Abstract
The molecular mechanisms regulating secretion of the orexigenic-glucoregulatory hormone ghrelin remain unclear. Based on qPCR analysis of FACS-purified gastric ghrelin cells, highly expressed and enriched 7TM receptors were comprehensively identified and functionally characterized using in vitro, ex vivo and in vivo methods. Five Gαs-coupled receptors efficiently stimulated ghrelin secretion: as expected the β1-adrenergic, the GIP and the secretin receptors but surprisingly also the composite receptor for the sensory neuropeptide CGRP and the melanocortin 4 receptor. A number of Gαi/o-coupled receptors inhibited ghrelin secretion including somatostatin receptors SSTR1, SSTR2 and SSTR3 and unexpectedly the highly enriched lactate receptor, GPR81. Three other metabolite receptors known to be both Gαi/o- and Gαq/11-coupled all inhibited ghrelin secretion through a pertussis toxin-sensitive Gαi/o pathway: FFAR2 (short chain fatty acid receptor; GPR43), FFAR4 (long chain fatty acid receptor; GPR120) and CasR (calcium sensing receptor). In addition to the common Gα subunits three non-common Gαi/o subunits were highly enriched in ghrelin cells: GαoA, GαoB and Gαz. Inhibition of Gαi/o signaling via ghrelin cell-selective pertussis toxin expression markedly enhanced circulating ghrelin. These 7TM receptors and associated Gα subunits constitute a major part of the molecular machinery directly mediating neuronal and endocrine stimulation versus metabolite and somatostatin inhibition of ghrelin secretion including a series of novel receptor targets not previously identified on the ghrelin cell.
Abbreviations: 7TM, seven transmembrane segment; BAC, bacterial artificial chromosome; CCK, cholecystokinin; CFMB, (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butamide; CGRP, calcitonin gene-related peptide; CHBA, 3-chloro-5-hydroxybenzoic acid; hrGFP, humanized Renilla reniformis green fluorescent protein; GLP-1, glucagon-like peptide 1; GIP, glucose-dependent insulinotropic polypeptide; PTx, Bordetella pertussis toxin; PYY, peptide YY
Keywords: Ghrelin, GPCR, Enteroendocrine, Secretion, G protein signaling, Metabolites
Graphical abstract
1. Introduction
Ghrelin is an octanoylated peptide produced mainly by gastric mucosal endocrine cells. Since its initial description as a potent stimulator of growth hormone secretion via engagement of the growth hormone secretagogue receptor [1], the known functions of ghrelin have broadened considerably to include a large number of physiologic processes and behaviors that influence eating, storage of energy, body weight, and blood glucose via mainly central but also peripheral mechanisms [2–4]. Plasma levels of ghrelin rise before expected meals and drop rapidly upon food intake [5,6] through molecular mechanisms that are poorly characterized. In contrast to most other enteroendocrine cells, gastric ghrelin cells are round, closed-type endocrine cells which do not contact the gastric lumen and are therefore not regulated directly by dietary components present in the stomach [7]. Gastric distension does not change plasma ghrelin levels; nor does a solution of glucose if it is retained in the stomach [5,8]. However, after reaching the small intestine, dietary glucose, lipids and amino acids all reduce plasma ghrelin [5,8–10], an effect most likely mediated through hormonal, neuronal and metabolite signals arising from nutrient sensing and absorption in the intestine. As examples, inhibition of ghrelin secretion by glucose and insulin has been demonstrated using primary cultures of rodent stomach cells [11,12], perfused rat stomach [13], and infusion studies in humans [14–18] – although this was not observed in all studies [19,20]. Several gastrointestinal peptides can suppress ghrelin secretion, including cholecystokinin (CCK) [21,22], glucose-dependent insulinotropic polypeptide (GIP) [23], glucagon-like peptide 1 (GLP-1) [13,24], peptide YY (PYY) [25] and somatostatin [20,26–28]. However, many of these effects may be indirect and/or have been difficult to reproduce and their physiological relevance remains unclear [13,22,28–30]. Also, metabolites efficiently decrease ghrelin secretion, as demonstrated by intravenous triglycerides [31] and total parenteral nutrition [32].
While known signals inhibiting ghrelin secretion are mainly endocrine and paracrine, known stimulatory signals are mainly neuronal. Both sympathetic nerve [33,34] and vagal stimulation [35,36] increase ghrelin secretion. Sympathetic stimulation most likely occurs directly at the ghrelin cell via β1 adrenergic receptors [33,37]. Vagal stimulation depends on cholinergic muscarinic mechanisms, but whether this effect is direct or indirect is unclear [35,37]. Inhibition of these pathways prevents fasting-induced elevation of plasma ghrelin levels [33,38].
Although we know much about how food, stress, sleep and circadian rhythmicity affects circulating ghrelin levels, the molecular machinery within the ghrelin cell which receives the stimulatory and inhibitory secretion signals remains mostly unknown. The large family of seven transmembrane segment (7TM), G protein-coupled receptors function as receivers for neurotransmitters, hormones and paracrine lipid messengers and as sensors for nutrients and metabolites [39,40]. In the present study, we identify the full repertoire of gastric ghrelin cell 7TM receptors and heterotrimeric GTP-binding protein Gα subunits and functionally characterize those that are both highly expressed and highly enriched in gastric ghrelin cells, in respect to their effects on ghrelin secretion.
2. Materials and methods
2.1. Transgenic and knockout mice
Ghrelin-hrGFP mice (line hrGFP10) [41], FFAR3-RFP mice, and FFAR2-RFP mice [42] were generated by pronuclear injection of recombined bacterial artificial chromosome (BAC) constructs into oocytes as described before. FFAR4 deficient mice and wild type age matched C57BL/6n mice were kindly provided by Merck (Kenilworth, NJ) through Taconic (Germantown, NY). FFAR2 deficient mice, FFAR3 deficient mice and ghrelin-Cre mice are described below. Rosa26-lox-STOP-lox-pertussis toxin S1 subunit mice (B6;129P2-Gt(ROSA)26Sortm1(ptxA)Cgh/Mmcd; stock #030678-UCD) were purchased from the Mutant Mouse Regional Resource Centers [43] and Rosa26-lox-STOP-lox-tdTomato reporter mice (B6J/N.Cg-Gt(ROSA)26Sortm14(CAG−tdTomato); stock #007908) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Animal procedures were conducted in accordance with the Danish Animal Research authorities (personal animal license 2012-15-2934-00221 issued by the Danish Committee for Animal Research) and the Institutional Animal Care and Use Committee of UT Southwestern Medical Center (permit numbers 2009-0377 and 2008-0107).
2.2. Compounds
The FFAR4 selective agonist compound B provided by Merck is example 209 in [44]. The FFAR3 selective agonist N-(2,5-dichlorophenyl)-4-(furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxamide (AR420626) and antagonist AR19 were synthesized as previously described [45]. The MC4R specific antagonist PG-901 was provided by Novo Nordisk A/S (Maaloev, DK). PYY3-36 and CP 55,940 were gifts from Christian Elling, 7TM Pharma (Hoersholm, DK). The following compounds were purchased: (S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butamide (CFMB) [Calbiochem/VWR (Herlev, DK)]; isoproterenol, sodium acetate, sodium propionate, sodium l-lactate, dopamine, acetylcholine, vincamine, and genistein [Sigma Aldrich (Broendby, DK)]; 3-chloro-5-hydroxybenzoic acid (CHBA) [Santa Cruz Biotechnology/AH Diagnostics (Aarhus, DK)]; the α-CGRP antagonist BIBN 4096, R-568 hydrochloride and the GPER agonist G-1 [Tocris Bioscience/R&D Systems Europe (Abingdon, UK)]; α-CGRP, CCK8, GIP, GLP-1, kisspeptin, neuromedin C, secretin, somatostatin, the somatostatin antagonist BIM-23627, and vasopressin [Bachem (Weil am Rhein, DE)], beraprost [Cayman Chemical/AH Diagnostics (Aarhus, DK)].
2.3. Fluorescence-activated cell sorting (FACS)
FACS was performed on dispersed gastric mucosal cells from ghrelin-hrGFP mice using previously described methods, separating them into hrGFP-positive and hrGFP-negative pools [41].
2.4. Quantitative PCR (qPCR)
RNA was isolated from sorted cells using STAT60 (Invitrogen/Life Technologies, Carlsbad, CA). The RNA was treated with Turbo DNA-free (Invitrogen/Life Technologies Europe, Naerum, DK), and converted to cDNA using Superscript III (Invitrogen). qPCR targeting G protein α subunits was performed as described previously [41] using primer sequences listed in Table S1. For the 7TM receptor analysis, cDNA was run on custom designed 384 well qPCR plates from Lonza (Copenhagen, DK) containing primers for 379 7TM receptors and 3 RAMPs together with primers for Rn18s and genomic DNA. Primer target regions are listed in Table S2. To gain intergenic comparable copy numbers, a genomic DNA sample was used as calibrator [46] and the relative copy number was calculated according to the formula:
CTtarget=the CT value of the 7TM receptor in the cDNA sample. CTDNA=the CT value of the 7TM receptor in a genomic DNA sample containing all assayed genes. NF is a GeNorm derived normalization factor using all genes with CT values below 35 in all samples. C is an arbitrary constant dependent on the DNA concentration, in this case C=11,585, consequently CT values of 35 are on average equal to one relative copy. Undetectable targets were assigned a CT value of 40.
2.5. Immunohistochemistry
Immunohistochemistry for ghrelin in gastric tissue from FFAR3-RFP mice and FFAR2-RFP mice was performed as described previously [47], using goat anti-ghrelin antibody [Santa Cruz Biotechnology (#sc-10368)], rat anti-RFP antibody [Chromotek (Planegg-Martinsried, DE)], Alexa Fluor® 488 anti-goat antibody, and Alexa Fluor® 594 anti-rat antibody [Invitrogen]. Immunohistochemistry to validate ghrelin-Cre mice was performed using stomachs and small intestines from adult (8–15 weeks old) gCre+/Tmt+ mice, as described in detail previously [41], using the above anti-ghrelin antibody and Alexa Fluor® 594 donkey anti-rabbit IgG (Invitrogen). All cells with either ghrelin-immunoreactivity alone, tdTomato fluorescence alone, or both were counted for three animals at three or more different levels, each separated by at least 100 µm, along the length of the gastric fundus or proximal duodenum.
2.6. IP3 accumulation
COS7 cells grown in Dulbecco's modified Eagle's medium (DMEM, low glucose) supplemented with 10% fetal bovine serum (FBS), 0.01 mg/ml penicillin/streptomycin, and 2 mM glutamine were transfected with 10 µg wild type receptor DNA in pCMV-Tag(2b) (Stratagene, La Jolla, CA) together with either 10 µg pCMVTag(2b) or 10 µg of the chimeric G protein Gα∆6qi4myr [48] using the calcium phosphate precipitation method+chloroquine. The next day, cells were seeded in 96 well plates (20.000 cells/well) with 5 µCi of myo-[3H]inositol/ml (Perkin Elmer, Broendby, DK). The following day, cells were washed and incubated at 37 °C for 30 min in Hank's buffer containing 10 mM LiCl, then 1.5 h with compound B at various concentrations, and finally lysed using 50 µl 10 mM cold formic acid on ice for 30 min. Twenty microliter were transferred to a 96 well plate+80 µl of YSi SPA beads (Perkin Elmer, diluted 1:8 to a final concentration of 1 mg YSi SPA beads/well). The plate was shaken for 30 min and centrifuged for 5 min at 1500 RPM and 3H was counted.
2.7. cAMP accumulation
FFAR2 and FFAR3 cDNAs were cloned into pCMV-Tag(2B). HEK293 cells were transfected with the plasmids using calcium phosphate precipitation. Cells were grown in DMEM GlutaMAX™-I supplemented with 10% FBS, 180 units/ml penicillin, 45 µg/ml streptomycin, and 0.5 mg/ml gentamicin to select for stable clones. Stably-transfected cells were seeded in 96-well plates (2.5×104 cells/well), incubated for 24 h, washed, and incubated in HEPES buffered saline with 300 µM 3-isobutyl-1-methylxanthine for 30 min at 37 °C. Twenty micromolar of forskolin (Sigma Aldrich) and various concentrations of propionate, CFMB, AR420626, and AR19 were added for 30 min at 37 °C, followed by the HitHunter™ cAMPXS+ assay (DiscoveRx, Birmingham, UK).
