Abstract
Scope
We previously demonstrated that lifelong feeding of diets enriched in n-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) significantly inhibits HER-2/neu-mediated mammary tumorigenesis in mice. Of interest is whether dietary n-3 fatty acids exert effects at early stages of mammary carcinogenesis.
Methods and results
Female 7 week old MMTV-HER-2/neu transgenic mice were randomized to AIN-based semipurified diets containing either fish or corn oil at 25% energy. Mice were evaluated at 25, 30 and 35 weeks with analysis of mammary glands for atypical ductal hyperplasia (hematoxylin and eosin), cell proliferation (Ki67 immunostaining), and fatty acid synthase and cyclooxygenase-2 gene expression (qRT-PCR). Tissue fatty acid profiles were quantitated by gas chromatography.
Atypia grade decreased significantly in mice fed fish oil (P=0.002). Mammary epithelial cells in mammary glands from mice fed fish oil also had an 8 fold lower percentage of Ki67 expression. COX-2 expression in mammary fat pads significantly decreased in mice fed fish versus corn oil enriched diets.
Conclusions
Dietary fish oil inhibits atypical ductal hyperplasia at early stages of HER-2/neu-mediated mammary carcinogenesis relative to corn oil diets. This histologic change is associated with suppression of mammary epithelial cell proliferation and decreased COX-2 expression in mammary tissue.
Keywords: n-3 PUFAs, HER-2/neu, breast cancer
Introduction
The human epidermal growth factor receptor 2 (HER-2/neu), also known as ErbB2, is a 185 kD transmembrane receptor tyrosine kinase that is involved in human mammary oncogenesis, amplified and overexpressed in 15–40% of invasive breast cancers [1]. HER-2/neu overexpression in breast cancer correlates with poor clinical outcome, as measured by disease recurrence, progression, and survival [2, 3]. Aberrant expression of HER-2/neu has also been observed in 50–60% of carcinoma in situ of the breast, at greater prevalence than in invasive disease, and thus may have a role in the earliest stages of mammary carcinogenesis [4–6].
In transgenic mice overexpressing the HER-2/neu protooncogene under the control of the mouse mammary tumor virus (MMTV) promoter, mammary epithelial-specific expression of this protooncogene leads to the induction of focal mammary tumors at 4 months with a median incidence of 205 days [7]. Mammary epithelium progresses through hyperplasia, atypical hyperplasia and carcinoma in situ to invasive tumors with a long latency and asynchronous appearance, mirroring features in human breast cancer. The histologic features of human lobular atypical hyperplasia and carcinoma resemble those arising in these HER-2/neu transgenic mice [8]. The tumors overexpress HER-2/neu and are negative for estrogen receptor (ER) and progesterone receptor (PR) expression. Transgenic mice carrying the unactivated HER-2/neu proto-oncogene under MMTV control thus represent a well-characterized model of mammary carcinogenesis that allows us to investigate the effects of putative preventive or therapeutic interventions on the development of pre-invasive disease.
Using this HER-2/neu transgenic model, we previously demonstrated the suppressive effects of fish oil versus corn oil enriched diets when fed over a lifetime on HER-2/neu mammary tumorigenesis, with increased tumor latency and decreased tumor multiplicity [9]. Mammary glands were also notable for increased atypia in corn oil fed mice. As HER-2/neu signaling appears critical during early mammary carcinogenesis, we conducted the current study to define the impact of n-3 and n-6 PUFAs on the development of pre-invasive disease and biomarkers of progression, prior to the appearance of clinically overt tumors. This effort may provide insight into strategies for women with premalignant conditions and/or other factors predisposing to breast cancer.
In this study, female MMTV-HER-2/neu transgenic mice were randomized to diets enriched with n-3 or n-6 PUFAs at 7 weeks of age and examined at 25, 30, or 35 weeks of age to investigate the effects of dietary fat content on pre-invasive stages of HER-2/neu-mediated mammary carcinogenesis.
