catheters in the jugular vein and carotid artery as previously described [21]. Surgeries were performed at the Vanderbilt Mouse Metabolic Phenotyping Center (MMPC). Briefly, mice were anesthetized and the carotid artery and jugular vein were catheterized. Free catheter ends were tunneled under the skin to the back of the neck, externalized, and sealed with steel plugs. These methods permit arterial sampling and are less stressful than cut-tail sampling. Mice were maintained on HFD and recovered for 5–7 days after surgery. Only mice returning to within 10% of pre-surgical body weight were studied. Body composition was determined on the day of study using an mq10 NMR analyzer (Bruker Optics).
2.3. Hyperinsulinemic-euglycemic clamp studies
A 2-h hyperinsulinemic-euglycemic clamp study was performed on mice fasted for 5 h as previously described [22]. A 3-μCi bolus of [3-3H] glucose was given at t=−90 min followed by a 0.05 μCi/min infusion until t=0. All blood samples were obtained via an arterial catheter. The clamp was begun at t=0 min with a continuous infusion of insulin (4 mU kg−1 min−1). The [3-3H] glucose infusion was increased to 0.1 μCi/min for the remainder of the experiment to prevent changes in specific activity. Euglycemia (100–150 mg/dl) was maintained by measuring blood glucose every 10 min by glucometer starting at t=0 min and infusing 50% dextrose by variable infusion. Mice received saline-washed erythrocytes from donors throughout the clamp (2.5 μl/min) to prevent a fall in hematocrit. Arterial samples to measure plasma hormones and glucose turnover were taken at t=60, 80, 90, and 100 min. At 120 min, mice were sacrificed and tissues were collected and frozen in liquid nitrogen. Calculations of EndoRa, Rd, and SI were performed as previously described [21], [23], [24].
2.4. Western blots
Whole cell extracts were performed on frozen liver and muscle tissue, and western blotting was performed as previously described [25]. Primary antibodies for Akt, P-Akt (Ser473), Erk1/2, and P-Erk1/2 (Thr202/Tyr204) were purchased from Cell Signaling Technology. Antibodies for actin and ERα were purchased from Santa Cruz Biotechnology. IR-Dye 800 anti-rabbit secondary antibody (LI-COR Biotechnology) was used for band visualization on an Odyssey imaging system.
2.5. Serum analysis
Serum insulin values were determined using a commercial ELISA kit (Millipore). CETP activity was measured using a commercially available kit (Roar Biochemical RB-CETP). Serum TG and cholesterol levels were measured using commercially available kits (Raichem). Serum estradiol was quantified using a commercially available kit (Calbiotech). Serum leptin, adiponectin, and IL-6 were quantified by luminex assay at the Vanderbilt Hormone Assay & Analytical Services Core.
2.6. Gene expression
RT-PCR was used to measure gene expression. RNA was extracted from tissues (RNeasy Mini, Qiagen) and cDNAs were synthesized using 1 μg RNA template (iScript cDNA synthesis kit, BioRad). RT-PCR was conducted using SYBR Green JumpStart Taq ReadyMix (Sigma) in a 20 μl reaction with 400 nM final primer concentration. Reactions were carried out for 50 cycles of 95 °C for 10 s, 58 °C for 45 s, and 72 °C for 60 s (MyIQ, Bio Rad). Ct values were analyzed using the efficiency corrected Pfaffl method and were normalized to cyclophilin A. Fold change was determined relative to fasted WT littermates. See Supplemental Table 1 for primer sequences.
2.7. Metabolite analysis
Metabolite analysis was performed by Metabolon Inc. (Durham NC). Briefly, aqueous and organic metabolites were methanol extracted and then analyzed both by LC/MS and GC/MS. Metabolites were identified by comparison to a library of known compounds and normalized to internal standards. Welch's two-sample t-test and an estimate of the false discovery rate (q-value) were used to take into account the multiple comparisons that normally occur in metabolomic-based studies. One animal was excluded as an outlier for hepatic bile acid data, as the gallbladder was ruptured during tissue collection.
2.8. Gut bile acid analysis
Bile acids were extracted from ileum tissue by ethanol extraction using a dounce homogenizer. Bile acids were quantified using a commercially available enzymatic assay (Crystal Chem).
2.9. Indirect calorimetry
Indirect calorimetry was performed on HFD-fed female CETP and WT littermates using a Promethion system in the Vanderbilt MMPC (Sable Systems International). The system allows for measurement of VO2, VCO2, food intake, feeding behavior, heat generation and activity level. Mice were individually housed in the system for a period of 4 days during which measurements were taken. The facility uses a standard 12 h light/dark cycle and measurements for light and dark phases are reported separately.
2.10. Statistics
Data are presented as mean±SEM. Data were analyzed by student's t-test, 1-way ANOVA using Tukey's post-test, or 2-way ANOVA using Bonferoni post-test as appropriate. p<0.05 is considered statistically significant.
3. Results
3.1. CETP expression does not alter high-fat diet induced weight or adiposity gain in males or females
Age- and weight-matched male and female CETP transgenic mice and their wild-type littermates (WT) lacking CETP expression were placed on HFD for 4 weeks to test their metabolic adaptation to obesity. As expected, CETP activity was significantly higher in CETP mice compared to WT in both males and females (males Figure 1A, females Figure 1E). There was no difference in CETP activity between transgenic males and females. Both male and female WT and CETP mice gained similar weight and had similar adiposity on HFD (males Figure 1B, females Figure 1F body weight: Table 1).
Figure 1.