2.8. Primary gastric mucosal cell cultures
The method was modified from [11,49] to obtain fragments of gastric glands instead of single cells from C57BL/6 mice [Charles River, Sulzfeld, DE]. Following cervical dislocation, the stomach was tied off at the esophagus and duodenum, excised, turned inside out through an incision in the nonglandular forestomach, rinsed with PBS, inflated with DMEM-F12 and placed in a 50 ml tube containing 10 ml DMEM-F12 with 20 mM HEPES, 1 mg/ml collagenase (Type XI – Sigma Aldrich), and 0.15% BSA (Sigma Aldrich). The tube was incubated in a 37 °C water bath shaking at 155 RPM for 40 min, after which the tube was shaken vigorously for 5 min. One milliliter FBS was added. The stomach was removed and the released cell clumps were allowed to sediment for 15 min. The pellet was dissolved in DMEM-F12 containing 10% FBS and 0.5 mM DTT (Invitrogen). The supernatant was centrifuged for 3 min at 0.2 RCF, and the pellet was mixed with the rest of the cell clumps, and the cell suspension was passed through a 500 µM nylon mesh. The cell clumps were allowed to sediment for 15 min, after which the pellet was resuspended in DMEM-F12 (which has 7.8 mM glucose) containing 10% FBS, 50 µM octanoate-BSA, 0.01 mg/ml penicillin/streptomycin and 2 mM glutamine [41]. The cell suspension was plated (400 µl/well) in 24 well Matrigel coated plates (200 µl Matrigel [BD Biosciences, Albertslund, DK] diluted 1:20 in DMEM-F12 and aspirated after at least 1 h) filled with 300 µl/well DMEM-F12 containing 10% FBS, 50 µM octanoate-BSA, 0.01 mg/ml penicillin/streptomycin and 2 mM glutamine. Cells pooled from 1 to 3 mice were seeded in 12–60 wells. To test Gαi/o dependence, pertussis toxin (PTx; 0.1 µg/ml, Calbiochem) was added to the medium. The plate was left for 10 min and then incubated overnight in humidified 90% air 10% CO2 at 37 °C.
2.9. Ex vivo ghrelin secretion studies
The day after the preparation of primary gastric mucosal cells, media was aspirated from the wells, and ligands were added in triplicate in 500 µl 37 °C DMEM (Invitrogen #11966) containing 5 mM glucose, 0.5% FBS and 0.15% fatty acid free BSA (Sigma Aldrich). To test Gαi dependence PTx (0.1 µg/ml) was added to the medium. Cells were incubated with ligands for 4 h, after which 250 µl supernatant was centrifuged for 3 min at 1500 RCF and 4 °C. Then, 200 µl supernatant was mixed with 20 µl 1 M HCl and kept at −80 °C until ghrelin was measured. Acyl-ghrelin was measured using “Acyl-ghrelin EIA” [SPI-Bio/AH Diagnostics (Aarhus, DK, #A05117)]. Total-ghrelin was measured with “Total Ghrelin ELISA” [Millipore (Hellerup, DK, #EZRGRT-91K)]. Secretory response of ghrelin cells to administered ligands was expressed as percentage of secreted ghrelin from cells exposed to medium alone.
2.10. In vivo ghrelin secretion studies
Compound B dose–response – Nine wk-old C57Bl/6J male mice (Taconic, Ry, DK) were fasted overnight in new cages with free access to water. Animals were orally dosed by gavage with compound B dissolved in 0.5% carboxymethylcellulose (CMC, Sigma) (3, 10, 30 mg/kg, n=6/group) or vehicle (0.5% CMC). Thirty minutes after dosing, blood samples were taken by retroorbital bleeding into EDTA coated tubes with aprotinin (500 KIU/ml, Sigma), centrifuged (1.8 g, 15 min. and 4 °C) and plasma stored at −20 °C before measurement of total ghrelin.
FFAR4 deficient mice – Nineteen-wk-old male FFAR4 deficient mice and age-matched wild type animals (C57Bl/6n, n=10) were fasted overnight in new cages with free access to water. A fasting blood sample was taken by retroorbital bleeding and processed as above for measurement of total ghrelin levels. Subsequently the animals were administered olive oil (10 ml/kg, Sigma Aldrich) by gavage, euthanized after 2 h, and trunk blood was collected.
2.11. FFAR2 and FFAR3 deficient mice
Exons encoding FFAR2 and FFAR3 were replaced by a cassette encoding neomycin phosphotransferase (neor) by homologous recombination in murine embryonic stem cells (ESC) (Figure S1A and B). Correct targeting in ESCs was verified by Southern blotting (Figure S1C). Correctly recombined ESC clones were injected into blastocysts from C57BL/6 donor mice. Chimeric blastocysts were then transferred into pseudopregnant surrogate mothers and chimeric male offspring was bred with C57BL/6 females to obtain F1 heterozygous progeny. Germline transmission of the mutated allele was verified by PCR and Southern blotting (Figure S1D). Mice were backcrossed with C57BL/6 mice for nine generations.
2.12. Ghrelin-Cre mice
Ghrelin-Cre transgenic mice were generated by microinjection of a Cre-modified, mouse ghrelin BAC (RP23-128E5; BACPAC Resources Center at Children's Hospital Oakland Research Institute) into pronuclei of fertilized one-cell stage embryos of C57BL6/J mice at the UTSW Medical Center Transgenic Core Facility. The same original RP23-128E5 BAC had previously been used to generate a previously-reported “R4” ghrelin-hrGFP line [41]. The construction of the ghrelin-Cre transgene was done using techniques described in more detail elsewhere [50], but in brief involved replacing the first 29 bp of the preproghrelin coding sequence with the coding sequence of Cre recombinase (Figure S2). Multiple potential ghrelin-Cre founder mice were generated, of which one (line E7) was used here based on its Cre activity expression pattern. Primers to confirm the genotype of mice harboring the ghrelin-Cre transgene were as follows: 5′-ggtcagcctaattagctctgtcat-3′ and 5′–TGCGAACCTCATCACTCGTTGCAT-3′. For immunohistochemical validation of transgene expression (described in Section 2.5), ghrelin-Cre mice were bred to Rosa26-lox-STOP-lox-tdTomato reporter mice, in which a transcriptional stop cassette is removed in the presence of Cre recombinase activity resulting in the expression of tdTomato fluorescence. Mice with one copy of each transgene are herein designated as gCre+/Tmt+ mice.
2.13. gCre+/PTx+ mice
Ghrelin-Cre mice were bred to Rosa26-lox-STOP-lox-pertussis toxin S1 subunit mice, in which a transcriptional stop cassette is removed in the presence of Cre recombinase activity resulting in the expression of the catalytic S1 subunit of PTx. Mice with one copy of each transgene are herein designated as gCre+/PTx+ mice. Correct targeting of PTx expression to ghrelin cells was confirmed by breeding gCre+/PTx+ mice to ghrelin-hrGFP mice to generate mice with the ghrelin hrGFP transgene±the ghrelin-Cre transgene±the Rosa26-lox-STOP-lox-PTx transgene. Stomachs from one mouse of each genotype were processed as above to obtain FACS-separated hrGFP-positive and hrGFP-negative pools. qPCR using PTx-specific primers (5′-CACACCGGCGCATTCC-3′ and 5′-TTGTGATAGACCCGCGTTACC-3′) was then performed on cDNA derived from the pools, as above.
Fresh blood from mice with ad lib access to regular chow was collected from tails of 6-wk-old and 14-wk-old gCre+/PTx+ mice and littermate controls of three different genotypes (gCre−/PTx−, gCre−/PTx+, and gCre+/PTx−). Blood was processed as previously described to inhibit degradation of acyl-ghrelin [41] prior to assay for acyl-ghrelin levels using ghrelin EIA kits as above. In addition, body weights for these animals were measured weekly, and body weights of gCre+/PTx+ mice were found to be indistinguishable from those of animals of the three control genotypes.
2.14. Statistics
Data was visualized and tested for significance using GraphPad Prism 6. Differences in ghrelin release from primary cells and mice treated with compound B were assessed using nonparametric one-way ANOVA (Kruskal–Wallis) with Dunn's posthoc test, unless otherwise stated in the figure legends. Differences in plasma ghrelin levels between gCre+/PTx+ mice and the 3 control genotypes at 6 and 14 weeks were assessed using repeated measures ANOVA with Tukey's posthoc test. The differences in plasma ghrelin levels between wild type and FFAR4 deficient mice before and after olive oil were assessed using ordinary two-way ANOVA with Sidak's posthoc test. Statistical significance was defined as p<0.05=⁎, p<0.01=⁎⁎, p<0.001=⁎⁎⁎, p<0.0001=⁎⁎⁎⁎ for all tests.
3. Results
3.1. 7TM receptor repertoire of gastric ghrelin cells
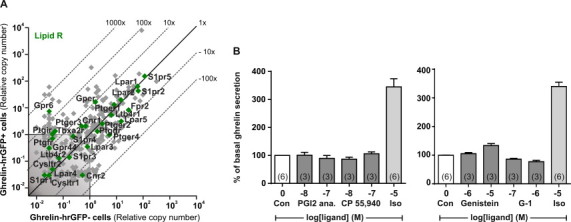
Ghrelin-hrGFP positive cells (herein designated “ghrelin cells”, as an almost 100% overlap between the expression of hrGFP and immunohistochemical staining for ghrelin was shown previously [41]), were purified by FACS from the stomachs of ghrelin-hrGFP reporter mice. cDNA from the purified ghrelin cells was analyzed by a qPCR array targeting 379 non-odorant 7TM receptors (Figure 1, gray and green symbols). Ninety 7TM receptors were stably expressed above noise level in the ghrelin cells. The mRNA for 29 of these receptors was enriched more than five-fold in the ghrelin cells as compared to the non-ghrelin (hrGFP-negative) mucosal cells. In the following sections a functional characterization of those 7TM receptors which were most enriched and/or highly expressed in ghrelin cells will be presented in groups according to their type of ligand: i.e. neurotransmitters, neuropeptides, nutrients/metabolites, peptide hormones and paracrine lipid messengers. The degree of expression in the ghrelin cells versus the surrounding control cells for all the receptors in each of these groups as well as the expression of orphan receptors from families A, B and C, respectively is listed in Table S3.
Figure 1.
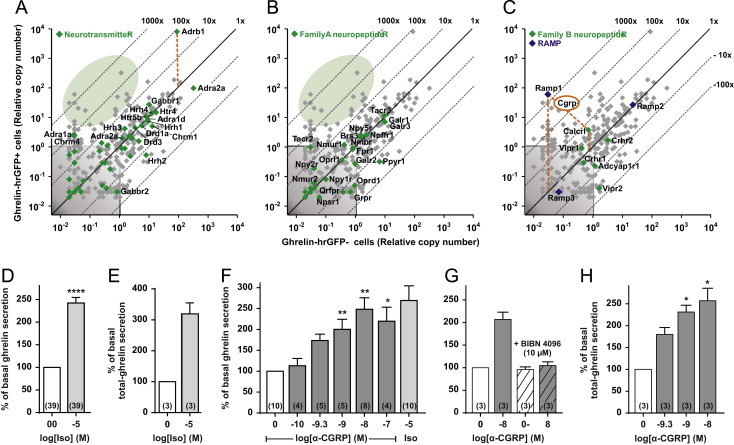
Expression and functional analysis of 7TM receptors for neurotransmitters and neuropeptides in gastric ghrelin cells. (A–C) qPCR expression data for 379 7TM receptors (gray plus green dots) and three RAMPs (blue dots, C) examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells. (A) Receptors for neurotransmitters, (B) family A neuropeptide receptors, and (C) family B neuropeptide receptors are highlighted with green dots and the receptor gene name indicated for all neuropeptide receptors but only for the enriched and/or highly expressed small molecule neurotransmitter receptors for clarity. The 45°-angled lines refer to the enrichment within ghrelin cells. The gray shaded area is considered as noise level. The orange dashed lines highlight particularly enriched receptors. The expression data for all the receptors and RAMPs are listed in Table S3. The green shaded areas highlight the lack of enriched neurotransmitter (A) and neuropeptide (B) receptors, respectively (i.e. only gray and no green dots in the shaded green areas). (D and E) Acyl-ghrelin/total ghrelin release from primary gastric mucosal cells treated with isoproterenol, (F and H) increasing concentrations of α-CGRP or isoproterenol, (G) or with BIBN 4096 alone (hatched) and before 10 nM of α-CGRP (gray, hatched). The data is normalized to basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is indicated in brackets in each bar.
3.2. Stimulation of ghrelin secretion through the β1-adrenergic receptor – the only enriched neurotransmitter receptor
In agreement with previous reports [12,33] the β1-adrenergic receptor (Adrb1) was nearly 100-fold enriched and was by far the most highly expressed among all the 7TM receptors in ghrelin cells. No other receptor for small molecule neurotransmitters was both highly expressed and enriched (the shaded green area in Figure 1A). While several neurotransmitter receptors such as the non-functional GABA b1 receptor protomer (Gabbr1), the histamine 1 and 4 receptors (Hrh4 and Hrh1), the serotonin 4 and 5b receptors (Htr4 and Htr5b) and the dopamine 1a and 3 receptors (Drd1a and Drd13) were relatively highly expressed in ghrelin cells, they also were highly expressed in non-ghrelin gastric mucosal cells, and thus not enriched within ghrelin cells.
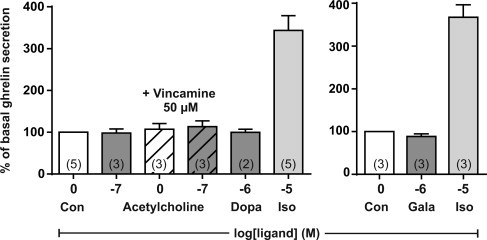
Dopamine stimulates ghrelin secretion from a ghrelinoma cell line [26], but not from the primary gastric mucosal cultures used here (Figure S3). Similarly, although cholinergic muscarinic activation has been reported to stimulate ghrelin secretion we did not observe any muscarinic receptor to be highly expressed and enriched in the ghrelin cells nor did acetylcholine affect ghrelin secretion (Figure S3).
The β-adrenergic agonist isoproterenol was an efficacious stimulator of both acyl and total ghrelin secretion (Figure 1D and E) and was consequently used as a positive control. All compounds identified in the present study similarly stimulated or inhibited both acyl and total ghrelin secretion, as shown for α-CGRP (Figure 1H) and lactate (Figure 3K).
