Abstract
The zebrafish has become an important in vivo model in biomedical research. Effective methods must be developed and utilized to deliver compounds or agents in solutions for scientific research. Current methods for administering compounds orally to adult zebrafish are inaccurate due to variability in voluntary consumption by the fish. A gavage procedure was developed to deliver precise quantities of infectious agents to zebrafish for study in biomedical research. Adult zebrafish over 6 months of age were anesthetized with 150 mg/L of buffered MS-222 and gavaged with 5 μl of solution using flexible catheter implantation tubing attached to a cut 22-G needle tip. The flexible tubing was lowered into the oral cavity of the zebrafish until the tip of the tubing extended past the gills (approximately 1 cm). The solution was then injected slowly into the intestinal tract. This method was effective 88% of the time, with fish recovering uneventfully. This procedure is also efficient as one person can gavage 20-30 fish in one hour. This method can be used to precisely administer agents for infectious diseases studies, or studies of other compounds in adult zebrafish.
Keywords: Basic Protocols, Issue 78, Developmental Biology, Anatomy, Physiology, Molecular Biology, Biomedical Engineering, Intestines, animal biology, animal models, zebrafish, gavage, Danio rerio, medaka, animal model
Introduction
Current methods for administering compounds orally to zebrafish include administering compounds top-coated onto feed, mixed into gelatin diets1, bioencapsulated into brine shrimp2, mixed with lipid encapsulated diets3, wax spray beads4 and via gluten-based diets.5 Limitations of these methods of oral administration include high leaching rates and incomplete or unpredictable compound consumption by individual fish. These variables are problematic because in infectious disease studies, knowing the infective dose administered to the fish may be critical for study success. Also, previous work has shown that certain compounds administered in water baths cause toxic lesion to zebrafish gills before the intestinal effects that may be under study occur.6
Gavage is a standard method used in other laboratory animal species to administer precise quantities of a product with a known concentration for study in biomedical and pharmaceutical research. Only recently have methods for gavaging zebrafish been described in the literature. One technique described is a two-person method using a 24-G catheter sheath attached to a 2-20 μl pipette to deliver 5 μl of solution.7 The method described had a mortality rate of 8.7% and 39% on the first and second trial, respectively; most of the mortality was attributed to gravid females. The second gavage technique described used blunt-tipped gavage syringes to administer 5 μl of solution to medakas (Oryzias latipes).8 Information on mortality was not provided and the exact process of performing the gavage was not described. According to the Canadian Council on Animal Care and the Laboratory Zebrafish, up to 1% of the fish's body weight can be administered via gavage.9,10 Our goal was to develop a repeatable, safe and efficient method of delivering precise volumes of compounds orally to adult zebrafish of both sexes and in all stages of reproduction. This procedure would be applicable for any study in which accurate oral dosing of a compound is required.
Protocol
1. Preparing the Anesthetic Solution
Prepare a solution of 150 mg/L of MS-222 in system water either from a stock solution or from the powdered form.
Verify the pH using a pH meter.
Buffer with sodium bicarbonate as needed until the pH is between 7-7.5.
Prepare a tank of system water free from MS-222 for the fish to recover in. The recovery time is typically less than 1 min.
2. Preparing the Gavage Apparatus
Cut the clear 22-G implantation tubing to a little more than approximate length from the mouth to the intestinal bulb of the fish. Initially, we suggest anesthetizing one fish for each new group being used to get the best estimate of the length needed.
Push the cut clear implantation tubing onto the ½-inch 22-G needle until it is secure and there is no risk of detaching. If a ½ 22-C needle is not available, cut a longer needle to approximately that length.
When complete, the catheter implantation tubing should extend about 1 cm from the tip of the needle, or shorter if the fish are smaller.
Attach the 22-G needle hub to a 1-cc luerlok syringe.
3. Preparing for Gavage
Cut a groove into a sponge with a scalpel blade.
Soak the sponge in system water free from MS-222.
Place the moistened sponge on a flat surface.
Draw up the appropriate 5 μl solution for gavage into the 22-G catheter tubing and a 1-cc syringe.
4. Anesthesia and Gavage
Fast the fish for at least 24 hr before the procedure.
Place the fish in the MS-222 solution until it loses its righting reflex, and does not respond to a tail fin pinch, but maintains opercular movement. This typically takes about 2-3 min.
Remove the fish from the anesthetic solution and place it into the groove in the sponge with the head slightly protruding from the sponge, but the gills covered by the sponge.
Move the sponge into a vertical position.
Open the zebrafish's mouth using the 22-G catheter tubing.
Gently insert the tubing until the tip is past the gills (approximately 1 cm or the length of the tubing). The implantation tubing should not need to be forced. Resistance suggests the tube may be hitting the gill arch or heart.
If there is resistance, gently withdraw, reposition and try again.
Inject the material slowly.
While injecting, make sure that the solution does not exit via the gills or the mouth.