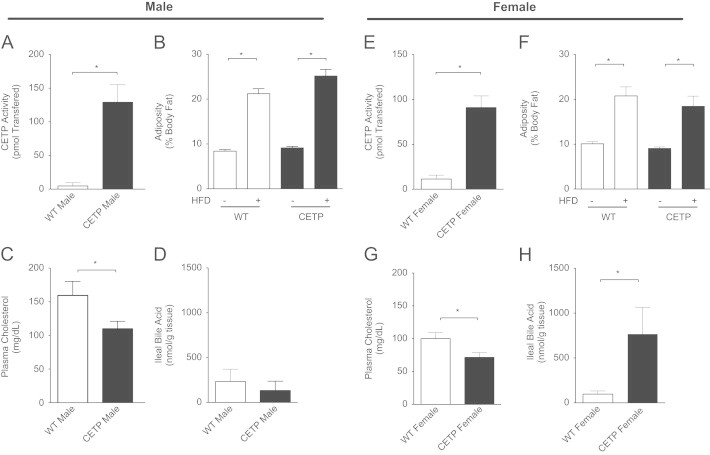
CETP expression alters serum cholesterol and ileal bile acids in obese mice. (A) CETP activity in WT littermates and CETP-transgenic males. (B) Adiposity before (−) and after (+) high-fat diet in male mice. (C) Serum cholesterol in male mice. (D) Total ileal bile acids in male mice. (E) CETP activity in WT littermates and CETP-transgenic females. (F) Adiposity before (−) and after (+) high-fat diet in female mice. (G) Serum cholesterol in female mice. (H) Total ileal bile acids in female mice. Data represent the mean±SEM from n=6–8 animals per group, *p<0.05.
Table 1.
Additional physiological parameters. Values represent mean±standard deviation.
| WT Female | CETP Female | WT Male | CETP Male | |
|---|---|---|---|---|
| Initial body weight (g) | 20.5±2.3 | 21.0.±1.8 | 24.0±2.1 | 25.4±3.6 |
| Post-HFD body weight (g) | 26.5±5.1† | 24.1±3.6† | 28.4±3.2† | 32.1±5.0⁎† |
| HFD fasting blood glucose (mg/dL) | 152±35.8 | 134±19.6 | 148±24.1 | 151±38.1 |
| HFD clamp insulin (ng/mL) | 4.8±1.6 | 2.6±1.1 | 2.8±0.6 | 3.7±1.0 |
| Chow clamp insulin (ng/mL) | 1.7±0.4 | 2.1±0.3 | ND | ND |
| HFD plasma free fatty acid (mmol/L) | 1.44±0.96 | 1.62±0.50 | 1.57±0.77 | 1.44±0.96 |
| HFD plasma LDL (mg/dL pooled) | 33.8 | 14.6 | 39.1 | 21.7 |
| HFD plasma HDL (mg/dL pooled) | 110.3 | 50.0 | 79.5 | 52.1 |
| HFD basal glucose EndoRa (mg kg−1 min−1) | 17.3±7.4 | 19.6±5.6 | 10.3±4.4 | 14.6±10.1 |
| HFD clamp glucose EndoRa (mg kg−1 min−1) | 2.2±2.1‡ | 3.9±2.8‡ | 3.7±4.8 | 2.9±5.7‡ |
| HFD clamp glucose Rd (mg kg−1 min−1) | 17.1±3.4 | 45.0±6.1⁎ | 16.9±4.4 | 17.19±4.8 |
| HFD clamp AUC GIR/glucose (mg2 min kg−1 dL−1) | 0.10±.03 | 0.25±.05⁎ | 0.09±.03 | 0.11±.02 |
| HFD fasting plasma triglyceride (mg/dL) | 73.8±22.6 | 53.5±21.0 | 46.5±13.0 | 65.0±15.0 |
| HFD clamp plasma triglyceride (mg/dl) | 51.7±23.1 | 36.6±5.9‡ | 43.8±18.6 | 50.0±11.3 |
| HFD liver cholesterol (mg/g tissue) | 2.75±1.5 | 4.27±2.1 | 2.47±0.8 | 3.86±1.6 |
| HFD liver triglyceride (mg/g tissue) | 54.8±21.7 | 75.1±9.4 | 79.2±20.5 | 53.8±14.2 |
| HFD liver diacylglycerol (mg/g tissue) | 2.67±0.63 | 2.67±0.61 | 3.61±1.53 | 2.53 1.02 |
| HFD plasma interleukin 6 (pg/mL) | 77.6±65.7 | 76.1±31.9 | 54.3±23.5 | 113.1±64.2 |
| HFD plasma leptin (ng/mL) | 12.9±5.2 | 10.6±5.8 | 12.7±0.1 | 18.6±5.0 |
| HFD plasma adiponectin (µg/mL) | 13.1±1.8 | 15.7±1.8 | 15.1±1.6 | 17.6±3.6 |
| HFD serum estradiol (pg/mL) | 6.8±3.1 | 6.9±2.0 | ND | ND |
p<0.05 versus WT. n=4–8 animals per group.
p<0.05 versus pre-HFD. n=4–8 animals per group.
p<0.05 versus clamp baseline. n=4–8 animals per group.
3.2. CETP expression reduces serum cholesterol in males and females and increases gut bile acid content in females only
To determine the effect of CETP expression on lipid and bile metabolism, we measured fasted serum lipids and ileal bile acids. We observed that CETP expression reduced serum cholesterol in both male and female mice (males Figure 1C, females Figure 1G, and Table 1). HDL and LDL cholesterol were both lower in CETP mice compared to WT, consistent with the changes in total cholesterol (Table 1). CETP-expressing males showed no difference in ileal bile acid content compared to WT males (Figure 1D). CETP-expressing females, however, showed a significant increase in ileal bile acid content compared to WT (Figure 1H). We saw a trend towards increased bile acid species in the serum of CETP females (Supp. Table 2). We did not see a change in total liver bile acids, but observed a modest increase in the measured hepatic bile acid species in the CETP females ((Supp. Table 2), p<0.05 for genotype effect). We did not observe a difference in plasma free fatty acid, triglyceride, or estradiol (Table 1). We saw a trend towards increased hepatic cholesterol content in both male and female CETP expressing mice, but this difference was not significant (Table 1). Overall, we observed a reduction in serum cholesterol in both male and female CETP mice, and an increase in gut bile acids in female CETP mice only.