Materials and methods
Mouse Experimental Procedure
Animal care and use were in accord with institution guidelines and approved by the Institutional Animal Care and Use Committee of The Ohio State University (OSU protocol 2001A0123). Virgin female FVB/N-TgN(MMTVneu)202Mul transgenic mice [7] were obtained from Jackson Laboratory (Bar Harbor, ME) and housed in groups of up to 5 in plastic shoebox cages with autoclaved bedding and filtered air, with 12 hours of darkness daily and an ambient air temperature of 22 ± 2°C. Mice had free access to diet and water. Mice were individually tagged and randomly distributed into treatment groups. Diets were replaced daily to limit oxidation of fatty acid species, with daily monitoring of food consumption. The health of the mice was monitored daily, and mice were weighed weekly. Animals were euthanized at 25 (n=61), 30 (n=72) or 35 (n=67) weeks of age (total n=200). Mammary glands were fixed in 10% buffered formalin for paraffin-embedding for hematoxylin and eosin staining and histopathologic evaluation, with a portion also snap-frozen in liquid nitrogen and stored at −80°C.
H&E-stained sections of mammary glands, without knowledge of diet groups, were evaluated for mammary gland proliferative lesions based on the consensus report from the 1999 Annapolis meeting on mammary gland pathology of genetically engineered mice [10]. Mammary gland atypical ductal hyperplasia was graded on a scale of normal, mild, moderate and severe based on the percentage of the mammary gland affected, thickness of the proliferating ductular epithelium, and cellular atypia (high nuclear to cytoplasmic ratio, cytoplasmic basophilia, cytomegaly, karyomegaly, and cellular and nuclear pleomorphism). Heterogeneity in glands was characterized as focal or multifocal features of the next higher grade, ranging from 0 (normal) to 12 (severe). Eight mammary glands were assessed per mouse.
Diets
AIN-93G-based diets[11] were prepared by Research Diets, Inc. (New Brunswick, NJ) with either 24.75 kcal% corn oil or 22.50 kcal% menhaden (fish) oil with 2.25 kcal% corn oil. 25 kcal% fat was selected for a diet of moderate fat content without significant imbalance in carbohydrate and protein content relative to chow diets, with corn oil as a source of n-6 PUFAs and fish oil as a source of n-3 PUFAs [9]. Diets were designed to contain 21.4% protein, 57.8% carbohydrate, and 11.6% fat by weight with 0.023 gram/kg t-butylhydroquinone (TBHQ) for stabilization of the oils. Per 950 g, the menhaden oil diets contained 9.5 g (2.1 kcal%) linoleic acid (LA), 14.2 g (32 kcal%) eicosapentaenoic acid (EPA), and 10.3 g (23.2 kcal%) docosahexaenoic acid (DHA). The corn oil diets contained 66.1 g LA/950 g (14.9 kcal%). Fish oil diets prepared for this study contained 0.0042% t-butylhydroquinone (TBHQ), as a deviation from the formulation of 0.0023% to match the corn oil diets (personal communication, Michael Pellizon, Research Diets). Diets were handled under low light conditions and stored at −20°C.
Fatty acid analysis
The fatty acid composition of mammary fat pad samples was determined by a two-step procedure as described previously [12]. Total lipids (both neutral and phospholipids) were analyzed. Total n-3:n-6 fatty acid ratios were calculated as the sum of (18:3n3, 20:4n3, 20:5n3, 22:5n3, 22:6n3)/sum of (18:2n6, 18:3n6, 20:2n6, 20:3n6, 20:4n6, 22:2n6, 22:4n6).
Immunohistochemical analyses
4 micron thick tissue sections of mammary glands (fourth or eighth/inguinal glands) were immunostained using primary antibody to Ki67 (Mib-1) as previously described [13].
Gene expression analyses
Portions of mammary tissue from the thoracic and inguinal glands were flash frozen in liquid nitrogen at the time of necropsy and stored at −80°C until analysis. Expressions of mRNA levels were analyzed by quantitative real-time PCR (Prism 7300 sequence detection system, Applied Biosystems, Foster City, CA). Total RNA was isolated by use of an RNeasy Lipid Tissue kit (Qiagen, Valencia, CA) and reversed transcribed with random hexamers using MultiScribe reverse transcriptase (Applied Biosystems). After cDNA synthesis, real-time PCR analysis was performed with predesigned primes and probes supplied by Applied Biosystems (TaqMan Gene Expression Assays) for samples in triplicate. Target gene expression was expressed as 2 –ΔΔCT by the comparative CT method and normalized to the expression of 18S ribosomal RNA, then to the corn oil group as the control.