Figure 3.
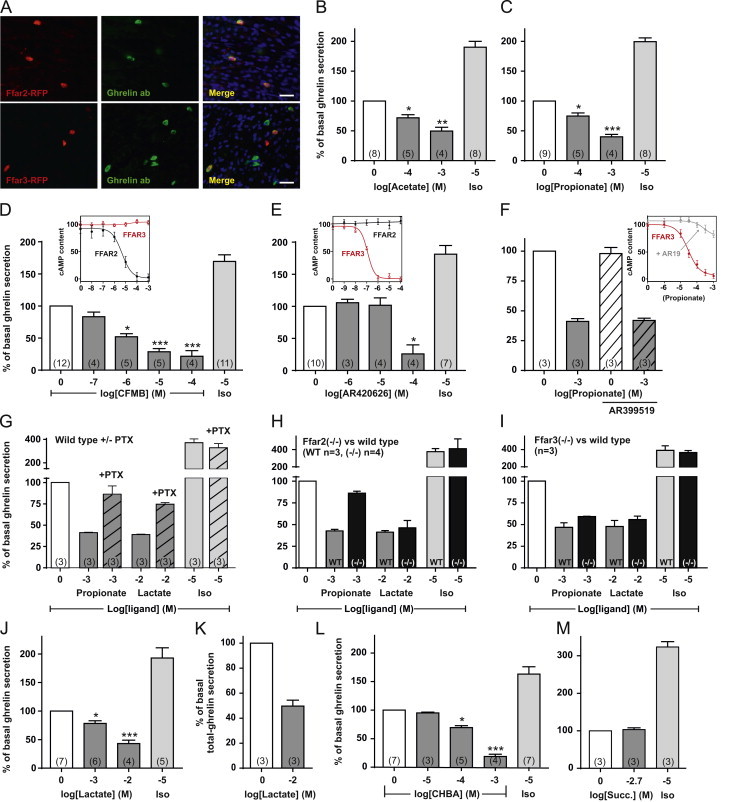
Functional analysis of FFAR2, FFAR3 and GPR81 in ghrelin secretion ex vivo. (A) Immunohistochemistry with antibodies against mRFP (red) and ghrelin (green) on gastric tissue from mice expressing mRFP from either the FFAR2 promoter (top) or the FFAR3 promoter (bottom) (see text for details). The scale bars in the merged pictures correspond to 25 µm. (B) Acyl-ghrelin release from primary gastric mucosal cells treated with various concentrations of acetate, (C) propionate, (D) CFMB, (E) AR420626, (F) propionate alone and after addition of 20 µM AR19 (hatched), (J) lactate, (L) CHBA, (M) succinate or 10 µM isoproterenol normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is written in brackets in each bar. (D, E, and F inserts) cAMP accumulation in HEK293 cells stably transfected with either FFAR2 (black) or FFAR3 (red) and treated with increasing concentrations of CMFB, AR420626, or propionate alone or after addition of 20 µM AR19 (gray). FFAR2-transfected cells treated with propionate and AR19 are shown in Figure S4. (H) Acyl-ghrelin release from primary gastric mucosal from wild type mice (gray), FFAR2 deficient mice (black), or (I) FFAR3 deficient mice (black) treated with 1 mM propionate, 10 mM lactate or 10 µM isoproterenol normalized to the basal secretion of ghrelin from vehicle-treated cells from wild type or knockout mice, respectively, and shown as means±SEM. (G) Acyl-ghrelin release from primary gastric mucosal cells treated with 1 mM propionate, 10 mM lactate or 10 µM isoproterenol alone or after addition of PTx (hatched) normalized to the basal secretion of ghrelin from vehicle-treated cells with and without PTx treatment, respectively (PTx did not affect basal ghrelin secretion – see legend of Figure 2), and shown as means±SEM. Number of repeated experiments is written in brackets in each bar. (K) Total-ghrelin release from primary gastric mucosal cells treated with 10 mM lactate and normalized to the basal secretion of total-ghrelin from vehicle-treated cells.
3.3. Stimulation of ghrelin secretion through the CGRP receptor – the only enriched neuropeptide receptor
Most neuropeptide receptors belong to the large family A, rhodopsin-like type of 7TM receptors. However, no family A neuropeptide receptor was enriched in the ghrelin cells (shaded green area in Figure 1B). A few family A neuropeptide receptors – including tachykinin NK3 receptor (Tacr3) and galanin receptors 1 and 3 (Galr1 and Galr3) – were highly expressed but not enriched in the ghrelin cells (Figure 1B). Despite the high expression of galanin receptors, 1 µM galanin did not affect ghrelin secretion from the primary cultures of gastric mucosal cells (Figure S3).
Among the family B neuropeptide receptors, the calcitonin receptor-like receptor (Calcrl) was the only ghrelin cell-enriched receptor albeit only about five-fold (Figure 1C). Several family B receptors including Calcrl must interact with ‘receptor activity-modifying proteins’ (RAMPs) to display their pharmacological properties. RAMP1 was the most highly enriched ghrelin cell mRNA examined in this study (~2400 fold; Figure 1C; Table S3). In contrast, RAMP3 could not be detected and RAMP2 was highly expressed but not enriched in ghrelin cells (Figure 1C). Importantly, heterodimers of Calcrl and RAMP1 comprise the calcitonin gene related peptide (CGRP) receptor [51]. Accordingly, stimulation of primary gastric mucosal cell cultures with the neuropeptide α-CGRP resulted in a dose-dependent stimulation of both acylated and total ghrelin secretion with an EC50 of 0.5 nM and reaching a similar Emax as observed with isoproterenol (Figure 1E–G). This effect was blocked by the CGRP receptor antagonist, BIBN 4096 (Figure 1E).
Thus, among about 40 known 7TM receptors for monoamine neuronal messengers and about 35–40 known neuropeptide 7TM receptors, Adrb1 and the heterodimeric CGRP receptor are utilized seemingly very selectively by ghrelin cells to efficaciously stimulate ghrelin secretion.
3.4. Expression of a selective repertoire of receptors for dietary and endogenous metabolites
A basic characteristic feature of enteroendocrine cells is their ability to sense nutrients and dietary metabolites, and we therefore examined ghrelin cell expression of 7TM receptors for such metabolites (Figure 2). Intestinal enteroendocrine cells, such as the CCK and GLP-1 cells, express three different receptors for the main metabolites of triglyceride, i.e. FFAR1 (GPR40, Ffar1) and FFAR4 (GPR120, Ffar4) recognizing long chain fatty acids (LCFAs), and GPR119 (Gpr119) sensing 2-acyl glycerols (Figure 2B) [52,53]. Of these, only FFAR4 was both highly expressed and enriched in ghrelin cells, whereas FFAR1 and GPR119 were undetectable (Figure 2A). Among the remaining metabolite receptors normally expressed in intestinal enteroendocrine cells, the short chain fatty acid (SCFA) receptor FFAR2 (GPR43, Ffar2) and the calcium sensing receptor (CaSR) were enriched in ghrelin cells. The SCFA receptor FFAR3 (GPR41, Ffar3) was also expressed in ghrelin cells, but was not enriched (Figure 2A). Most surprisingly and unique for the ghrelin cell, the lactate receptor GPR81, which has not been described in other enteroendocrine cell types, was highly enriched in the ghrelin cells (~1300 fold). This makes GPR81 the most enriched of all 7TM receptors in ghrelin cells (Figure 2A, Table S3). The function of these nutrient receptors is assessed below.
Figure 2.
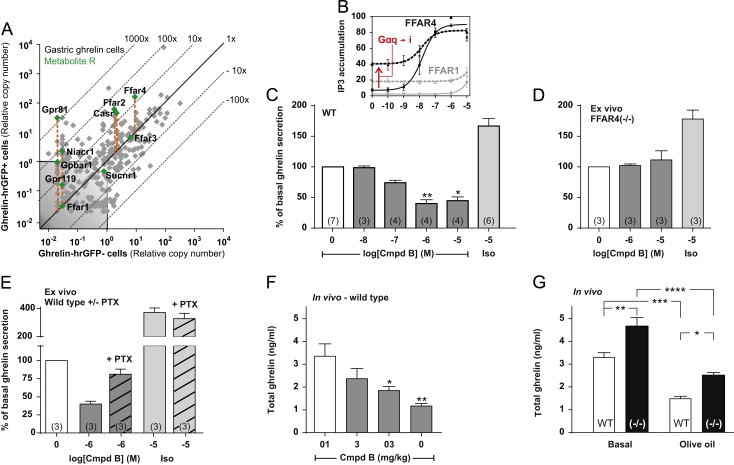
Expression of 7TM receptors for metabolites in gastric ghrelin cells and functional analysis of FFAR4 in ghrelin secretion in vitro, ex vivo and in vivo. (A) qPCR data for 379 7TM receptors examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells. Receptors for nutrients and dietary metabolites are highlighted with green dots. (B) Inositol triphosphate accumulation in COS7 cells transiently transfected with either FFAR4 (black, solid), FFAR1 (gray, solid), FFAR4 together with Gα∆6qi4myr (black, dotted) or FFAR1 together with Gα∆6qi4myr (gray, dotted) and treated with increasing concentrations of the FFAR4 specific agonist compound B normalized to the maximum compound B-induced FFAR4 activation. (C) Acyl-ghrelin release from primary gastric mucosal cells from wild type or (D) FFAR4 deficient mice treated with increasing concentrations of compound B or 10 µM isoproterenol normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is indicated in brackets in each bar. (E) Acyl-ghrelin release from primary gastric mucosal cells treated with 1 µM of compound B or 10 µM of isoproterenol alone, or after addition of pertussis toxin (PTx, hatched) normalized to the basal secretion of ghrelin from vehicle-treated cells with and without PTx treatment, respectively (the PTx treatment did not affect ghrelin secretion from vehicle-treated cells (−PTx: 648±86 pg/ml, +PTx: 607±66 pg/ml)), and shown as means±SEM. Number of repeated experiments is indicated in brackets in each bar. (F) Total-ghrelin concentrations in plasma from mice orally dosed with increasing concentrations of compound B after an overnight fast. (G) Total-ghrelin concentrations in plasma from wild type mice (white) or FFAR4 deficient mice (black) after an overnight fast (basal) and two hours after an oral triglyceride load (120 min).
3.5. LCFA receptor FFAR4 inhibits ghrelin secretion both ex vivo and in vivo
The repertoire of endogenous lipid ligands for FFAR1 and FFAR4 overlaps just as certain commonly used pharmacological compounds (i.e. GW9508) are dual specific FFAR1/FFAR4 agonists [54]. In the present study we instead used a novel, synthetic small molecule agonist, compound B, to target FFAR4. Compound B activates the Gαq-coupled FFAR4 with a potency (EC50=15 nM) more than three orders of magnitude greater than that for FFAR1, as determined in inositol triphosphate accumulation assays in transfected COS7 cells (Figure 2B). As shown below FFAR4 apparently does not stimulate ghrelin secretion as expected for a Gαq-coupled receptor but instead inhibit secretion. However, co-transfection with a chimeric G protein, which directs Gαi/o-coupling to the Gαq pathway, markedly increased the basal signaling of FFAR4, indicating that FFAR4 is able to couple to Gαi besides Gαq.
Compound B inhibited basal ghrelin secretion from primary gastric mucosal cells in a dose-dependent manner with an EC50 value of approximately 100 nM (Figure 2C). Importantly, the inhibitory effect of compound B on ghrelin secretion was mediated through FFAR4 as no effect of the compound was observed in cells isolated from FFAR4 deficient mice (Figure 2D). Furthermore, the inhibitory effect on ghrelin secretion of the selective FFAR4 agonist was blunted by treatment with PTx, indicating a Gαi/o-mediated effect (Figure 2E). The inhibitory effect of FFAR4 activation was also observed in vivo in mice, as oral dosing of 3–30 mg/kg of compound B dose-dependent decreased plasma ghrelin under fasting conditions (Figure 2F).
Interestingly, plasma ghrelin in fasted FFAR4 deficient mice was significantly higher (1.4 fold) than that observed in wild-type mice, suggesting that ghrelin secretion under normal conditions is under a tonic FFAR4-mediated inhibition (Figure 2G). However, FFAR4 was not required for the triglyceride induced inhibition of ghrelin secretion, as a similar reduction in plasma ghrelin was observed in FFAR4 deficient and wild type mice after an olive oil challenge (Figure 2G).
It is concluded that the usually Gαq-coupled FFAR4 receptor for LCFAs inhibits ghrelin secretion through a Gαi/o-coupled mechanism.
3.6. Inhibition of ghrelin secretion through the short chain fatty acid (SCFA) receptor FFAR2
qPCR analysis demonstrated both FFAR2 and FFAR3 expression in the ghrelin cells, although the expression level and degree of enrichment (about 20-fold) was much greater for FFAR2 than for FFAR3 (Figure 2A, Table S3). This expression pattern was confirmed by immunohistochemical analysis of gastric tissue from two strains of transgenic reporter mice expressing monomeric red fluorescent protein (mRFP) under the control of transcriptional elements for ffar3 and ffar2, respectively [42]. In the gastric mucosa the majority of FFAR2-mRFP positive cells also stained with ghrelin antibody and the majority of ghrelin immunoreactive cells were FFAR2-mRFP positive (Figure 3A, upper panels). In contrast, only about half of ghrelin cells expressed the FFAR3-mRFP reporter protein and FFAR3-mRFP was observed in some cells which did not stain for ghrelin (Figure 3A, lower panels) – many of which were gastrin cells (data not shown). Thus, the expression analysis of ghrelin cells and the immunohistochemistry on gastric tissue from the FFAR3-mRFP and FFAR2-mRFP mice consistently showed that FFAR2 is expressed and particularly enriched in ghrelin cells.