3.3. CETP expression reduces fasting insulin in female mice
Since bile acids are known to affect insulin sensitivity, we measured fasting glucose and insulin levels in the CETP mice and WT littermates. After 4 weeks of HFD-feeding, there was no difference in fasting blood glucose between CETP and WT mice (males Figure 2A, females Figure 2C). While there was not a difference in fasting insulin between CETP and WT males (Figure 2B), CETP female mice had lower fasting insulin levels than WT (Figure 2D). Thus, despite weight and adiposity gain on HFD, female CETP mice maintained lower fasting insulin levels, suggesting improved insulin sensitivity in the female animals.
Figure 2.
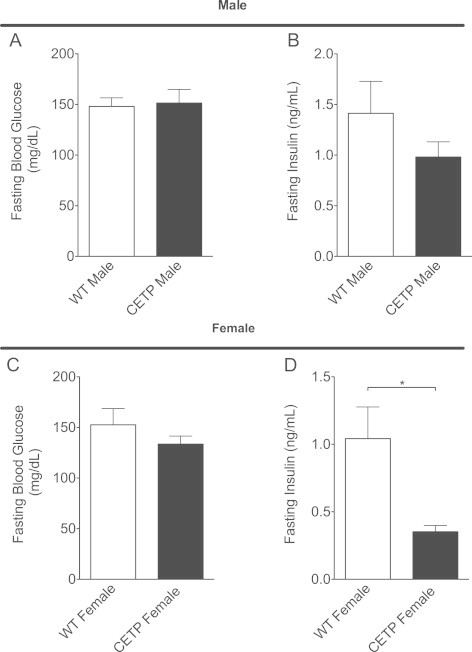
CETP expression decreases fasting plasma insulin in female mice. (A) Fasting blood glucose in male mice. (B) Fasting plasma insulin in male mice. (C) Fasting blood glucose in female mice. (D) Fasting plasma insulin in female mice. Data represent the mean±SEM from n=6–8 animals per group, *p<0.05.
3.4. Expression of CETP protects against diet-induced insulin resistance in female mice
Since the changes in fasting insulin suggested improved insulin sensitivity in the CETP female mice, we measured insulin sensitivity using a hyperinsulinemic-euglycemic clamp in mice fed HFD for 4 weeks (Figure 3A). The hyperinsulinemic-euglycemic clamp technique assesses insulin sensitivity by determining the glucose infusion rate (GIR) required to maintain euglycemia in response to a physiologic increase in serum insulin; increased GIR corresponds to greater insulin sensitivity. There was no significant difference in GIR between the CETP and WT males during the clamp (Figure 3B and C). Male CETP mice showed no change in insulin sensitivity index (SI) compared to WT (Figure 3D), which is defined by the GIR divided by the average insulin level in the steady-state clamp period.
Figure 3.
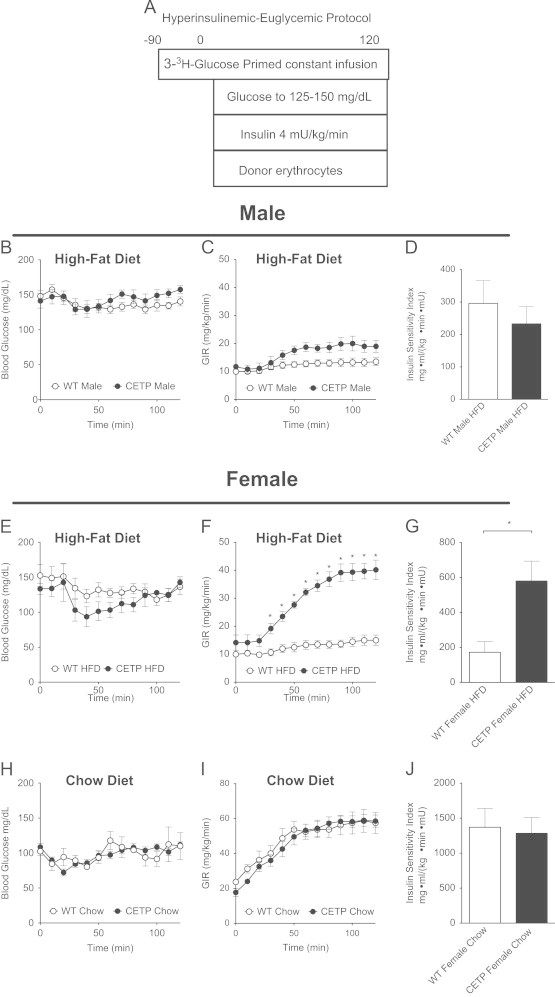
CETP expression protects female mice against diet-induced insulin resistance. (A) Hyperinsulinemic-euglycemic clamp study design. (B) Clamp blood glucose in HFD-fed males. (C) Glucose infusion rate (GIR) required to maintain euglycemia during hyperinsulinemic clamp in HFD-fed males. (D) Insulin sensitivity index based on steady-state GIR/insulin for HFD-fed males. (E) Clamp blood glucose in HFD-fed females. (F) GIR for HFD-fed females. (G) Insulin sensitivity index for HFD-fed females. (H) Blood glucose during clamp in chow-fed females. (I) GIR for chow-fed females. (J) Insulin sensitivity index for chow-fed females. Data represent the mean±SEM from n=6–8 animals per group, *p<0.05.