Statistical analysis
Data for body weight and fatty acid composition are presented as mean values ± standard deviations (SD), and differences between fatty acid means were analyzed using the nonparametric Wilcoxon rank sum test. For these comparisons, Holm’s method of p-value adjustment was used to control the overall Type I error rate. For the analysis of mammary gland atypia, we fit a linear mixed effects model to assess the effects of diet on tumor grade. The model included the age of the mouse, initial weight, batch, and gland as covariates; there were no significant two-way interactions with diet. Relevant estimates from the model were calculated with 95% confidence intervals (CI). Similarly, Ki67 expression in mammary duct cells was assessed using a mixed effects logistic regression model to allow for dependencies among glands from the same mouse. Differences in gene expression between the fish oil and corn oil diets by qRT-PCR were assessed using linear mixed effects models on the log-transformed fold change data, with mouse age considered as a covariate. From the models, average differences in expression for each target between the two diets were estimated, with 95% CI.
Results
Dietary treatment of HER-2/neu transgenic mice
The average daily food intake was 2.1 ± 0.6 grams of study diet per mouse calculated from diet remaining/number of mice per cage, without significant difference between the diet groups by Student’s t test (p=0.72). There were no differences in initial body weights between mice in the two diet groups (p=0.071). Overall the mice gained slightly more weight when fed fish oil vs. corn oil diets by the end of the treatment period (p=<0.001). Terminal body weights for the combined age groups of 25, 30 and 35 weeks differed by 7%, with average weights 26.7 ± 3.0 and 24.9 ± 3.0 in the fish and corn oil diet groups, respectively. The weight difference was noted as early as 9 weeks following the initiation of study diets with attenuation at 35 weeks (Figure 1). The body weight differences related at least in part to greater abdominal and mammary fat pad adiposity in fish oil fed versus corn oil fed mice (data not shown). Diet-associated toxicities were not observed.
Figure 1.
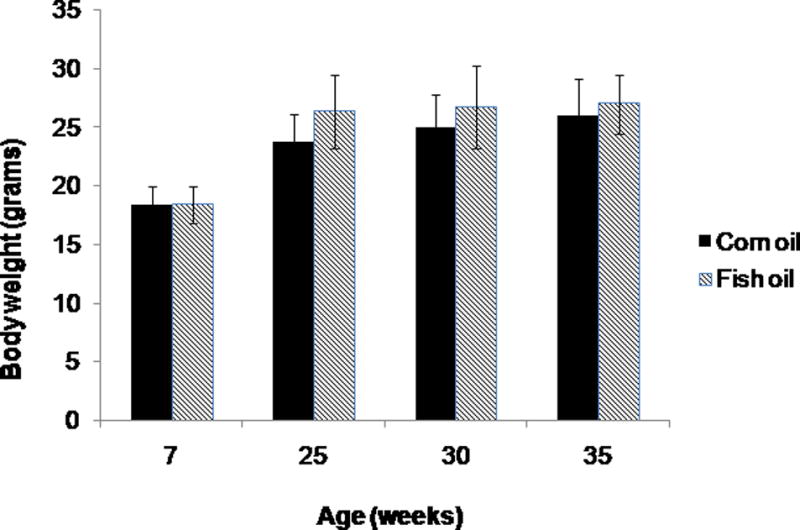
Body weights of HER-2/neu mice in corn and fish oil diet groups differed significantly at 25 weeks (p=0.0004) and 30 weeks (p=0.03) of age but not at 7 or 35 weeks. Error bars indicate standard deviation.
Fatty acid analysis of mammary fat pads showed significantly increased DHA and EPA content in mice fed fish oil (n=23) compared to the corn oil enriched diet (n= 24), with increased total n-3 fatty acid composition (p<0.001, Table 1). Mammary fat pad content of DHA and EPA was 17 and 33 fold higher, respectively, in fish oil compared to corn oil fed mice.
Table 1.