The SCFAs, acetate and propionate decreased ghrelin secretion from primary gastric mucosal cells with similar efficacy (Figure 3B and C). As acetate is a poor ligand for FFAR3, these data suggest that the SCFA effect is mediated through FFAR2.
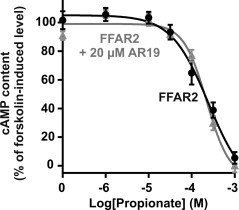
To better differentiate between the two SCFA receptors, we employed CFMB as an established, selective FFAR2 agonist [55] and two novel, selective ligands for FFAR3, the agonist AR420626 and the antagonist AR19 [45]. The selectivity of the three compounds first was probed in cAMP accumulation assays performed in stably transfected HEK293 cells, where CFMB had an IC50 value of 4.7 µM on FFAR2 and did not activate FFAR3 in concentrations up to 1 mM (Figure 3D, insert). In contrast, AR420626 had an IC50 value of 117 nM on FFAR3 and did not activate FFAR2 in concentrations up to 100 µM (Figure 3E, insert). AR19 selectively inhibited the propionate-induced activation of FFAR3 (Figure 3F, insert), but had no effect on FFAR2 (Figure S4).
In the primary gastric mucosal cells, the FFAR2 selective agonist CFMB efficiently decreased basal ghrelin secretion in a dose-dependent manner with an EC50 of ~1 µM (Figure 3D). In contrast, the FFAR3 selective agonist AR420626 only had an effect on ghrelin secretion at 100 µM suggesting an off-target effect (Figure 3E), while the FFAR3 selective antagonist AR19 did not have any effect on propionate-induced inhibition of ghrelin secretion (Figure 3F). The propionate induced reduction in ghrelin secretion is mediated through a Gαi/o-coupled mechanism, as the inhibitory response was reduced from 59% to 14% by treatment with PTx (Figure 3G).
That propionate mainly inhibits ghrelin secretion via FFAR2 is supported by a markedly attenuated inhibition in gastric mucosal cells isolated from FFAR2 deficient mice (Figure 3H); i.e. a reduction in ghrelin secretion of only 14% as compared to 57% in cells isolated from wild type littermate controls (Figure 3H). In contrast, in cells isolated from a FFAR3 deficient mouse, propionate still inhibited ghrelin secretion rather efficiently, by 41% (Figure 3I). It should be noted, that the expression of FFAR2 has been reported to be reduced in FFAR3 deficient mice [56] and that the slight reduction in propionate-induced inhibition of ghrelin secretion might result from a lower level of FFAR2. Lactate served as a positive control which efficiently reduced ghrelin secretion (see below) in cells from both the FFAR2 and FFAR3 deficient mice (Figure 3H and I).
It is concluded that SCFAs such as propionate and acetate inhibit ghrelin secretion efficiently mainly through the FFAR2 receptor and a Gαi/o mechanism.
3.7. Inhibition of ghrelin secretion through the highly enriched lactate receptor GPR81
Treatment of primary gastric mucosal cells with lactate at concentrations of 1 and 10 mM, which are considered to be physiologically relevant concentrations [57], resulted in a dose-dependent and efficient inhibition of both acyl-ghrelin and total ghrelin secretion (Figure 3J and K). The inhibitory effect of lactate on ghrelin secretion was blunted by treatment with PTx indicating a Gαi mediated effect (Figure 3I). To probe the specific role of GPR81 in the inhibition of ghrelin secretion, we tested the effect of the selective GPR81 agonist 3-chloro-5-hydroxybenzoic acid (CHBA) [58]. As observed with lactate, CHBA reduced basal ghrelin secretion of ghrelin in a dose-dependent and more potent manner inhibiting basal ghrelin secretion down to 20% of vehicle-treated cells at a dose of 1 mM (Figure 3L). Since lactate as well as propionate and acetate all are small organic acids, we tested whether another small organic acid, succinate, had a similar inhibitory effect on ghrelin secretion. Succinate which signals through GPR91, a 7TM receptor expressed below the noise level in ghrelin cells (Sucnr1, Figure 2A), had no effect on ghrelin secretion (Figure 3M).
Thus, ghrelin secretion is inhibited by lactate acting through the highly enriched GPR81 receptor.
3.8. Inhibition of ghrelin secretion by agonists for the calcium sensing receptor, CaSR
The calcium sensing receptor, CaSR, which is a family C 7TM receptor implicated in sensing both calcium ions and aromatic amino acids [59], is expressed and enriched in ghrelin cells to the same degree as FFAR2 (Figure 2A). Treatment of primary gastric mucosal cells with calcium chloride decreased ghrelin secretion dose-dependently, albeit only at supraphysiological concentrations of 20–40 mM (Figure 4A). The calcimimetic R-568 [60], which is an allosteric agonist analog of Cinacalcet® at the CaSR, also inhibited ghrelin secretion in a dose-dependent manner in the presence of 1.8 mM CaCl2 in the medium (Figure 4B). Surprisingly, increasing calcium by adding 4 mM – which by itself did not have any effect on ghrelin release – shifted the effect of R-568 from inhibition to slight stimulation of ghrelin secretion (Figure 4C).
Figure 4.

Functional analysis of CaSR in ghrelin secretion ex vivo. (A) Acyl-ghrelin release from primary gastric mucosal cells treated with various concentrations of R-568, (B) CaCl2, 10 µM isoproterenol, or (C) R-568 alone, 4 mM CaCl2 alone or R-568 together with 4 mM CaCl2 (gray, hatched). The data has been normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is written in brackets in each bar.
Thus, CaSR activation, which stimulates secretion of other gut hormones [61–63], appears to mainly inhibit ghrelin secretion but is apparently balancing between inhibition and stimulation in a complex manner.
3.9. 7TM receptors for peptide hormones on ghrelin cells
Expression data for peptide hormone receptors belonging to the large rhodopsin-like, family A type of 7TM receptors are shown in Figure 5A. The melanocortin receptor 4 (MC4R, Mc4r) was highly expressed and enriched in ghrelin cells, i.e ~1240 fold, followed by vasopressin receptor 1b (Avpr1b) and kisspeptin receptor (Kiss1r). In the primary gastric mucosal cells, α-melanocyte stimulating hormone (α-MSH) efficiently stimulated ghrelin secretion in a dose-dependent manner with an EC50 of approximately 10 nM (Figure 5B). The α-MSH-induced ghrelin secretion could be blocked by the MC4R specific antagonist PG-901 [64,65] (Figure 5B). Although the Gαq-coupled vasopressin 1b receptor was expressed and enriched close to the level of GPR81, vasopressin had only a limited stimulatory effect on ghrelin release and at supra-physiological concentrations only (Figure 5C). Kisspeptin did not affect ghrelin release (Figure 5C).
Figure 5.
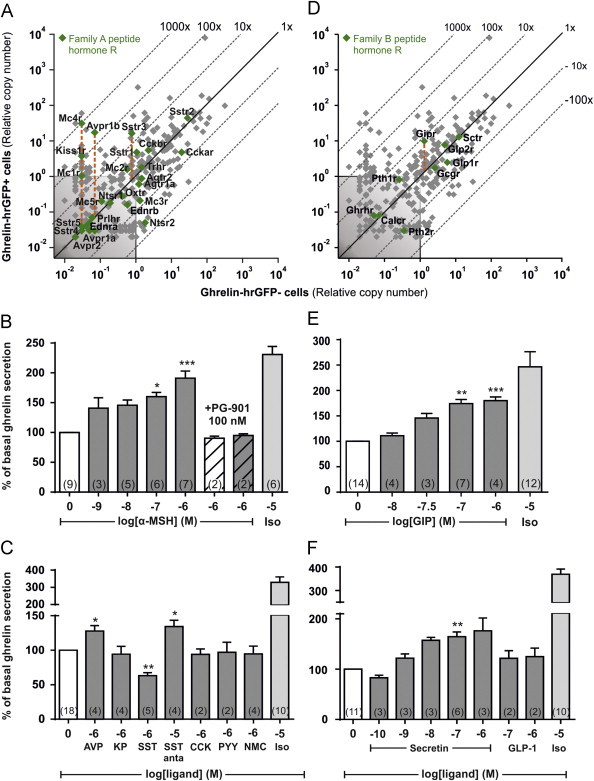
Expression and functional analysis of 7TM receptors for peptide hormones in gastric ghrelin cells. (A and D) qPCR data for 379 7TM receptors examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells. Family A receptors for peptide hormones and (D) family B receptors for peptide hormones have been marked with green. (B) Acyl-ghrelin release from primary gastric mucosal cells treated with increasing concentrations of α-MSH, 100 nM PG-901 alone (hatched) or before 1 µM α-MSH, (C) 1 µM vasopressin (AVP), kisspeptin (KP), somatostatin (Sst), 10 µM of the somatostatin antagonist BIM-23627, 1 µM CCK8, PYY3-36, neuromedin C (NMC) (significance tested with Mann–Whitney), (E) increasing concentrations of GIP, (F) secretin, GLP-1 or 10 µM isoproterenol. The data has been normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is written in brackets in each bar.
Three of the five somatostatin receptors SSTR1, SSTR2, and SSTR3 were expressed at relatively high levels in the ghrelin cells and of these SSTR1 and SSTR3 were enriched (Figure 5A), as demonstrated previously [11,66]. As expected, somatostatin inhibited ghrelin secretion from primary gastric mucosal cells, though at relatively high concentration (Figure 5C). Interestingly, the broad-spectrum somatostatin antagonist BIM-23627 stimulated basal ghrelin release (Figure 5C), indicating that in the employed ex vivo gastric mucosal cell preparation there is an inhibitory somatostatin tone which is eliminated by the somatostatin antagonist. The IC50 value for BIM-23627 on SSTR2 and SSTR3 is in the nanomolar range in contrast to almost 3 µM on SSTR1 [67]. The high concentration of BIM-23627 needed to block the inhibitory tone of somatostatin on ghrelin secretion suggests that SSTR1 contributes to the inhibition.
The two CCK receptors, CCK1R (Cckar) and CCK2R (Cckbr) both were relatively highly expressed but not enriched in ghrelin cells. However, CCK did not affect ghrelin secretion from the gastric mucosal cell cultures. Although PYY and neuromedin C have previously been suggested as regulators of ghrelin secretion [25,28], expression of the corresponding Y1 (Npy1r), Y2 (Npy2r) and BB2 (Grpr) receptors were not detectable in the ghrelin cells and neither PYY nor neuromedin C affected ghrelin secretion (Figure 5C).
Among the family B peptide hormone receptors, the Gαs-coupled GIP receptor (Gipr) was the only one enriched in ghrelin cells (about 8-fold), whereas the secretin receptor (Sctr) and the GLP-2 receptor (Glp2r) both were expressed to the same level but not enriched (Figure 5D, Table S3). Of these peptide hormones, GIP stimulated ghrelin secretion from the primary gastric mucosal cells 1.8 fold with an EC50 value of 29 nM (Figure 5E). Secretin also stimulated ghrelin secretion with an Emax similar to GIP and an EC50 value of 1.8 nM (Figure 5F). In contrast, no effect of GLP-1 on ghrelin secretion was observed, which is in agreement with the observation of low GLP-1 receptor expression and a negative enrichment in ghrelin cells (Figure 5D and F), which has also been reported previously [66].
It is concluded that in respect of receptors for peptide hormones ghrelin secretion is stimulated by the GIP, secretin and MC4 receptor and inhibited by one or more of somatostatin receptors SSTR1, SSTR2, and SSTR3.
3.10. 7TM receptors for lipid messengers in ghrelin cells
7TM receptors for paracrine “lipid messengers”, including the sphingosine-1-phosphate receptors 2 and 5 (S1pr2, S1pr5) and the lysophosphatidic acid receptors 1 and 2 (Lpar1, Lpar2), are among the most highly expressed receptors in gastric mucosal cells – both in ghrelin cells and in the surrounding cells (Figure S5A). Only two paracrine lipid messenger receptors are enriched in ghrelin cells – GPR6 (~275 fold), which is thought to be activated by sphingosine-1-phosphate [68] and the G protein-coupled estrogen receptor GPER (Gper) (~10 fold) (Figure S5A). No selective ligand is available for GPR6 to our knowledge, however we tested the effect of the phytoestrogen genistein, which is known to activate both GPER and cytosolic estrogen receptors [69], and the GPER-specific agonist G-1 [70]. Genistein stimulated ghrelin secretion slightly, whereas G-1 inhibited ghrelin secretion slightly (Figure S5B). This discrepancy might be explained by the effect of genistein on cytosolic estrogen receptors.
The cannabinoid receptor 1 (Cnr1), which previously has been suggested to stimulate ghrelin secretion based on injections of cannabinoid receptor 1 agonists in rats [71] was expressed at low levels in ghrelin cells. That said, the cannabinoid receptor 1 agonist CP 55,940 did not affect ghrelin secretion from primary gastric mucosal cells (Figure S5B). One study demonstrated co-localization of the prostaglandin I2 receptor mRNA and ghrelin mRNA and decreased plasma ghrelin following administration of a stable prostaglandin I2 analog (beraprost) in rats [72]. Here, the prostaglandin I2 receptor (Ptgir) was expressed below noise level in ghrelin cells, and beraprost did not affect ghrelin release (Figure S5B).