In contrast, CETP females showed a markedly increased GIR compared to WT (Figure 3E and F). Additionally, SI was increased by approximately 3-fold in the CETP females compared to WT (Figure 3G). The endogenous rate of glucose production EndoRa, an index of liver insulin action, was suppressed by insulin in all groups. However, the dose of insulin used, which raises insulin to a post-prandial level, was optimized to study muscle metabolism (Table 1). Glucose rate of disappearance (Rd), an index of muscle insulin action, was significantly increased in CETP females compared to WT (Table 1). Rd in CETP expressing females was significantly higher than both WT females and CETP-expressing males. Overall, the clamp results show that CETP expression significantly improves insulin sensitivity in obese female mice fed HFD.
To determine if CETP-mediated effects on insulin sensitivity are dependent on diet composition, we performed a parallel hyperinsulinemic-euglycemic clamp study on CETP and WT females fed a standard chow diet (Figure 3A). We focused on the female mice because of the large phenotype observed in the HFD-fed females. In contrast to the HFD-fed group, there was no difference in GIR or SI between the chow-fed CETP and WT mice, suggesting that the improvement in insulin sensitivity observed with CETP expression depends on HFD-feeding (Figure 3H, I, and J). Thus, CETP protects against the effect of HFD on insulin sensitivity in females; it has no effect on insulin sensitivity in lean chow-fed females. The interaction between CETP and diet in females correlates with a mechanism mediated by gut bile acids, which are known to be increased by HFD.
3.5. CETP expression increases insulin-stimulated phosphorylation of hepatic Akt and Erk1/2 in HFD-fed mice
To define changes in insulin signaling compared to the fasted state, we added an additional non-insulin treated cohort of CETP and WT female mice that were HFD-fed for 4 weeks and then fasted for 5 h with no insulin treatment prior to sacrifice. We performed western blot analysis and found that insulin-stimulated Akt phosphorylation was greater in clamped female CETP mice compared to WT (Figure 4A and B). We did see a trend towards increased hepatic AKT phosphorylation in the CETP males, although this result was not statistically significant (Supp. Figure 1). Erk1/2 phosphorylation was increased in the insulin-clamped CETP females compared to clamped WT females (Figure 4A and C). Fasting Erk1/2 phosphorylation was similar between fasted CETP and WT females, suggesting that insulin is required for increased Erk1/2 phosphorylation in CETP female mice (Figure 4A and C). Erk1/2 phosphorylation was not increased in CETP males (Supp. Figure 1). To further investigate the sex difference, we performed western blots for estrogen receptor alpha in liver and muscle of female CETP and WT mice. We did not observe a difference in ERα expression between CETP and WT in either liver or muscle (Supp. Figure 2). Overall, we observed that CETP expression improves the insulin signal to Akt in the context of HFD-feeding. Female CETP mice had an additional robust signal to increase signaling to Erk1/2, likely contributing to the marked sex-difference seen in our clamp study. These signaling changes suggest an intersection between insulin signaling to AKT and gut bile signaling through FGF15, which results in increased ERK1/2 phosphorylation in the liver [4], [11].
Figure 4.
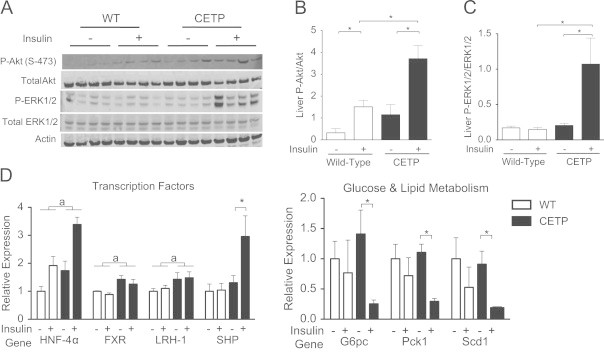
CETP expression alters hepatic insulin signaling and bile acid signal related gene expression. (A) Immunoblot analysis of Akt, P-Akt (S-473), Erk1/2, P-Erk1/2, and actin in liver whole-cell extract from fasted and clamped CETP and WT female mice on a high fat diet. (B) Quantification of P-Akt to Akt ratio. (C) Quantification of P-Erk1/2 to Erk1/2 ratio. (D) Gene expression for genes whose products control glucose metabolism, bile acid metabolism, and gene transcription determined by qRT-PCR from hepatic RNA. Data represents the mean±SEM from n=4 animals per group. Asterix (*) indicates p<0.05 for insulin-treated versus fasted, brackets (a) indicate statistical significance (p<0.05) for genotype effect by 2-way ANOVA.
3.6. CETP expression alters expression of genes involved in liver glucose metabolism
As insulin sensitivity was only altered in the female CETP mice, we focused our further mechanistic studies on female CETP mice versus female WT. To determine the effect of CETP expression on hepatic gene expression, we performed qRT-PCR on hepatic mRNA from fasted and insulin-clamped CETP and WT female mice. We determined mRNA levels for genes whose products are involved in glucose and lipid metabolism and regulate gene transcription (Figure 4D). After HFD-feeding, insulin failed to suppress glucose-6-phosphatase (G6pc), and phosphoenol pyruvate carboxylase (Pepck) expression in the WT mice, consistent with the severe insulin resistance noted in the clamp for WT animals. Pepck and G6pc gene products are central to control of hepatic gluconeogenesis. CETP mice showed improved insulin-suppression of mRNA for G6pc and Pepck compared to WT. Additionally, insulin-mediated suppression of RNA for sterol CoA desaturase expression, a gene whose product is involved in de-novo lipogenesis, was improved in the CETP mice compared to WT. Female CETP mice had increased expression of Hnf4α, Fxr, and Lrh-1, as well as increased Shp under insulin treatment. These transcription factors regulate genes whose products control bile, glucose and lipid metabolism. Based on our observed changes in ileal bile acids, liver protein, and mRNA, CETP expression may improve insulin action on control points of hepatic glucose metabolism, potentially through bile acid signaling initiated in the gut.