Fatty acid composition of mammary fat pads for corn oil and fish oil diet group
| Fatty acid | Corn Oil (n=24) |
Fish Oil (n=23) |
|
|---|---|---|---|
| C14:0 | myristic acid | 1.59 ± 0.39 | 6.05 ± 0.70 |
| C16:0 | palmitic acid | 17.07 ±1.73 | 26.6 ± 1.89 |
| C16:1n7 | palmitoleic acid | 6.05 ± 0.75 | 17.82 ± 1.93 |
| C16:3n4 | hexadecatrienoic acid | 0.14 ± 0.02 | 0.78 ± 0.08 |
| C18:0 | stearic acid | 1.51 ± 0.64 | 2.23 ± 0.47 |
| C18:1n9 | oleic acid | 32.77 ± 0.92 | 26.83 ± 2.09 |
| C18:1n7 | vaccenic acid | 1.22 ± 0.10 | 3.85 ± 0.27 |
| C18:2n6 | linoleic acid | 38.17 ± 2.7 | 11.96 ± 1.49 |
| C18:3n6 | gamma linolenic acid | 0.14 ± 0.04 | 0.24 ± 0.03 |
| C18:3n3 | alpha linolenic acid | 0.27 ± 0.03 | 0.59 ± 0.16 |
| c9t11 CLA | conjugated linoleic acid | 0.07 ± 0.01 | 0.15 ± 0.11 |
| C20:0 | arachidic acid | 0.11 ± 0.03 | 0.1 ± 0.02 |
| C20:1n9 | cetoleic acid | 0.38 ± 0.08 | 0.62 ± 0.1 |
| C20:2n6 | eicosadienoic acid | 0.07 ± 0.01 | 0.09 ± 0.02 |
| C20:3n6 | dihomo-gamma-linolenic acid | 0.04 ± 0.01 | 0.08 ± 0.02 |
| C20:4n6 | arachidonic acid | 0.14 ± 0.09 | 0.18 ± 0.07 |
| C20:4n3 | eicosatetraenoic acid | 0.08 ± 0.03 | 0.26 ± 0.09 |
| C20:5n3 | eicosapentaenoic acid | 0.02 ± 0.02 | 0.70 ± 0.40 |
| C22:4n6 | adrenic acid | 0.01 ± 0.01 | 0.02 ± 0.01 |
| C22:5n3 | docosapentaenoic acid | 0.13 ± 0.05 | 0.29 ± 0.11 |
| C22:6n3 | docosahexaenoic acid | 0.03 ± 0.03 | 0.57 ± 0.35 |
| Total Saturated Fatty Acids (sum of %) | 20.26 ± 2.67 | 34.98 ± 2.41 | |
| Total Monounsaturated Fatty Acids (sum of %) | 40.42 ± 1.05 | 49.12 ± 2.11 | |
| Omega 3 Fatty Acids (sum of %) | 0.54 ± 0.11 | 2.40 ± 0.87 | |
| Omega 6 Fatty Acids (sum of %) | 38.7 ± 2.61 | 12.85 ± 1.55 | |
| n-3/n-6 ratio | 0.014 ± 0.00 | 0.19 ± 0.07 | |
Fatty acids are expressed as percentage of total fatty acids in mammary fat pad tissue (mean ± standard deviation). Statistical significance was determined by the Wilcoxon rank sum test, with an adjusted p<0.001 for all corn oil versus fish oil diet group comparisons (using Holm’s method) except arachidonic acid (p=0.005), conjugated linoleic acid (p=0.0.007), and arachidic and adrenic acids (p=0.431 for both).
Dietary fish oil inhibited HER-2/neu-mediated mammary gland atypia
The treatment periods of 25, 30 and 35 weeks of age were selected as time points during the phase of pre-invasive mammary gland histopathology. Overall, 7% (14 of 200) of the mice developed mammary tumors during the treatment period, with 4.1% (4/97) in the fish oil group and 9.7% (10/103) in the corn oil group. 13 of the 14 tumors were detected at necropsy at 25 weeks (n=1), 30 weeks (n=6) and 35 weeks (n=6). Only one tumor had been detected during routine monitoring at 27 weeks, which was in a mouse randomized to the 30 week corn oil diet treatment.