3.11. A number of orphan 7TM receptors are enriched in the ghrelin cells
Orphan family A receptors account for a large fraction of those 7TM receptors enriched more than five-fold enriched, as ten of the 29 enriched receptors are orphan family A receptors (Figure 6, Table S3). In contrast, basically no orphan family A receptors are among the enriched receptors expressed in CCK and GLP-1 cells of the small intestine (data not shown). Among the most highly enriched orphan receptors in the ghrelin cells are GPR142 (530-fold), GPR27 (285-fold), GPR37 (240-fold), and GPR21 (200-fold). Of these, GPR27, GPR37 and GPR142 are also among the most highly expressed 7TM receptors in ghrelin cells (Figure 6).
Figure 6.
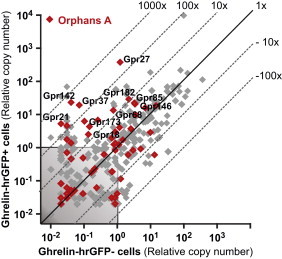
Expression of orphan family A 7TM receptors in ghrelin cells. qPCR data for 379 7TM receptors examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells. Orphan receptors from family A are marked with red. Receptors labeled with names are stably expressed and more than fivefold enriched in ghrelin cells.
3.12. Gα proteins expressed in gastric ghrelin cells
The expression of 17 Gα protein genes was determined in FACS-separated ghrelin cells. As expected, the ubiquitously expressed and widely distributed Gαs, Gαi, Gαq, Gα11, Gα12 and Gα13 [73] were all expressed in ghrelin cells at high levels, with Gαq being the most enriched (~20 fold) (Figure 7A, Table S4). Among members of the Gαi/o family that inhibit adenylyl cyclase, GαoA, GαoB, and GαZ, which in general are rather restricted in their cellular expression pattern [73], were highly enriched in ghrelin cells (77-fold to 270-fold) (Figure 7A, Table S4). Cone transducin (Gαt2) was enriched in ghrelin cells, which is in agreement with Janssen et al. [74]. Gustducin (Gαt3) was not detectable in ghrelin cells, but was expressed in the non-ghrelin gastric mucosal cells, which is inconsistent with results from Janssen et al. [74].
Figure 7.
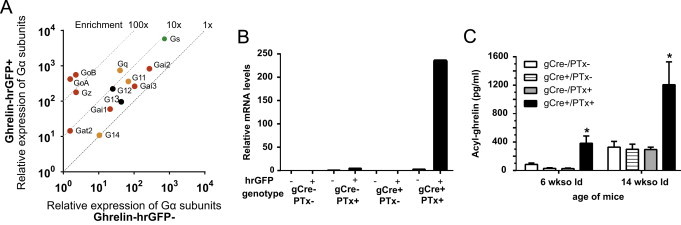
Expression of Gα subunits and pertussis toxin in ghrelin cells. (A) Relative mRNA expression of 13 Gα subunits examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells. Cyclophilin was used as a reference gene. Gα subunits with a mean CT value above 35 in either of the cell pools have been left out, but can be seen in Table S4. (B) Relative mRNA expression of the PTx catalytic S1 subunit within ghrelin cells (hrGFP+) and non-ghrelin cells (hrGFP−) in the four different genotypes (gCre−/PTx−, gCre+/PTx−, gCre−/PTx+, and gCre+/PTx+). (C) Levels of plasma acyl-ghrelin in 6-wk-old and 14-wk-old ad lib-fed male in the four different genotypes (gCre−/PTx−, gCre+/PTx−, gCre−/PTx+, and gCre+/PTx+). The data is shown as means±SEM.
Thus, three non-common Gαi/o proteins are highly expressed and enriched in ghrelin cells.
3.13. Elevated circulating ghrelin in mice with ghrelin cell-selective pertussis toxin expression
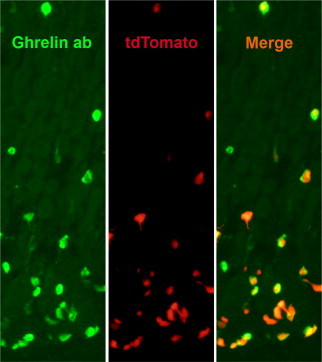
To confirm the important role for Gαi/o coupled signaling in ghrelin secretion, we generated a novel ghrelin-Cre mouse line in which Cre recombinase is expressed under the control of the ghrelin promoter (Figure S2). Crosses of this ghrelin-Cre line to Rosa26-lox-STOP-lox-tdTomato reporter mice in which tdTomato expression marks the location of Cre recombinase activity demonstrated a high degree of co-localization of Cre activity with ghrelin-immunoreactivity (Figure S6). Thus, nearly all (95.6±3.6%) cells with tdTomato fluorescence were also immunoreactive for ghrelin, and vice versa (97.3±0.9%). We also detected tdTomato fluorescence within a subset (69±6%) of the highly dispersed and less numerous population of ghrelin-immunoreactive cells present in the duodenum (data not shown). We failed to observe tdTomato expression in the adult brain or the adult or fetal pancreas of ghrelin/tdTomato mice (data not shown).
The ghrelin-Cre line was crossed to a mouse line which Cre-dependent expression of the PTx catalytic S1 subunit (ROSA26-lox-STOP-lox-PTx) [43]. Mice harboring both the PTx transgene and a rat insulin promoter transgene previously has been shown to have constitutive hyperinsulinemia and marked elevations in glucose-stimulated insulin secretion due to pancreatic β-cell-selective PTx expression [43]. The four different genotypes resulting from these crosses were studied, including mice with and without the ghrelin-Cre transgene and the ROSA26-lox-STOP-lox-PTx transgene. We confirmed the expected ghrelin cell-selective expression of the PTx catalytic S1 subunit within ghrelin cells by determining its relative expression in FACS-separated hrGFP-positive versus hrGFP-negative gastric mucosal cells derived from the above four genotypes when placed on a ghrelin-hrGFP background (Figure 7B). Although body weights of gCre+/PTx+ mice were indistinguishable from those of animals of the three control genotypes (data not shown), the plasma acyl-ghrelin was markedly elevated in 6-wk-old and 14-wk-old ad lib-fed male mice harboring both transgenes (gCre+/PTx+) (Figure 7C).
4. Discussion
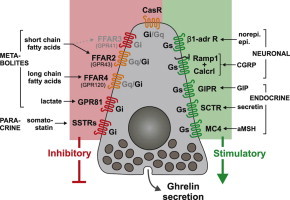
To understand the molecular basis for how ghrelin secretion is regulated at the cellular level, we identified the full repertoire of 7TM receptors and G proteins in gastric ghrelin cells and functionally characterized the majority of the receptors which were highly enriched, highly expressed and/or previously-implicated in the control of ghrelin secretion. Thus a comprehensive picture is presented of the G protein-coupled receptor signaling machinery controlling hormone secretion directly at the ghrelin cell (Figure 8).
Figure 8.
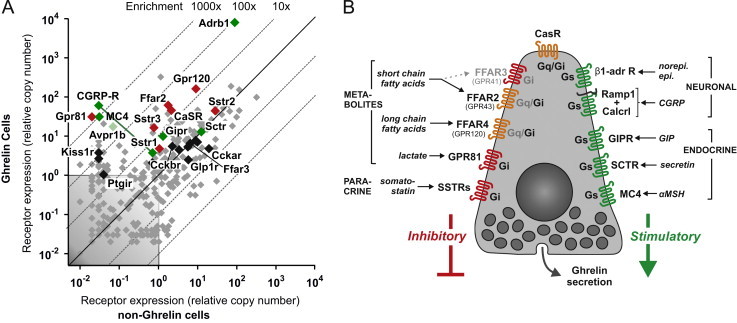
Overview of 7TM receptors involved in the control of ghrelin secretion directly at the ghrelin cell level. (A) Overview of expression of 7TM receptors involved in control of hormone secretion in FACS-separated ghrelin-hrGFP positive cells (Y-axis) versus expression in ghelin-hrGFP negative cells (X-axis) isolated from gastric mucosa (see Figure 1 and text for details). Receptors stimulating ghrelin secretion are marked in green, receptors inhibiting ghrelin release are marked in red, receptors functionally tested without effect on ghrelin secretion are marked in black and receptors not tested functionally are marked in gray. (B) Schematic overview of the 7TM receptors judged to be either stimulating (in green to the right) or inhibiting (red or orange to the left and top) ghrelin secretion directly on the ghrelin cell. The main signaling pathway (Gαs or Gαi) employed by each of the receptors in the ghrelin cell is indicated inside the receptor in black. No Gαq-coupled receptor effectively stimulating ghrelin secretion was identified but some receptors are known to be able to signal through Gαq and stimulate secretion in other cell systems (indicated in orange). Main endogenous ligands are indicated for each receptor.
4.1. The functional 7TM receptor repertoire of the ghrelin cell
Among the 24 7TM receptors which were functionally probed, we identified 11 likely to be of physiological importance for the regulation of ghrelin secretion and identified a number of others – including a few orphan receptors – as potentially important. The majority of the eleven receptors were not only highly expressed but also highly enriched in the gastric ghrelin cells (Figure 8A), and furthermore many were not anticipated to be involved in regulating ghrelin secretion at all or at the level of the ghrelin cell. Among the five receptors stimulating ghrelin secretion, two were receptors for neuronal messengers, including the dominating β1-adrenergic receptor and surprisingly, the hetero-dimeric receptor for the sensory neuropeptide CGRP, and three were receptors for peptide hormones: the receptors for the gut hormones GIP and secretin and surprisingly the MC4 receptor (Figure 8B). In addition to receptors for somatostatin, three of the receptors inhibiting ghrelin secretion were metabolite sensors including the SCFA receptor FFAR2, the LCFA receptor FFAR4 and surprisingly the lactate receptor GPR81 (Figure 8B). The receptors stimulating ghrelin secretion were all known to be Gαs-coupled while experiments with pertussis toxin demonstrated that all the inhibitory receptors on the ghrelin cell act through Gαi/o. This includes classical Gαq signaling receptors such as FFAR2 and FFAR4 which normally would be expected to stimulate hormone secretion. This shift towards Gαi/o coupling could be a consequence of the high ghrelin cell expression and enrichment of the three non-classical Gαi/o subunits, GαoA, GαoB or Gαz.
4.2. Neuronal stimulation of ghrelin secretion through β1 adrenergic and CGRP receptors
As reported previously, the increase in ghrelin secretion in response to an overnight fast is likely mediated by sympathetic nerve-derived norepinephrine acting on β1-adrenergic receptors expressed on the ghrelin cell [33,34]. In the present study we find that the Adbr1 by far is the most dominating 7TM receptor among all the 379 receptors, in respect of level of expression (Figure 8A). Interestingly, it is also the only small molecule neurotransmitter receptor enriched in the ghrelin cell.
Among the many neuropeptide receptors, only the receptor for the sensory neuropeptide CGRP is highly expressed and enriched in ghrelin cells (Figure 8A). In the heart, epinephrine and CGRP are co-expressed [75] and co-signal [76], although here, we did not detect any additive or synergistic effects of CGRP and isoproterenol on ghrelin release (data not shown). In the intact gastric mucosa, CGRP is found in sensory, spinal afferent neurons [77], which however also function as efferent neurons releasing CGRP to exert local tissue protective functions, e.g. by inhibition of gastric acid secretion [78]. We find that CGRP potently stimulates ghrelin release with an efficacy similar to isoproterenol, an effect which to our knowledge has not been described before. Of interest, exogenously administered ghrelin has been shown to protect against ischemia/reperfusion injury [79] and ethanol-induced gastric ulcers [80], in both cases through activation of gastric sensory neurons. Thus, CGRP-mediated stimulation of ghrelin release might be part of a gastro-protective, positive feedback loop. Another important CGRP-mediated gastro-protective mechanism is release of somatostatin [81]. Interestingly, although somatostatin can inhibit ghrelin release [20,26–28], the system is balanced in a way that the direct stimulatory effect of CGRP on ghrelin secretion prevails over the indirect inhibitory effect via somatostatin apparently to provide a gastro-protective effect of both peptides.
Based on reports in the literature on cholinergic, muscarinic stimulation of ghrelin secretion [35,37], it was surprising not to find any muscarinic receptor to be stably expressed and enriched in ghrelin cells and not to find any stimulatory effect of acetylcholine in the primary cultures of gastric mucosal cells. This indicates that the cholinergic, muscarinic effect on ghrelin secretion must be indirect.
4.3. Inhibition of ghrelin secretion by FFAR4 and LCFAs
Plasma levels of ghrelin are reduced by dietary triglycerides [10,82,83] and by intralipid infusion [84,85]. Moreover, LCFAs inhibit ghrelin secretion from FACS-purified ghrelin cells [66]. Here, we find that out of the three known receptors for triglyceride metabolites (FFAR1, FFAR4 and GPR119 [52]), only FFAR4 is expressed in gastric ghrelin cells, whereas all are highly expressed in another enteroendocrine cell – the L cell. Furthermore, a novel selective, synthetic non-lipid FFAR4 agonist suppresses ghrelin secretion in a FFAR4-dependent manner both ex vivo and in vivo. The higher fasting level of ghrelin observed in FFAR4 deficient mice suggests that FFAR4 conveys a baseline inhibitory tone on the ghrelin secretion.