3.7. CETP expression increases insulin-stimulated phosphorylation of muscle Akt in HFD-fed mice
The large increase in GIR and Rd in CETP females was likely due to increases in muscle insulin sensitivity, since muscle is the largest depot for insulin-stimulated glucose uptake. We measured phosphorylated Akt in gastrocnemius muscle from fasted and insulin-clamped CETP and WT females. We observed an increase in Akt phosphorylation in response to the insulin in CETP but not WT females (Figure 5A and B). We saw a trend towards increased Akt phosphorylation in muscle of CETP males, but this result was not significant (Supp. Figure 1). This result suggests that CETP expression ameliorates the HFD-induced insulin resistance to Akt in muscle of female mice.
Figure 5.
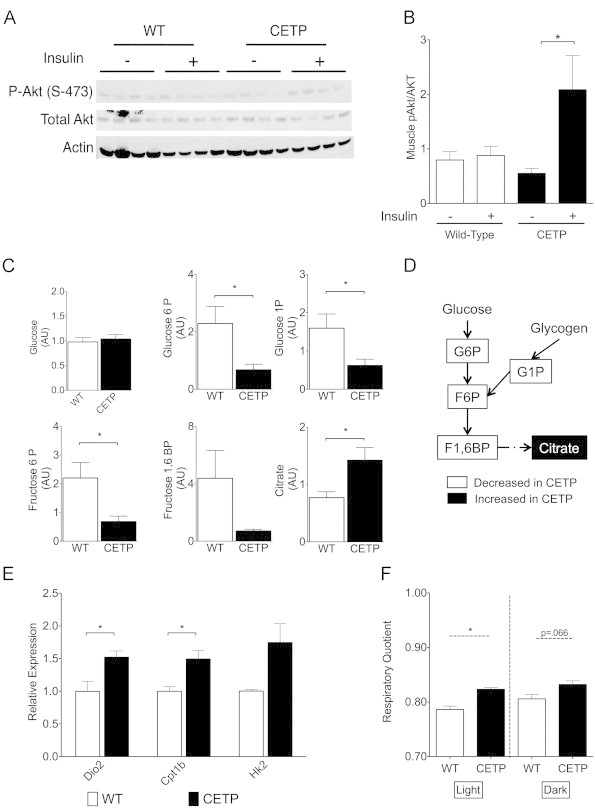
CETP-expressing mice on a high-fat diet display improved muscle insulin signaling and altered glucose metabolism. (A) Immunoblot analysis of Akt, P-Akt (S-473), and actin in muscle whole-cell extract from fasted and clamped CETP and WT female mice. (B) Quantification of P-Akt to Akt ratio. (C) Metabolite analysis of glycolytic and TCA cycle intermediates in muscle of fasted CETP and WT female mice. (D) Schematic representation of changes in glycolytic and TCA cycle intermediates. (E) Gene expression for Dio2, Cpt1b, and Hk2 determined by qRT-PCR from muscle RNA extracts. (F) Respiratory quotient determined by indirect calorimetry for HFD fed CETP and WT female mice. Data represent the mean±SEM from n=4 animals per group, except for (F) which is n=3 animals per group. *p<0.05.
3.8. CETP expression reduces muscle glycolytic intermediates
Having observed improved muscle insulin signaling and increased glucose disposal, we measured glycolytic intermediates in muscle tissue from fasted CETP and WT females (Figure 5C and D). CETP animals had significantly decreased levels of glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P). We also saw a trend towards decreased fructose 1,6 bisphosphate (F1,6BP) in the CETP females (p=0.11). Glycogen-derived glucose-1-phosphate (G1P) was also significantly decreased. Muscle glucose was not altered. Levels of citrate were increased in the CETP mice, an indicator of increased glucose and/or lipid oxidation. The reduced glycolytic intermediates despite increased citrate (an inhibitor of glycolysis) suggest that CETP expression promotes muscle TCA cycle flux in the fasting state. We examined soleus muscle mRNA for genes whose products promote TCA cycling, and found significantly increased gene expression for Dio2, carnitine palmitoyltransferase 1b (Cpt1b), and a trend towards increased hexokinase 2 (Hk2) gene expression in CETP mice (Figure 5E: p=0.058 for Hk2). Taken together, the reduction in muscle glycolytic intermediates along with increased gene expression for Dio2 and Cpt1b suggests that CETP expression causes increased muscle substrate oxidation. These changes likely contribute to improved insulin sensitivity in CETP females.
Having observed a significant difference in insulin sensitivity in HFD-fed CETP females as well as altered muscle glucose metabolism, we performed indirect calorimetry on HFD-fed CETP and WT females to measure energy expenditure. Food intake and energy expenditure were similar between CETP and WT, although CETP females ate more frequently than WT (Supp. Figure 3). Additionally, the increase in respiratory quotient showed that CETP females had increased carbohydrate oxidation compared to WT (Figure 5F). The finding that CETP expression increases the relative utilization of carbohydrate compared to lipid is consistent with our observation that CETP alters muscle glucose oxidation. Activation of glycolysis and Dio2 are known effects of bile acid signaling in muscle, and these observations are consistent with our model that bile signaling is responsible for improved insulin sensitivity in the CETP females.