Remove the fish from the sponge and place into the recovery tank.
Recovery typically occurs in less than 1 min and is indicated by the fish swimming upright and maintaining equilibrium.
Monitor fish for regurgitation as shown by visualizing the fish actively expelling material from its mouth, or no opercular movement.
Fish can be returned to their regular tank once they have recovered.
Representative Results
Both sexes of fish, including gravid females were successfully gavaged (Figure 1). A successful procedure takes less than one minute with no liquid seen exiting the gills or the mouth. The tubing enters easily, without force, with no blood seen upon catheter removal. The procedure is rapid, requiring approximately 10 min to gavage 3 to 4 fish, with an average of 30-45 sec per fish.
Zebrafish should not be gavaged with more than 5 μl of any solution. Gavage with 10 μl solutions resulted in only a 50% success rate (Figure 2). Gavage of 10 μl was also completely unsuccessful in gravid females. In this study, the adult fish weighed an average of 0.2 g each. Using the CCAC's recommendation, this would mean that 4 μl of solution could be administered to each fish. The current use of 5 μl would be consistent with these recommendations. This technique had approximately a 10% mortality rate mostly attributable to gravid females which were found dead the next day. While this mortality rate is significantly lower than other reported gavage techniques, ideally, grossly gravid females should not be gavaged unless it is necessary for study purposes.
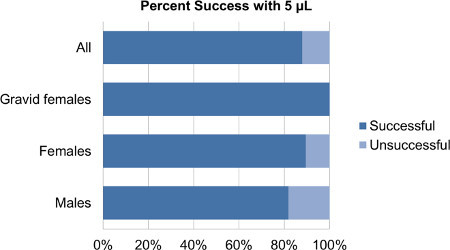 Figure 1. Comparison of percent success between different reproductive groups using 5 μl of solution. The percent success of all groups combined was 88%. The percent success for gravid females was 100%; for non-gravid females 90% and for males 82%.
Figure 1. Comparison of percent success between different reproductive groups using 5 μl of solution. The percent success of all groups combined was 88%. The percent success for gravid females was 100%; for non-gravid females 90% and for males 82%.
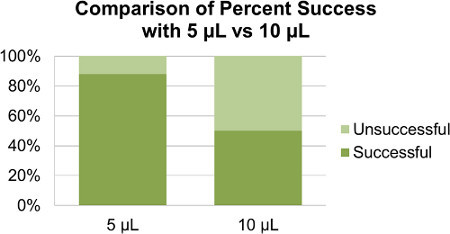 Figure 2. Comparison of overall percent success using 5 μl versus 10 μl. The total percent success using 5 μl was 88%. The total percent success using 10 μl was 50%.
Figure 2. Comparison of overall percent success using 5 μl versus 10 μl. The total percent success using 5 μl was 88%. The total percent success using 10 μl was 50%.
Discussion
Significance
This technique is an improvement and provides several advantages over the two previously described techniques for gavaging adult zebrafish. First, the use of a sponge to restrain the fish allows one person to perform the procedure, as opposed to a previously described technique requiring two people.7 Second, catheter implantation tubing is more flexible than IV catheters, which can help minimize secondary trauma due to perforation similarly to using blunt-tipped syringes.7,8 Third, by using a clear tubing, the solution can easily be visualized entering the fish. And finally, the gavage apparatus is stable allowing it to be reused quickly and efficiently when many fish need to be gavaged. The gavage technique in general reduces the variation in consumption and concentration seen in other methods of oral compound administration to zebrafish, and as such may also reduce the amount of fish needed to perform an experiment.1-5
Critical Steps
It is also important to fast the fish for at least 24 hr since with a 12 hr fast not all zebrafish have an empty intestinal bulb. The 24 hr fast will prevent regurgitation and loss of the solution through the mouth and gills due to a partially full intestinal tract13. The most common problem is liquid exiting through the mouth or the gills, indicating that the tubing was not inserted far enough down the intestinal tract. Liquid exiting via the mouth or gills can be avoided by observing the correct placement of the tubing as well as using a tubing of sufficient length to bypass the gills. More severe complications may include gill trauma, internal hemorrhage, intestinal perforation, regurgitation and sudden death. These complications may or may not be observed immediately. Typically, sudden death occurs hours after the procedure is completed, while the fish looked normal during initial recovery.
The sponges were soaked in bleach and then in sodium thiosulfate solution prior to coming into contact with the fish. This removed any agents or chemicals that may have been harmful to the fish. The MS-222 stock solution should be stored in a dark container, away from light and for up to 5 days in the refrigerator. If a brownish tint is observed the solution should be discarded, as the efficacy is reduced.11,12
Limitations
This protocol does require anesthesia of the zebrafish to ensure success. This may be disadvantageous in certain circumstances where the anesthetic agent may have an adverse effect on the research outcomes being measured.12 Currently, there is no gavage protocol for zebrafish that does not require anesthesia.7,8 If MS-222 anesthesia is known to affect measured variables, other anesthetics may be used, or one of the previously mentioned methods of voluntary consumption methods can be utilized.1-5
This technique, as with previously described techniques is challenging when performed on gravid females.7 A possible cause of high mortality in this group may be intestinal perforation leading to sepsis due to the intestinal tract being displaced by the eggs in the coelom. Similarly, if the eggs as well as intestine are perforated, then an acute egg-associated coelomitis may also occur. Such events may be minimized by spawning the gravid females before gavaging. Alternatively, a smaller gauge catheter may be used to perform the procedure.