Altogether, these observations might suggest that FFAR4 could be responsible for the decrease in plasma ghrelin after a lipid challenge. However, the drop in plasma ghrelin after oral dosing of olive oil was still observed in FFAR4 deficient mice (Figure 2F). In fact, the physiological significance of a postprandial inhibition of ghrelin secretion by LCFA acting directly on the ghrelin cell is questionable, as the plasma level of free fatty acids (FFA) in general does not increase but instead decreases after meal intake [86]. Moreover, gastric ghrelin cells are not anatomically designed to sense the content of the stomach directly as they are of the closed type of enteroendocrine cells (Figure 8B). Nevertheless, Lu and coworkers have suggested that LCFAs possibly could be sensed directly from the stomach lumen, as they observed lower ghrelin levels in pyloric ligated mice gavaged with olive oil, or that up-regulation of LCFA uptake proteins in the gastric mucosa after a meal creates a local increase in LCFAs [66].
4.4. Inhibition of ghrelin secretion by FFAR2 and SCFAs
SCFAs are mainly generated in the lumen of the colon from digestion of complex carbohydrates by the gut microbiota [87]. Depending on the amount and type of dietary fiber this can lead to an increase in peripheral blood SCFAs [88–90] and there are reports demonstrating that high fiber diets may reduce plasma ghrelin levels [90–92]. Interestingly, carbohydrate fermentation is not the only source of SCFAs as plasma acetate during alcohol consumption can reach levels up to 1 mM [93], and alcohol consumption is associated with decreased ghrelin levels [94].
Our data using novel selective ligands and tissue from receptor deficient mice indicate that the inhibitory effects of SCFAs such as propionate are mediated through FFAR2 and a Gαi/o signaling pathway. Lu and coworkers also found FFAR2 to be enriched in ghrelin cells [66], but did not observe any effect of SCFAs on ghrelin release. This could however be explained by the fact that they used butyrate and pentanoate, which have lower potency and efficacy on FFAR2 compared to acetate and propionate [95], and the relatively low doses of ligand they used.
4.5. Inhibition of ghrelin secretion by GPR81 and lactate
GPR81, which generally is not expressed in enteroendocrine cells (data not shown), was surprisingly found to be the most highly enriched 7TM receptor in the ghrelin cells and its ligand, lactate efficiently inhibited ghrelin release. Lactate is produced through bacterial fermentation of fiber in the colon [96,97], through oxidation of glucose and glutamine in the small intestine [98–100], and the majority of glucose taken up by adipocytes under the influence of insulin is converted to lactate [101]. The postprandial production and absorption of lactate into the portal vein is mirrored in the peripheral circulation reaching concentrations up to 1.9 mM [96,97], which should be enough to inhibit ghrelin secretion. However, much higher plasma concentrations of lactate are obtained during exercise (up to 25 mM) [57], and plasma ghrelin levels are suppressed during acute exercise [102–105]. It has been suggested that the decrease in ghrelin during exercise is related to decreased vagal activity and increased sympathetic tone [104], which however is not likely since sympathetic activity efficiently stimulates ghrelin secretion [12,33,34]. We would suggest instead that ghrelin levels decrease during exercise due to the increase in lactate acting directly through GPR81 on the ghrelin cells and that ghrelin is suppressed despite the exercise-associated increase in sympathetic signaling. Interestingly, chronic hypoxia which also is associated with high lactate production has been shown to markedly reduce plasma ghrelin levels in rats [106], as has short-term hypoxia in humans [107].
4.6. CaSR balancing between inhibition and stimulation of ghrelin secretion
The CaSR was originally characterized as a sensor of extracellular Ca2+ concentrations in the parathyroid gland and kidney [108]. However, CaSR is expressed also in enteroendocrine cells [61,62] and is activated by aromatic amino acids and could be considered to be part of the otherwise rather elusive protein sensing machinery [109]. Thus CaSR agonists including aromatic amino acids stimulate secretion of gastrin [61], CCK [62], and GLP-1 [63]. However, in the ghrelin cell, the role of CaSR is more complex, as we observed that R-568 can both inhibit and stimulate ghrelin secretion dependent upon the concentration of Ca2+. CaSR couples both to Gαq and Gαi and this potential for biased signaling has been described in a number of previous studies in other cell types [110–112]. Apparently CaSR couples through the stimulatory Gαq in other enteroendocrine cells but mainly through Gαi in ghrelin cells, similar to FFAR2 (Figure 8B).
4.7. Inhibition of ghrelin secretion by somatostatin receptors
It is not surprising that ghrelin secretion is inhibited by somatostatin [20,26–28]. But, in view of the fact that only a single neurotransmitter and neuropeptide receptor is highly expressed and enriched in the ghrelin cells, it is surprising that three of the five somatostatin receptor subtypes all are relatively highly expressed in the ghrelin cell (Figure 8A [11,66]). Selective agonists/antagonists are needed to determine the relative contribution of each receptor to the suppression of ghrelin release.
Somatostatin only reduced ghrelin secretion partly, as compared to for example lactate, even using a high concentration. This could indicate that ghrelin secretion is already suppressed via a tonic paracrine inhibition resulting from somatostatin secreted from nearby cells, as described previously [113]. The fact that ghrelin secretion increased after treatment with the broad spectrum somatostatin antagonist supports this notion. This observation also underlines the fact that we in general should be careful to exclude indirect effects in our primary cultures of gastric mucosal cells. Therefore we measured somatostatin release from the primary gastric mucosal cultures in parallel with measuring ghrelin. Importantly, neither the inhibitory effects of FFAR4, FFAR2 and GPR81 activation nor the stimulatory effects of CGRP, MC4, and GIP receptor activation on ghrelin release are caused by alterations in somatostatin secretion (data not shown).
4.8. Regulation of ghrelin secretion by peptide hormones
The two most highly enriched and highly expressed peptide hormone receptors were surprisingly the MC4 receptor and the vasopressin V1b receptor (Figure 8). Although activation of these receptors stimulated ghrelin release, their physiological relevance is still rather unclear. In particular, the maximal efficacy for the Gαq coupled V1b receptor was rather low. In contrast, the Gαs coupled MC4 receptor displayed a higher efficacy. Furthermore, because the relevant agonist could be a locally produced β-defensin, which is up-regulated during gastric infection [114] the MC4 receptor could be part of a gastro-protective mechanism, as discussed for CGRP above.
Among the receptors for gut hormones, only the GIP receptor was both highly expressed and enriched in the ghrelin cell, as shown previously [66]. GIP also acted as a potent and efficacious ghrelin secretagogue in the primary cultures of gastric mucosal cells as previously demonstrated in the perfused rat stomach [13]. This is in contrast to a previous in vivo study in which GIP inhibited ghrelin secretion indirectly possibly through modulation of the fatty acid pool [23]. However, we now know that GIP acts directly on the ghrelin cell to efficiently stimulate hormone secretion, a mechanism which needs to be taken into account in understanding both the physiology of both GIP and ghrelin. In the case of the secretin receptor the physiological relevance is more unclear as secretin is circulating in very low concentrations and although a stimulatory effect of secretin previously was reported it was only at rather high doses dose [28].
The peptide hormones PYY and pancreatic polypeptide (PP) acting through Y2 and Y4 receptors, respectively both efficiently decrease plasma ghrelin in vivo [25,115]. However, since neither of these receptors are expressed on gastric ghrelin cells, the inhibitory effects of PYY and PP must be indirect. Similarly, CCK1 receptor antagonist can block the inhibitory effect of intra-duodenal LCFA on ghrelin secretion [21]. Yet, although both of the CCK receptors are relatively highly expressed – but not enriched – in ghrelin cells, we did not observe any effect of CCK on ghrelin secretion, suggesting that the effects of CCK and receptor antagonists must be indirect.
Also of interest, although the glucagon receptor was not found at high or enriched levels in the FACS-separated ghrelin cells, previous studies using primary cultures of dispersed fetal rat stomach cells did demonstrate glucagon-stimulated ghrelin secretion, and a potentiation of this effect by norepinephrine [116]. As such, other 7TM receptors expressed at low levels in ghrelin cells may still play modulatory roles in the secretory machinery or there may be species differences or different expression patterns in fetal cells.
4.9. The ghrelin cell is enriched in Gαi/o coupling mechanisms
Hormone secretion from the ghrelin cell is apparently almost completely controlled by a balance between Gαs and Gαi signaling. Thus, all identified stimulatory receptors are Gαs-coupled and all identified inhibitory receptors act through PTx-sensitive Gαi/o-signaling mechanisms, indicating that cAMP is the major second messenger controlling ghrelin secretion (Figure 8B). Remarkably, no Gαq-coupled receptor was found to be effectively stimulating hormone secretion in the ghrelin cell. Receptors such as FFAR2, FFAR4, and the CaSR, which in other endocrine cells are known to couple mainly through Gαq and calcium mobilization [56,117], in the ghrelin cell inhibit hormone secretion through a Gαi/o mechanism (Figure 8B). As previously shown with β-cell expression and insulin secretion [43], specific expression of PTx in ghrelin cells resulted in a marked elevation of ghrelin plasma levels, underlining the importance of Gαi/o (Gαz is not targeted by PTx) as a strong regulatory mechanism for ghrelin secretion.
The lack of functional Gαq/Gα11 signaling and the dominating Gαi/o-coupling of receptors in the ghrelin cell is not due to lack of Gαq/Gα11 as these subunits are highly expressed in the ghrelin cells just like the common Gαi-subunits also are expressed normally in the ghrelin cells. Importantly however, three Gαi/o proteins, GαoA, GαoB, and Gαz, which usually are expressed mainly in neurons but also, for example in pancreatic islets [73,118], are surprisingly highly expressed and enriched in ghrelin cells (Figure 8). GαoB and Gαz have been implicated in inhibition of insulin release from pancreatic β-cells [119,120].
Thus, we conclude that the ghrelin cell is remarkably tuned for Gαi/o coupling and suggest that this is due to the high expression of the ‘non-common’ Gαi/o subunits GαoA, GαoB, and perhaps Gαz.
4.10. Orphan receptors enriched in ghrelin cells
Several orphan 7TM receptors are among the most highly expressed and enriched receptors in ghrelin cells. Among these, GPR142 and GPR27 are also highly expressed in pancreatic β-cells, where GPR142 acts as a receptor for novel synthetic glucose-dependent insulin secretagogues [121] and GPR27 functions as a positive regulator of insulin production [122]. Moreover, GPR37 was very recently “deorphanized” when the peptide prosaptide was shown to activate the receptor [123]. It remains to be determined if these orphan receptors mediate ghrelin release.
5. Conclusions and perspectives
A rather comprehensive picture is presented of the repertoire of 7TM receptors for neurotransmitters, neuropeptides, hormones, paracrine lipid messengers and in particular metabolites, and the corresponding Gα subunits that are expressed and functionally control ghrelin secretion directly at the level of the ghrelin cell (Figure 8B). This provides a new and significantly expanded basis for understanding the physiology of the cell producing the important orexigenic-glucoregulatory hormone ghrelin and thereby an expanded basis for selecting targets and developing novel therapeutic agents to control ghrelin secretion. The observation that certain receptors inhibit ghrelin secretion while stimulating secretion in other endocrine cell types and the differential expression of certain G protein α-subunits, which possibly could explain this difference in signaling, could open for a knowledge-based discovery process for signaling biased and tissue selective pharmaceuticals in general.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
We are grateful for the expert technical assistance from Susanne Hummelgaard. The Novo Nordisk Foundation Center for Basic Metabolic Research (http://www.metabol.ku.dk) is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen. The project was also supported by the UNIK project for Food, Fitness & Pharma (http://www.foodfitnesspharma.ku.dk) from the Danish Ministry of Science, Technology and Innovation. T.W.S and K.L.E were further supported by grants from the Lundbeck Foundation and from the Danish Medical Research Council. M.S.E was supported by a PhD scholarship from the Faculty of Health and Medical Sciences, University of Copenhagen. P.K.P. received funding from the Endocrine Fellows Foundation. A.K.W. was supported by NIH (T32DA7290). K.A. was supported by an EMBO long-term fellowship.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Jeffrey M. Zigman, Email: Jeffrey.Zigman@UTSouthwestern.edu.
Thue W. Schwartz, Email: TWS@sund.ku.dk.
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2013.08.006.
Appendix A. Supplementary materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Figure S1.
Ex vivo ghrelin secretion. Acyl-ghrelin release from primary gastric mucosal cells treated with acetylcholine, the M4 allosteric agonist vincamine, acetylcholine and vincamine (gray, hatched), dopamine, galanin or isoproterenol. The data has been normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is written in brackets in each bar.
Figure S2.
Propionate-induced cAMP accumulation. cAMP accumulation assays in HEK293 cells stably transfected with FFAR2 and treated with increasing concentrations of propionate alone (black) or after addition of 20 µM AR19 (gray).
Figure S3.