4. Discussion
Our studies demonstrate a novel role for CETP expression to ameliorate insulin resistance in obese female mice. We propose that the increase in gut bile acids in the CETP females contributes to the improved insulin sensitivity in the setting of obesity. Our data is consistent with the growing body of evidence that bile acids initiate signaling pathways in the gut that promote insulin sensitivity [3], [4], [5]. CETP expression did not alter ileal bile acid levels in male mice, and, correspondingly, there was no alteration in insulin sensitivity in the male CETP mice. Additionally, we did not observe a difference in insulin sensitivity between female CETP mice and female WT littermates fed chow, suggesting that CETP may have metabolic benefits that promote insulin sensitivity only in the context of a lipid-rich diet that would promote bile acid secretion.
We propose that the metabolic effect of CETP in female mice is due to bile acid signaling that originates in the gut. We observe multiple alterations in metabolism in the CETP mice that are similar to the effects of gut bile acids to improve insulin sensitivity in both humans and mouse models. Our observation of improved insulin sensitivity and increased Akt phosphorylation in muscle is similar to the effects of oral taurodeoxycholic acid treatment in humans [5]. CETP expressing female mice showed increased Erk1/2 phosphorylation in the liver, which is a known effect of FGF15 signaling from the gut to the liver [4], [11], [12]. A limitation of our data is that we were unable to precisely quantify specific bile acid species that are ligands for bile acid receptors, such as chenodeoxycholic acid and deoxycholic acid for FXR (reviewed in [26]).
We also saw a large increase in the rate of glucose disappearance during our clamp studies, an index of muscle glucose disposal. The increase in Rd corresponded with decreased glycolytic intermediate metabolites and an increase in the TCA cycle intermediate citrate as well as increased respiratory quotient. The increased glucose disposal in CETP female mice is consistent with human studies in which patients with type-2 diabetes were treated with bile acid sequestrants, which lead to increased gut bile content [10]. These effects on glucose metabolism also correlate with known effects of bile acid signaling through Gpbar1 to induce Dio2, which was upregulated in the CETP female animals. GPBAR1 signaling in the gut can also promote the secretion of glucagon-like peptide 1 (Glp1) from enteroendocrine cells [3]. Glp1 is known to improve insulin sensitivity in both humans and mouse models of diabetes [27], [28]. GPBAR1 is also expressed in enteric nervous system and may have an effect on the afferent signal from the gut to the central nervous system [29]. Collectively, our results show a strong correlation between CETP expression in females and increased bile acid signaling that may result in increased glucose disposal, oxidation of carbohydrate, and improved insulin sensitivity. Further studies are required to determine if bile acid signaling has a truly causative role in the metabolic effects of CETP.
The role of CETP in human health has been a topic of considerable controversy, and there are significant unresolved questions about its effects on lipid and glucose homeostasis. CETP promotes the movement of cholesterol esters and triglycerides between lipoproteins, resulting in a net flux of cholesterol from HDL to LDL. Since low HDL levels are a well-known risk factor for CHD, CETP inhibitor drugs were developed with the goal of increasing HDL and reducing CHD. While the inhibitors successfully raised HDL in humans, the clinical benefit of these drugs on cardiovascular disease has not yet been established [30], [31]. Our data suggest that CETP may have additional effects not directly related to lipoproteins, such as improvements in whole body glucose metabolism with obesity.
We observed a pronounced sexual dimorphism in insulin sensitivity between the male and female CETP mice, which is consistent with the observed sex-specific roles of CETP between men and women in human studies [32], [33], [34]. In men, studies suggest a neutral or harmful effect of CETP on glucose homeostasis. An association study in men showed a correlation between high levels of CETP activity and markers of insulin resistance [35]. An insulin clamp study performed in men showed no correlation between CETP activity and insulin sensitivity [36]. A post-hoc analysis of the ILLUMINATE CETP inhibitor trial showed that diabetic patients treated with the CETP inhibitor torcetrapib had a modest reduction in hemoglobin A1c [34]. This study involved 70% men, and only examined individuals with long-standing obesity, diabetes, and established cardiovascular disease. In our studies, CETP expression did not improve insulin sensitivity in males. Although the GIR was higher in CETP males in our clamp study, mice in the CETP group also had higher blood glucose levels in the clamp period. Two measures of insulin sensitivity, SI and the AUC of the GIR/glucose, were not different in male CETP vs. WT. Mice in our study were fed HFD for 4 weeks. A longer duration of HFD-feeding may have revealed an effect of CETP on insulin sensitivity in males.
Reciprocally, studies in females suggest beneficial effects of CETP on cholesterol efflux and glucose metabolism. Higher CETP activity is associated with increased transfer of CE from macrophages to HDL in women but not men [37], [38]. This improved cholesterol transport capacity with increased CETP was independent of HDL cholesterol [37]. In women, CETP polymorphisms that increase CETP activity correlated with reduced risk of ischemic heart disease [39]. Higher CETP activity is associated with improved glucose metabolism after roux-en-Y gastric bypass in women but not men [32], [33]. In our studies, we found that female CETP mice had 3-fold improvement in insulin sensitivity with HFD-feeding compared to WT, showing a strong protection of female CETP mice against diet-induced insulin resistance. In our studies there was a significant sex-difference in gut bile acid content that correlates with the observed increases in bile acid related signaling in the CETP expressing females. Estrogen may augment the bile signal in female CETP mice, as both CETP and estrogen signaling through ERα increase bile secretion [40], [41]. We observed alterations in signaling pathways that were unique to the females, such as increased hepatic Erk phosphorylation, and increased Shp mRNA, pathways that are shared between estrogen and bile-signaling pathways [11], [42]. CETP has also been shown to augment estradiol delivery to tissues, enhancing estrogen signaling [43], [44]. We did not, however, observe an increase in ERα levels in liver or muscle of CETP-expressing mice, and CETP-expressing females had levels of circulating estradiol similar to WT. Overall, we propose that the mechanism for the observed sex difference in insulin sensitivity lies in the alteration in gut bile acids observed in the CETP expressing females.