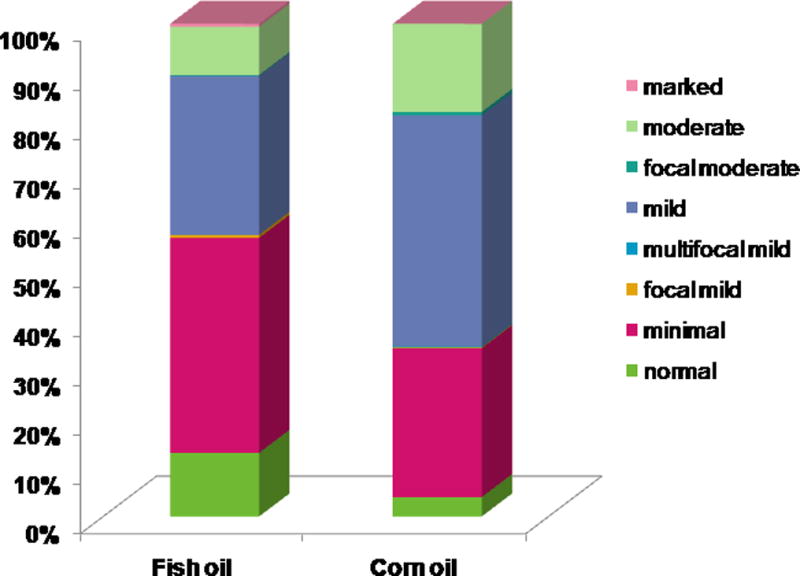
Hematoxylin and eosin (H&E) stained tissue sections of mammary glands were scored for atypical ductal hyperplasia ranging from 0 (normal) to 12 (severe). As shown in Figure 2, mammary glands in the fish and corn oil fed groups had an average grade of 4.23 ± 0.66 (minimal with focal mild atypia) versus 5.39 ± 0.66 (minimal with multiple foci of mild atypia), respectively, with an overall estimated difference of 1.0 (95% CI: −1.6, −0.3; p=0.007). There were no significant differences between two replicate experiments (p=0.446), age groups of 25, 30, 35 weeks (overall p=0.345) or location of the gland on the left or right side (p=0.723).
Figure 2.
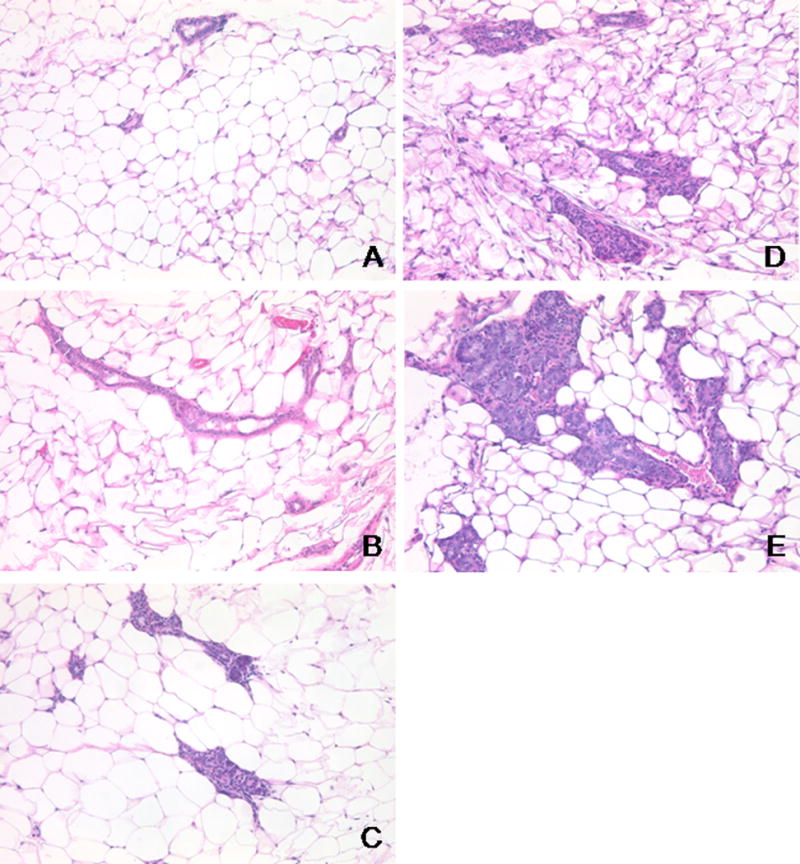
A. Photomicrographs of representative H&E stained MMTV-HER-2/neu mammary glands for different atypia grades of normal (A), minimal (B), mild (C), moderate (D) and marked (E), taken at 40× magnification.
B. A greater proportion of the fish oil fed mice (n=65) had glands with lower atypia scores compared to mammary glands from the corn oil diet group (n=70). Eight mammary glands per mouse at 25 (n=40), 30 (n=50) and 35 (n=45) weeks of age were assessed for grade by H&E histology.