Expression and functional analysis of lipid 7TM receptors in ghrelin cells. (A) qPCR data for 379 7TM receptors examined in FACS-separated hrGFP-positive (Y-axis) and hrGFP-negative (X-axis) gastric mucosal cells from ghrelin-hrGFP mice. Lipid receptors are marked with green. (B) Acyl-ghrelin release from primary gastric mucosal cells treated with a stable prostaglandin I2 analog (PGI2, beraprost), a CB1 agonist (CP 55,940), the GPER agonists genistein and G-1 or isoproterenol. The data has been normalized to the basal secretion of ghrelin from vehicle-treated cells and shown as means±SEM. Number of repeated experiments is written in brackets in each bar.
Figure S4.
Ghrelin-immunoreactivity and tdTomato fluorescence. Ghrelin-immunoreactivity (green) and tdTomato fluorescence (red) within gastric mucosal cells. Co-localization (yellow) is visualized in the merged picture (right).
References
- 1.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Kumar J., Chuang J.C., Na E.S., Kuperman A., Gillman A.G., Mukherjee S. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite. 2013;64:81–88. doi: 10.1016/j.appet.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhulst P.J., Depoortere I. Ghrelin's second life: from appetite stimulator to glucose regulator. World Journal of Gastroenterology. 2012;18(25):3183–3195. doi: 10.3748/wjg.v18.i25.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchner H., Heppner K.M., Tschop M.H. The role of ghrelin in the control of energy balance. Handbook of Experimental Pharmacology. 2012;209:161–184. doi: 10.1007/978-3-642-24716-3_7. [DOI] [PubMed] [Google Scholar]
- 5.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 6.Cummings D.E., Purnell J.Q., Frayo R.S., Schmidova K., Wisse B.E., Weigle D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 7.Date Y., Kojima M., Hosoda H., Sawaguchi A., Mondal M.S., Suganuma T. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 8.Williams D.L., Cummings D.E., Grill H.J., Kaplan J.M. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144(7):2765–2767. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 9.Overduin J., Frayo R.S., Grill H.J., Kaplan J.M., Cummings D.E. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146(2):845–850. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 10.Foster-Schubert K.E., Overduin J., Prudom C.E., Liu J., Callahan H.S., Gaylinn B.D. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. Journal of Clinical Endocrinology and Metabolism. 2008;93(5):1971–1979. doi: 10.1210/jc.2007-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata I., Park W.M., Walker A.K., Piper P.K., Chuang J.C., Osborne-Lawrence S. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. American Journal of Physiology – Endocrinology and Metabolism. 2012;302(10):E1300–E1310. doi: 10.1152/ajpendo.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon J., Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology. 2012;153(8):3646–3656. doi: 10.1210/en.2012-1040. [DOI] [PubMed] [Google Scholar]
- 13.Lippl F., Kircher F., Erdmann J., Allescher H.D., Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regulatory Peptides. 2004;119(1–2):93–98. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Anderwald C., Brabant G., Bernroider E., Horn R., Brehm A., Waldhausl W. Insulin-dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes. 2003;52(7):1792–1798. doi: 10.2337/diabetes.52.7.1792. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan D.E., Evans M.L., Monsod T.P., Rife F., Heptulla R.A., Tamborlane W.V. The influence of insulin on circulating ghrelin. American Journal of Physiology – Endocrinology and Metabolism. 2003;284(2):E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 16.Leonetti F., Iacobellis G., Ribaudo M.C., Zappaterreno A., Tiberti C., Iannucci C.V. Acute insulin infusion decreases plasma ghrelin levels in uncomplicated obesity. Regulatory Peptides. 2004;122(3):179–183. doi: 10.1016/j.regpep.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Saad M.F., Bernaba B., Hwu C.M., Jinagouda S., Fahmi S., Kogosov E. Insulin regulates plasma ghrelin concentration. Journal of Clinical Endocrinology and Metabolism. 2002;87(8):3997–4000. doi: 10.1210/jcem.87.8.8879. [DOI] [PubMed] [Google Scholar]
- 18.McCowen K.C., Maykel J.A., Bistrian B.R., Ling P.R. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. Journal of Endocrinology. 2002;175(2) doi: 10.1677/joe.0.175r007. (R7-11) [DOI] [PubMed] [Google Scholar]
- 19.Caixas A., Bashore C., Nash W., Pi-Sunyer F., Laferrere B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. Journal of Clinical Endocrinology and Metabolism. 2002;87(4):1902. doi: 10.1210/jcem.87.4.8538. [DOI] [PubMed] [Google Scholar]
- 20.Schaller G., Schmidt A., Pleiner J., Woloszczuk W., Wolzt M., Luger A. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes. 2003;52(1):16–20. doi: 10.2337/diabetes.52.1.16. [DOI] [PubMed] [Google Scholar]
- 21.Degen L., Drewe J., Piccoli F., Grani K., Oesch S., Bunea R. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2007;292(4):R1391–R1399. doi: 10.1152/ajpregu.00734.2006. [DOI] [PubMed] [Google Scholar]
- 22.Brennan I.M., Otto B., Feltrin K.L., Meyer J.H., Horowitz M., Feinle-Bisset C. Intravenous CCK-8, but not GLP-1, suppresses ghrelin and stimulates PYY release in healthy men. Peptides. 2007;28(3):607–611. doi: 10.1016/j.peptides.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Rudovich N.N., Nikiforova V.J., Otto B., Pivovarova O., Gogebakan O., Erban A. Metabolomic linkage reveals functional interaction between glucose-dependent insulinotropic polypeptide and ghrelin in humans. American Journal of Physiology – Endocrinology and Metabolism. 2011;301(4):E608–E617. doi: 10.1152/ajpendo.00154.2011. [DOI] [PubMed] [Google Scholar]
- 24.Hagemann D., Holst J.J., Gethmann A., Banasch M., Schmidt W.E., Meier J.J. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regulatory Peptides. 2007;143(1–3):64–68. doi: 10.1016/j.regpep.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Batterham R.L., Cohen M.A., Ellis S.M., Le Roux C.W., Withers D.J., Frost G.S. Inhibition of food intake in obese subjects by peptide YY3-36. New England Journal of Medicine. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 26.Iwakura H., Ariyasu H., Hosoda H., Yamada G., Hosoda K., Nakao K. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology. 2011;152(7):2619–2625. doi: 10.1210/en.2010-1455. [DOI] [PubMed] [Google Scholar]
- 27.Shimada M., Date Y., Mondal M.S., Toshinai K., Shimbara T., Fukunaga K. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochemical and Biophysical Research Communications. 2003;302(3):520–525. doi: 10.1016/s0006-291x(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 28.de la Cour C.D., Norlen P., Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regulatory Peptides. 2007;143(1–3):118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Murakami N., Hayashida T., Kuroiwa T., Nakahara K., Ida T., Mondal M.S. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. Journal of Endocrinology. 2002;174(2):283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 30.Ito T., Thidarmyint H., Murata T., Inoue H., Neyra R.M., Kuwayama H. Effects of peripheral administration of PYY3-36 on feed intake and plasma acyl-ghrelin levels in pigs. Journal of Endocrinology. 2006;191(1):113–119. doi: 10.1677/joe.1.06855. [DOI] [PubMed] [Google Scholar]
- 31.Gomez G., Englander E.W., Greeley G.H., Jr. Nutrient inhibition of ghrelin secretion in the fasted rat. Regulatory Peptides. 2004;117(1):33–36. doi: 10.1016/j.regpep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Qader S.S., Salehi A., Hakanson R., Lundquist I., Ekelund M. Long-term infusion of nutrients (total parenteral nutrition) suppresses circulating ghrelin in food-deprived rats. Regulatory Peptides. 2005;131(1–3):82–88. doi: 10.1016/j.regpep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhao T.J., Sakata I., Li R.L., Liang G., Richardson J.A., Brown M.S. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundinger T.O., Cummings D.E., Taborsky G.J., Jr. Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147(6):2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- 35.Broglio F., Gottero C., Van Koetsveld P., Prodam F., Destefanis S., Benso A. Acetylcholine regulates ghrelin secretion in humans. Journal of Clinical Endocrinology and Metabolism. 2004;89(5):2429–2433. doi: 10.1210/jc.2003-031517. [DOI] [PubMed] [Google Scholar]
- 36.Ao Y., Go V.L., Toy N., Li T., Wang Y., Song M.K. Brainstem thyrotropin-releasing hormone regulates food intake through vagal-dependent cholinergic stimulation of ghrelin secretion. Endocrinology. 2006;147(12):6004–6010. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- 37.Hosoda H., Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regulatory Peptides. 2008;146(1–3):12–18. doi: 10.1016/j.regpep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Williams D.L., Grill H.J., Cummings D.E., Kaplan J.M. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144(12):5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 39.Engelstoft M.S., Egerod K.L., Holst B., Schwartz T.W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metabolism. 2008;8(6):447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Tolhurst G., Reimann F., Gribble F.M. Intestinal sensing of nutrients. Handbook of Experimental Pharmacology. (209) 20122012:309–335. 309–335. doi: 10.1007/978-3-642-24716-3_14. [DOI] [PubMed] [Google Scholar]
- 41.Sakata I., Nakano Y., Osborne-Lawrence S., Rovinsky S.A., Lee C.E., Perello M. Characterization of a novel ghrelin cell reporter mouse. Regulatory Peptides. 2009;155(1–3):91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nohr M.K., Pedersen M.H., Gille A., Egerod K.L., Engelstoft M.S., Husted A.S. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 43.Regard J.B., Kataoka H., Cano D.A., Camerer E., Yin L., Zheng Y.W. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. Journal of Clinical Investigation. 2007;117(12):4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi, D.F., Song, J., Ma, J., Novack, A., Pham, P., Nashashibi, I., Rabbat, C.J., and Chen, X., 2010. GPR120 receptor agonists and uses thereof. Metabolex, Inc. WO 2010/080537.
- 45.Boatman, P.D., Bruce, M.A., Chu, Z.L., and Leonard, J.N., 2006. GPR41 and modulators thereof for the treatment of insulin-related disorders. PCT International Application. p. WO 2006052566.