Our finding that CETP expression promotes insulin sensitivity does not necessarily suggest that CETP inhibition would be harmful with regard to glucose homeostasis. CETP has an extracellular role in lipid transfer between lipoproteins but also functions intracellularly to promote CE uptake, and direct partitioning of CE between subcellular compartments [45], [46]. If our effects were mediated by intracellular CETP, we would not expect CETP inhibition to adversely impact the phenotype [45]. Additionally, several CETP inhibitors promote RCT, culminating in increased CE delivery to the liver and bile secretion [47], [48]. Such drugs would be predicted to increase bile acid signaling and perhaps improve insulin sensitivity.
In humans, dietary weight-loss strategies are complicated by weight regain. Pharmacologic strategies to achieve weight loss often have significant side effects on the cardiovascular system or central nervous system. Strategies to improve insulin sensitivity in the setting of obesity would be an attractive alternative to weight loss. The idea of “metabolically-healthy obesity” is an important goal in the prevention of diabetes and cardiovascular risk associated with obesity. We observe a metabolically-healthy obese phenotype in female CETP mice. The model shown here, though dissimilar to the human in some regards, demonstrates an important proof-of-principle that augmenting aspects of CETP function, such as bile acid signaling, may be a therapeutic approach to improve insulin sensitivity and lessen the negative metabolic impact of obesity.
Conflict of interest
The authors have no conflicts of interest related to this article.
Acknowledgments
We acknowledge excellent assistance by the Vanderbilt Mouse Metabolic Phenotyping Center (DK59637) and the Vanderbilt Hormone Assay and Analytical Services Core (DK59637 and DK20593).
Sources of funding: This work was supported by the Department of Veterans Affairs Career Development Award and Merit Award (BX002223), the American Heart Association (10GRNT3650024), the Vanderbilt Diabetes Research and Training Center Pilot and Feasibility Program (DK20593), the Vanderbilt Digestive Diseases Research Center Pilot and Feasibility Program (DK058404). D.C. was supported by the Vanderbilt Molecular Endocrinology Training Grant, B.P by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program, and M.M. is supported by NRSA F31 DK093330-02. O.M. was supported by DK020593, DK059637, DK043748 and DK078188.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found in the online version at 10.1016/j.molmet.2013.08.007.
Appendix A. Supplementary materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Wadden T.A. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Annals of Internal Medicine. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 2.Wing R.R., Phelan S. Long-term weight loss maintenance. American Journal of Clinical Nutrition. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabolism. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potthoff M.J., Boney-Montoya J., Choi M., He T., Sunny N.E., Satapati S. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metabolism. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kars M., Yang L., Gregor M.F., Mohammed B.S., Pietka T.A., Finck B.N. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rader D.J., Alexander E.T., Weibel G.L., Billheimer J., Rothblat G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. Journal of Lipid Research. 2009;50:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera A.V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M.F., Jafri K. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New England Journal of Medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe M., Morimoto K., Houten S.M., Kaneko-Iwasaki N., Sugizaki T., Horai Y. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PloS One. 2012;7:e38286. doi: 10.1371/journal.pone.0038286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potthoff M.J., Potts A., He T., Duarte J.A., Taussig R., Mangelsdorf D.J. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2013;304:G371–G380. doi: 10.1152/ajpgi.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beysen C., Murphy E.J., Deines K., Chan M., Tsang E., Glass A. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55:432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 11.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F., Suino-Powell K. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin D.J., Osborne T.F. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. Journal of Biological Chemistry. 2009;284:11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. Journal of Endocrinology. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 15.El Bouhassani M., Gilibert S., Moreau M., Saint-Charles F., Treguier M., Poti F. Cholesteryl ester transfer protein expression partially attenuates the adverse effects of SR-BI receptor deficiency on cholesterol metabolism and atherosclerosis. Journal of Biological Chemistry. 2011;286:17227–17238. doi: 10.1074/jbc.M111.220483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanigawa H., Billheimer J.T., Tohyama J.I., Zhang Y.Z., Rothblat G., Rader D.J. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 17.Marotti K.R., Castle C.K., Boyle T.P., Lin A.H., Murray R.W., Melchior G.W. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 1993;364:73–75. doi: 10.1038/364073a0. [DOI] [PubMed] [Google Scholar]
- 18.Marotti K.R., Castle C.K., Murray R.W., Rehberg E.F., Polites H.G., Melchior G.W. The role of cholesteryl ester transfer protein in primate apolipoprotein A-I metabolism. Insights from studies with transgenic mice. Arteriosclerosis and Thrombosis. 1992;12:736–744. doi: 10.1161/01.atv.12.6.736. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz C.C., VandenBroek J.M., Cooper P.S. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. Journal of Lipid Research. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y., Wang N., Ramakrishnan R., Sehayek E., Huszar D., Breslow J.L. Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. Journal of Biological Chemistry. 1999;274:33398–33402. doi: 10.1074/jbc.274.47.33398. [DOI] [PubMed] [Google Scholar]