If zebrafish of a smaller size are used care should be taken to adjust the size of the tubing and the needle so that the diameter and length will fit into their mouth and intestinal tract.
The efficiency of the procedure can be further increased if a second individual were to monitor anesthesia and ensure a constant supply of anesthetized fish to the individual performing the gavage. This technique is relatively easy to master and does not require extensive practice to gain proficiency. Additionally, the procedure does not require specialized equipment or facilities.
Trouble-shooting
If the zebrafish do not become anesthetized within 2-3 min, change the anesthetic solution or use a freshly prepared stock solution. If the tubing does not slide easily down the intestinal tract of the zebrafish, smaller diameter tubing should be used. It may be beneficial to practice using a small number of similarly sized fish in order to ensure the correct diameter and length of the tubing as well as to evaluate whether the volume administered is appropriate for the zebrafish's size. The tubing may also be marked to note the ideal penetration depth for the size of fish being utilized.
Conclusion
Once this technique is mastered, it can be used to deliver a precise amount of various compounds or infectious agents to adult zebrafish efficiently and consistently, making it useful for various research protocols. This technique may also minimize the number of fish needed to perform experiments, and allow precise time measurements of the effects of various compounds on the fish.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The Rockefeller University Comparative Bioscience Center provided support for this project. We thank Janelle Monnas for technical assistance.
References
- Royes J-AB, Chapman F. Preparing Your Own Fish Feeds. Department of Fisheries and Aquatic Sciences, Florida Cooperative Extension Service, Insitute of Food and Agricultural Sciences, University of Florida; 2009. [Google Scholar]
- Gomez-Gil B, Cabanillas-Ramos J, Paez-Brambila A, Roque A. Standardization of the bioencapsulation of enrofloxacin and oxytetracycline in Artemia fransciscana Kellogg. Aquaculture. 1906;196:1–12. [Google Scholar]
- Langdon C. Microparticle types for delivering nutrients to marine fish larvae. Aquaculture. 2003;227:259–275. [Google Scholar]
- Langdon C, Nordgreen A, Hawkyard M, Hamre K. Evaluation of wax spray beads for delivery of low-molecular weight, water soluble nutrients and antibiotics to Artemia. Aquaculture. 2008;284:151–158. [Google Scholar]
- Zang L, Morikane D, Shimada Y, Tanaka T, Nishimura N. A Novel Protocol for the Oral Administration of Test Chemicals to Adult Zebrafish. Zebrafish. 2011;8:203–210. doi: 10.1089/zeb.2011.0726. [DOI] [PubMed] [Google Scholar]
- Goldsmith JR, Jobin C. Think Small: Zebrafish as a Model System of Human pathology. Journal of Biomedicine and Biotechnology. 2012. [DOI] [PMC free article] [PubMed]
- Tysnes KR, Jorgensen A, Poppe T, Midtlyng PJ, Robertson LJ. Preliminary expermients on use of zebrafish as a laboratory model for Giardia duodenalis infection. Acta Parasitologica. 2012;57:1–6. doi: 10.2478/s11686-012-0001-1. [DOI] [PubMed] [Google Scholar]
- Marie B, Huet H, et al. Effects of a toxic cyanobacterial bloom (Planktothris agardhii) on fish: Insights from histopathological and quantitative proteomic assessments following the oral exposure of medaka fish (Oryzias latipes) Aquatic Toxicology. 2012;114-115:39–48. doi: 10.1016/j.aquatox.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care. CCAC guidelines on: the care and use of fish in research, teaching and testing. Ottawa, Ontario, Canada: CCAC; 2005. [Google Scholar]
- Harper C, Lawrence C. The Laboratory Zebrafish. Boca Raton, Florida, USA: CRC Press; 2011. [Google Scholar]
- De Tolla LJ, Srinivas S, et al. Guidelines for the Care and Use of Fish in Research. ILAR Journal. 1995;37(4) doi: 10.1093/ilar.37.4.159. [DOI] [PubMed] [Google Scholar]
- Topic Popovic N, Strunjak-Perovic I, et al. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. Journal of Applied Ichthyology. 2012;28:553–564. [Google Scholar]
- Field HA, Kelley KA, Martell L, Goldstein AM, Serluca FC. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterology & Motility. 2009;21:304–312. doi: 10.1111/j.1365-2982.2008.01234.x. [DOI] [PubMed] [Google Scholar]