- 46.Hald A., Rono B., Lund L.R., Egerod K.L. LPS counter regulates RNA expression of extracellular proteases and their inhibitors in murine macrophages. Mediators of Inflammation. 2012;2012:157894. doi: 10.1155/2012/157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nohr M.K., Secher A., Sakata I. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kostenis E. Potentiation of GPCR-signaling via membrane targeting of G-protein α subunits. Journal of Receptors and Signal Transduction. 2002;22:267–281. doi: 10.1081/rrs-120014601. [DOI] [PubMed] [Google Scholar]
- 49.Gliddon B.L., Nguyen N.V., Gunn P.A., Gleeson P.A., van Driel I.R. Isolation, culture and adenoviral transduction of parietal cells from mouse gastric mucosa. Biomedical Materials. 2008;3(3):034117. doi: 10.1088/1748-6041/3/3/034117. [DOI] [PubMed] [Google Scholar]
- 50.Yanovsky Y., Zigman J.M., Kernder A., Bein A., Sakata I., Osborne-Lawrence S. Proton- and ammonium-sensing by histaminergic neurons controlling wakefulness. Frontiers in Systems Neuroscience. 2012;6:23. doi: 10.3389/fnsys.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar A., Goel A., Paul S. Re: Urodynamic Testing – is it a useful tool in the management of children with cutaneous stigmata of occult spinal dysraphism?: L. T. Lavallee, M. P. Leonard, C. Dubois and L. A. Guerra. Journal of Urology. 2013;189:678–683. doi: 10.1016/j.juro.2012.08.203. ([190(2): p. 812–3]) [DOI] [PubMed] [Google Scholar]
- 52.Garg M., Singh V., Kumar A., Re Osman. Clinically insignificant residual fragments: an acceptable term in the computed tomography era? Urology. 2013;82(2):496–497. doi: 10.1016/j.urology.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 53.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briscoe C.P., Peat A.J., McKeown S.C., Corbett D.F., Goetz A.S., Littleton T.R. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. British Journal of Pharmacology. 2006;148(5):619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee T., Schwandner R., Swaminath G., Weiszmann J., Cardozo M., Greenberg J. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Molecular Pharmacology. 2008;74(6):1599–1609. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- 56.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodwin M.L., Harris J.E., Hernandez A., Gladden L.B. Blood lactate measurements and analysis during exercise: a guide for clinicians. Journal of Diabetes Science and Technology. 2007;1(4):558–569. doi: 10.1177/193229680700100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dvorak C.A., Liu C., Shelton J., Kuei C., Sutton S.W., Lovenberg T.W. Identification of hydroxybenzoic acids as selective lactate receptor (GPR81) agonists with antilipolytic effects. ACS Medicinal Chemistry Letters. 2012;3(8):637–639. doi: 10.1021/ml3000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conigrave A.D., Quinn S.J., Brown E.M. l-amino acid sensing by the extracellular Ca2+-sensing receptor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemeth E.F., Steffey M.E., Fox J. The parathyroid calcium receptor: a novel therapeutic target for treating hyperparathyroidism. Pediatric Nephrology. 1996;10(3):275–279. doi: 10.1007/BF00866757. [DOI] [PubMed] [Google Scholar]
- 61.Feng J., Petersen C.D., Coy D.H., Jiang J.K., Thomas C.J., Pollak M.R. Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17791–17796. doi: 10.1073/pnas.1009078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liou A.P., Sei Y., Zhao X., Feng J., Lu X., Thomas C. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to l-phenylalanine in acutely isolated intestinal I cells. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2011;300(4):G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mace O.J., Schindler M., Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. Journal of Physiology. 2012;590(Pt 12):2917–2936. doi: 10.1113/jphysiol.2011.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grieco P., Han G., Weinberg D., Van der Ploeg L.H., Hruby V.J. Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochemical and Biophysical Research Communications. 2002;292(4):1075–1080. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 65.Moller C.L., Raun K., Jacobsen M.L., Pedersen T.A., Holst B., Conde-Frieboes K.W. Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Molecular and Cellular Endocrinology. 2011;341(1-2):9–17. doi: 10.1016/j.mce.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Lu X., Zhao X., Feng J., Liou A.P., Anthony S., Pechhold S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2012;303(3):G367–G376. doi: 10.1152/ajpgi.00541.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tulipano G., Soldi D., Bagnasco M., Culler M.D., Taylor J.E., Cocchi D. Characterization of new selective somatostatin receptor subtype-2 (sst2) antagonists, BIM-23627 and BIM-23454. Effects of BIM-23627 on GH release in anesthetized male rats after short-term high-dose dexamethasone treatment. Endocrinology. 2002;143(4):1218–1224. doi: 10.1210/endo.143.4.8716. [DOI] [PubMed] [Google Scholar]
- 68.Ignatov A., Lintzel J., Kreienkamp H.J., Schaller H.C. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochemical and Biophysical Research Communications. 2003;311(2):329–336. doi: 10.1016/j.bbrc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Maggiolini M., Vivacqua A., Fasanella G., Recchia A.G., Sisci D., Pezzi V. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. Journal of Biological Chemistry. 2004;279(26):27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 70.Bologa C.G., Revankar C.M., Young S.M., Edwards B.S., Arterburn J.B., Kiselyov A.S. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chemical Biology. 2006;2(4):207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 71.Zbucki R.L., Sawicki B., Hryniewicz A., Winnicka M.M. Cannabinoids enhance gastric X/A-like cells activity. Folia Histochemica et Cytobiologica. 2008;46(2):219–224. doi: 10.2478/v10042-008-0033-4. [DOI] [PubMed] [Google Scholar]
- 72.Madison L.D., Scarlett J.M., Levasseur P., Zhu X., Newcomb K., Batra A. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. Journal of Endocrinology. 2008;196(2):263–273. doi: 10.1677/JOE-07-0478. [DOI] [PubMed] [Google Scholar]
- 73.Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiological Reviews. 2005;85(4):1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 74.Janssen S., Laermans J., Verhulst P.J., Thijs T., Tack J., Depoortere I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(5):2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang M.H., Nguyen V., Wu Y., Rastogi S., Lui C.Y., Birnbaum Y. Reducing ischaemia/reperfusion injury through delta-opioid-regulated intrinsic cardiac adrenergic cells: adrenopeptidergic co-signalling. Cardiovascular Research. 2009;84(3):452–460. doi: 10.1093/cvr/cvp233. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen V.T., Wu Y., Guillory A.N., McConnell B.K., Fujise K., Huang M.H. Delta-opioid augments cardiac contraction through beta-adrenergic and CGRP-receptor co-signaling. Peptides. 2012;33(1):77–82. doi: 10.1016/j.peptides.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Green T., Dockray G.J. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neuroscience Letters. 1987;76(2):151–156. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 78.Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Current Opinion in Pharmacology. 2007;7(6):563–569. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konturek P.C., Brzozowski T., Walter B., Burnat G., Hess T., Hahn E.G. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology. 2006;536(1–2):171–181. doi: 10.1016/j.ejphar.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 80.Sibilia V., Rindi G., Pagani F., Rapetti D., Locatelli V., Torsello A. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144(1):353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 81.Zaki M., Coudron P.E., McCuen R.W., Harrington L., Chu S., Schubert M.L. H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2013;304(8):G715–G722. doi: 10.1152/ajpgi.00187.2012. [DOI] [PubMed] [Google Scholar]
- 82.Erdmann J., Lippl F., Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regulatory Peptides. 2003;116(1–3):101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 83.Tannous dit El Khoury D., Obeid O., Azar S.T., Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Annals of Nutrition and Metabolism. 2006;50(3):260–269. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 84.Gormsen L.C., Gjedsted J., Gjedde S., Vestergaard E.T., Christiansen J.S., Jorgensen J.O. Free fatty acids decrease circulating ghrelin concentrations in humans. European Journal of Endocrinology. 2006;154(5):667–673. doi: 10.1530/eje.1.02146. [DOI] [PubMed] [Google Scholar]
- 85.Gormsen L.C., Nielsen C., Gjedsted J., Gjedde S., Vestergaard E.T., Christiansen J.S. Effects of free fatty acids, growth hormone and growth hormone receptor blockade on serum ghrelin levels in humans. Clinical Endocrinology (Oxford) 2007;66(5):641–645. doi: 10.1111/j.1365-2265.2007.02786.x. [DOI] [PubMed] [Google Scholar]
- 86.Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sommer F., Backhed F. The gut microbiota – masters of host development and physiology. Nature Reviews Microbiology. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 88.Muir J.G., Lu Z.X., Young G.P., Cameron-Smith D., Collier G.R., O'Dea K. Resistant starch in the diet increases breath hydrogen and serum acetate in human subjects. American Journal of Clinical Nutrition. 1995;61(4):792–799. doi: 10.1093/ajcn/61.4.792. [DOI] [PubMed] [Google Scholar]
- 89.Peters S.G., Pomare E.W., Fisher C.A. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut. 1992;33(9):1249–1252. doi: 10.1136/gut.33.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tarini J., Wolever T.M. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition, and Metabolism. 2010;35(1):9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 91.Vitaglione P., Lumaga R.B., Stanzione A., Scalfi L., Fogliano V. beta-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite. 2009;53(3):338–344. doi: 10.1016/j.appet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Gruendel S., Garcia A.L., Otto B., Mueller C., Steiniger J., Weickert M.O. Carob pulp preparation rich in insoluble dietary fiber and polyphenols enhances lipid oxidation and lowers postprandial acylated ghrelin in humans. Journal of Nutrition. 2006;136(6):1533–1538. doi: 10.1093/jn/136.6.1533. [DOI] [PubMed] [Google Scholar]
- 93.Lundquist F., Tygstrup N., Winkler K., Mellemgaard K., Munck-Petersen S. Ethanol metabolism and production of free acetate in the human liver. Journal of Clinical Investigation. 1962;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szulc M., Mikolajczak P.L., Geppert B., Wachowiak R., Dyr W., Bobkiewicz-Kozlowska T. Ethanol affects acylated and total ghrelin levels in peripheral blood of alcohol-dependent rats. Addiction Biology. 2013;(6):689–701. doi: 10.1111/adb.12025. [DOI] [PubMed] [Google Scholar]
- 95.Nilsson N.E., Kotarsky K., Owman C., Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochemical and Biophysical Research Communications. 2003;303(4):1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 96.Theil P.K., Jorgensen H., Serena A., Hendrickson J., Bach Knudsen K.E. Products deriving from microbial fermentation are linked to insulinaemic response in pigs fed breads prepared from whole-wheat grain and wheat and rye ingredients. British Journal of Nutrition. 2011;105(3):373–383. doi: 10.1017/S0007114510003715. [DOI] [PubMed] [Google Scholar]
- 97.Bach Knudsen K.E., Jorgensen H., Canibe N. Quantification of the absorption of nutrients derived from carbohydrate assimilation: model experiment with catheterised pigs fed on wheat- or oat-based rolls. British Journal of Nutrition. 2000;84(4):449–458. [PubMed] [Google Scholar]
- 98.Windmueller H.G., Spaeth A.E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. Journal of Biological Chemistry. 1980;255(1):107–112. [PubMed] [Google Scholar]
- 99.Vaugelade P., Posho L., Darcy-Vrillon B., Bernard F., Morel M.T., Duee P.H. Intestinal oxygen uptake and glucose metabolism during nutrient absorption in the pig. Proceedings of the Society for Experimental Biology and Medicine. 1994;207(3):309–316. doi: 10.3181/00379727-207-43821. [DOI] [PubMed] [Google Scholar]
- 100.Fleming S.E., Zambell K.L., Fitch M.D. Glucose and glutamine provide similar proportions of energy to mucosal cells of rat small intestine. American Journal of Physiology. 1997;273(4 Pt 1):G968–G978. doi: 10.1152/ajpgi.1997.273.4.G968. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed K., Tunaru S., Tang C., Muller M., Gille A., Sassmann A. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metabolism. 2010;11(4):311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Broom D.R., Stensel D.J., Bishop N.C., Burns S.F., Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of Applied Physiology. 2007;102(6):2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- 103.Broom D.R., Batterham R.L., King J.A., Stensel D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2009;296(1):R29–R35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- 104.Shiiya T., Ueno H., Toshinai K., Kawagoe T., Naito S., Tobina T. Significant lowering of plasma ghrelin but not des-acyl ghrelin in response to acute exercise in men. Endocrine Journal. 2011;58(5):335–342. doi: 10.1507/endocrj.k11e-021. [DOI] [PubMed] [Google Scholar]
- 105.Balaguera-Cortes L., Wallman K.E., Fairchild T.J., Guelfi K.J. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Applied Physiology, Nutrition, and Metabolism. 2011;36(6):958–966. doi: 10.1139/h11-121. [DOI] [PubMed] [Google Scholar]
- 106.Chaiban J.T., Bitar F.F., Azar S.T. Effect of chronic hypoxia on leptin, insulin, adiponectin, and ghrelin. Metabolism. 2008;57(8):1019–1022. doi: 10.1016/j.metabol.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Wasse L.K., Sunderland C., King J.A., Batterham R.L., Stensel D.J. Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. Journal of Applied Physiology. 2012;112(4):552–559. doi: 10.1152/japplphysiol.00090.2011. [DOI] [PubMed] [Google Scholar]
- 108.Brown E.M., Pollak M., Hebert S.C. Sensing of extracellular Ca2+ by parathyroid and kidney cells: cloning and characterization of an extracellular Ca(2+)-sensing receptor. American Journal of Kidney Diseases. 1995;25(3):506–513. doi: 10.1016/0272-6386(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 109.Reimann F., Tolhurst G., Gribble F.M. G-protein-coupled receptors in intestinal chemosensation. Cell Metabolism. 2012;15(4):421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 110.Davey A.E., Leach K., Valant C., Conigrave A.D., Sexton P.M., Christopoulos A. Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor. Endocrinology. 2012;153(3):1232–1241. doi: 10.1210/en.2011-1426. [DOI] [PubMed] [Google Scholar]
- 111.Rey O., Young S.H., Yuan J., Slice L., Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. Journal of Biological Chemistry. 2005;280(24):22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 112.Makita N., Sato J., Manaka K., Shoji Y., Oishi A., Hashimoto M. An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5443–5448. doi: 10.1073/pnas.0701290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saffouri B., Weir G., Bitar K., Makhlouf G. Stimulation of gastrin secretion from the perfused rat stomach by somatostatin antiserum. Life Science. 1979;25(20):1749–1753. doi: 10.1016/0024-3205(79)90478-8. [DOI] [PubMed] [Google Scholar]
- 114.Bauer B., Wex T., Kuester D., Meyer T., Malfertheiner P. Differential expression of human beta defensin 2 and 3 in gastric mucosa of Helicobacter pylori-infected individuals. Helicobacter. 2013;18(1):6–12. doi: 10.1111/hel.12000. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi H., Kurose Y., Suzuki Y., Kojima M., Yamaguchi T., Yoshida Y. Changes in blood pancreatic polypeptide and ghrelin concentrations in response to feeding in sheep. Journal of Animal Science. 2010;88(6):2103–2107. doi: 10.2527/jas.2009-1920. [DOI] [PubMed] [Google Scholar]
- 116.Gagnon J., Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154(2):666–674. doi: 10.1210/en.2012-1994. [DOI] [PubMed] [Google Scholar]
- 117.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 118.Zigman J.M., Westermark G.T., LaMendola J., Steiner D.F. Expression of cone transducin, Gz alpha, and other G-protein alpha-subunit messenger ribonucleic acids in pancreatic islets. Endocrinology. 1994;135(1):31–37. doi: 10.1210/endo.135.1.8013366. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y., Park S., Bajpayee N.S., Nagaoka Y., Boulay G., Birnbaumer L. Augmented glucose-induced insulin release in mice lacking G(o2), but not G(o1) or G(i) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1693–1698. doi: 10.1073/pnas.1018903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kimple M.E., Joseph J.W., Bailey C.L., Fueger P.T., Hendry I.A., Newgard C.B. Galphaz negatively regulates insulin secretion and glucose clearance. Journal of Biological Chemistry. 2008;283(8):4560–4567. doi: 10.1074/jbc.M706481200. [DOI] [PubMed] [Google Scholar]
- 121.Lizarzaburu M., Turcotte S., Du X., Duquette J., Fu A., Houze J. Discovery and optimization of a novel series of GPR142 agonists for the treatment of type 2 diabetes mellitus. Bioorganic & Medicinal Chemistry Letters. 2012;22(18):5942–5947. doi: 10.1016/j.bmcl.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 122.Ku G.M., Pappalardo Z., Luo C.C., German M.S., McManus M.T. An siRNA screen in pancreatic beta cells reveals a role for Gpr27 in insulin production. PLoS Genetics. 2012;8(1):e1002449. doi: 10.1371/journal.pgen.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meyer R.C., Giddens M.M., Schaefer S.A., Hall R.A. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9529–9534. doi: 10.1073/pnas.1219004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material