- 21.Ayala J.E., Bracy D.P., Mcguinness O., Wasserman D.H. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 22.Berglund E.D., Li C.Y., Poffenberger G., Ayala J.E., Fueger P.T., Willis S.E. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L., Brown W.C., Cai Q., Krust A., Chambon P., McGuinness O.P. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes. 2013;62:424–434. doi: 10.2337/db11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala J.E., Bracy D.P., Julien B.M., Rottman J.N., Fueger P.T., Wasserman D.H. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes. 2007;56:1025–1033. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- 25.Martinez M.N., Emfinger C.H., Overton M.H., Hill S., Ramaswamy T.S., Cappel D.A. Obesity and altered glucose metabolism impact HDL composition in CETP transgenic mice: a role for ovarian hormones. Journal of Lipid Research. 2012;53:379–389. doi: 10.1194/jlr.M019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Aguiar Vallim T.Q., Tarling E.J., Edwards P.A. Pleiotropic roles of bile acids in metabolism. Cell Metabolism. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zander M., Madsbad S., Madsen J.L., Holst J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q., Brubaker P.L. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 29.Poole D.P., Godfrey C., Cattaruzza F., Cottrell G.S., Kirkland J.G., Pelayo J.C. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterology and Motility: the Official Journal of the European Gastrointestinal Motility Society. 2010;22(814-25):e227–e228. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barter P.J., Caulfield M., Eriksson M., Grundy S.M., Kastelein J.J., Komajda M. Effects of torcetrapib in patients at high risk for coronary events. New England Journal of Medicine. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz G.G., Olsson A.G., Abt M., Ballantyne C.M., Barter P.J., Brumm J. Effects of dalcetrapib in patients with a recent acute coronary syndrome. New England Journal of Medicine. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 32.Asztalos B.F., Swarbrick M.M., Schaefer E.J., Dallal G.E., Horvath K.V., Ai M. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. Journal of Lipid Research. 2010;51:2405–2412. doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purnell J.Q., Kahn S.E., Albers J.J., Nevin D.N., Brunzell J.D., Schwartz R.S. Effect of weight loss with reduction of intra-abdominal fat on lipid metabolism in older men. Journal of Clinical Endocrinology & Metabolism. 2000;85:977–982. doi: 10.1210/jcem.85.3.6402. [DOI] [PubMed] [Google Scholar]
- 34.Barter P.J., Rye K.A., Tardif J.C., Waters D.D., Boekholdt S.M., Breazna A. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 2011;124:555–562. doi: 10.1161/CIRCULATIONAHA.111.018259. [DOI] [PubMed] [Google Scholar]
- 35.Dullaart R.P., Sluiter W.J., Dikkeschei L.D., Hoogenberg K., Van Tol A. Effect of adiposity on plasma lipid transfer protein activities: a possible link between insulin resistance and high density lipoprotein metabolism. European Journal of Clinical Investigation. 1994;24:188–194. doi: 10.1111/j.1365-2362.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 36.Maclean P. Plasma cholesteryl ester transfer protein activity is not linked to insulin sensitivity. Metabolism. 2001;50:783–788. doi: 10.1053/meta.2001.24205. [DOI] [PubMed] [Google Scholar]
- 37.Villard E.F., El Khoury P., Duchene E., Bonnefont-Rousselot D., Clement K., Bruckert E. Elevated CETP activity improves plasma cholesterol efflux capacity from human macrophages in women. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:2341–2349. doi: 10.1161/ATVBAHA.112.252841. [DOI] [PubMed] [Google Scholar]
- 38.Villard E.F., Khoury P.E., Frisdal E., Bruckert E., Clement K., Bonnefont-Rousselot D. Genetic determination of plasma cholesterol efflux capacity is gender-specific and independent of HDL-cholesterol levels. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013 doi: 10.1161/ATVBAHA.112.300979. [DOI] [PubMed] [Google Scholar]
- 39.Agerholm-Larsen B., Tybjaerg-Hansen A., Schnohr P., Steffensen R., Nordestgaard B.G. Common cholesteryl ester transfer protein mutations, decreased HDL cholesterol, and possible decreased risk of ischemic heart disease: the Copenhagen City Heart Study. Circulation. 2000;102:2197–2203. doi: 10.1161/01.cir.102.18.2197. [DOI] [PubMed] [Google Scholar]
- 40.Wang H.H., Afdhal N.H., Wang D.Q. Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. Journal of Lipid Research. 2006;47:778–786. doi: 10.1194/jlr.M500454-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Hayek T., Azrolan N., Verdery R.B., Walsh A., Chajek-Shaul T., Agellon L.B. Hypertriglyceridemia and cholesteryl ester transfer protein interact to dramatically alter high density lipoprotein levels, particle sizes, and metabolism. Studies in transgenic mice. Journal of Clinical Investigation. 1993;92:1143–1152. doi: 10.1172/JCI116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai K., Harnish D.C., Evans M.J. Estrogen receptor alpha regulates expression of the orphan receptor small heterodimer partner. Journal of Biological Chemistry. 2003;278:36418–36429. doi: 10.1074/jbc.M303913200. [DOI] [PubMed] [Google Scholar]
- 43.Gong M., Wilson M., Kelly T., Su W., Dressman J., Kincer J. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. Journal of Clinical Investigation. 2003;111:1579–1587. doi: 10.1172/JCI16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provost P.R., Lavallee B., Belanger A. Transfer of dehydroepiandrosterone- and pregnenolone-fatty acid esters between human lipoproteins. Journal of Clinical Endocrinology and Metabolism. 1997;82:182–187. doi: 10.1210/jcem.82.1.3693. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier A., Lau P., Zha X., Milne R., McPherson R. Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:2177–2184. doi: 10.1161/01.ATV.0000183613.13929.13. [DOI] [PubMed] [Google Scholar]
- 46.Vassiliou G., McPherson R. Role of cholesteryl ester transfer protein in selective uptake of high density lipoprotein cholesteryl esters by adipocytes. Journal of Lipid Research. 2004;45:1683–1693. doi: 10.1194/jlr.M400051-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Niesor E.J., Magg C., Ogawa N., Okamoto H., von der Mark E., Matile H. Modulating cholesteryl ester transfer protein activity maintains efficient pre-beta-HDL formation and increases reverse cholesterol transport. Journal of Lipid Research. 2010;51:3443–3454. doi: 10.1194/jlr.M008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro-Perez J., Briand F., Gagen K., Wang S.P., Chen Y., McLaren D.G. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. Journal of Lipid Research. 2011;52:1965–1973. doi: 10.1194/jlr.M016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material