In addition, there was a significant decreasing linear trend in mammary gland atypia in a cephalad to caudad direction, with breast tissue in the cervical location notable for the highest grade of atypia (test for trend p-value < 0.001). The incidence of mammary tumors or microscopic nodules did not follow this cephalad to caudad progression of atypical ductal hyperplasia. Also, mice with greater initial body weight tended to have slightly lower atypia grades of approximately −0.2 (95% CI: −0.4, −0.1; p=0.010).
Dietary fish oil inhibits mammary epithelial cell proliferation but not HER-2/neu expression
Ki67 protein is expressed by proliferating cells and serves as a biomarker of tumor cell replication and progression and also response to therapy [14–16]. To test whether dietary fish oil altered cell proliferation in MMTV-HER-2/neu mammary glands relative to corn oil based diets, we performed Ki67 immunostaining of formalin fixed mammary glands and assessed epithelial cell staining (Figure 3). Mammary glands from mice fed fish oil diets had a lower percentage of Ki67 positive cells per duct relative to mammary glands from the corn oil diet group with the odds of having Ki67 positive cells per duct area 8 times higher for mice fed corn oil compared to fish oil (odds ratio (OR) = 7.97; 95% CI: 2.54, 24.96; p=0.004). By immunostaining HER-2/neu expression did not differ significantly by intensity or localization of staining between the diet groups (data not shown).
Figure 3.
Ki-67 expression in mammary glands of HER-2/neu mice. 4 micron thick sections of mammary glands from HER-2/neu mice fed corn or fish oil diets at 25 (n=6), 30 (n=8) and 35 (n=7) weeks of age were immunostained for Ki67 and analyzed by quantitative image analysis. The percent of Ki67 expression was calculated as the ratio of Ki67+ duct cell to total duct cell area (n=10 corn oil fed mice with assessment of 138 ducts and n=11 fish oil fed mice with 120 observations).
HER-2/neu mediated gene expression in mammary tissue
Downstream targets for HER-2/neu mediated signaling include fatty acid synthase (FASN) and cyclooxygenase 2 (COX-2) [17, 18]. Dietary PUFAs and/or HER-2/neu have been associated with modulation of adipoctyokine expression (adiponectin and leptin) as well as peroxisome proliferator receptor gamma (PPARγ) [19–21]. For exploratory analyses of potential dietary PUFA effects on HER-2/neu signal transduction, total RNA isolated from mammary tissue samples from both diet groups was analyzed by qRT-PCR techniques. COX-2 expression in mammary fat pad tissue was significantly higher in mice fed corn oil relative to fish oil enriched diets without significant alteration of the other selected markers (Table 2).
Table 2.
Gene expression in mammary fat pads
| Genes | Fold change (Fish Oil vs. Corn Oil) | 95% CI | p-value |
|---|---|---|---|
| Adiponectin | 1.06 | (0.64, 1.4) | 0.762 |
| COX-2 | 0.49 | (1.09, 3.83) | 0.029 |
| FASN | 1.33 | (0.4, 1.43) | 0.353 |
| Leptin | 1.95 | (0.26, 1.03) | 0.058 |
| PPAR | 1.49 | (0.35, 1.29) | 0.205 |
Gene expression in mammary tissue samples from HER-2/neu mice fed corn and fish oil diets by qRT-PCR for adiponectin, cyclooxygenase 2 (COX-2), fatty acid synthase (FASN), leptin, and peroxisome proliferator activated receptor gamma (PPARγ). Triplicate samples of mammary fat pad tissue from 65 mice fed corn oil (n=34) and fish oil (n=31 diets) at 25 (n=33), 30 (n=24) and 35 (n=8) weeks of age were evaluated for COX-2, FASN, leptin, adiopnectin and PPARγ by qRT-PCR. As gene fold changes were almost identical after age adjustment, the table presents univariable estimates without age adjustment.
Discussion
The study data indicate that the diets enriched with n-3 relative to n-6 PUFAs inhibited early stages of HER-2/neu-mediated mammary carcinogenesis, with decreased development of mammary gland atypia as a histopathologic precursor to invasive cancer. We previously demonstrated a 30% reduction in the incidence of invasive mammary cancers in MMTV-HER-2/neu mice fed diets enriched in n-3 vs. n-6 PUFA lipids for their lifetime, along with decreased tumor multiplicity and mammary gland histopathology [9]. As we consider future human intervention trials of bioactive nutrients, women with premalignant conditions such as atypical ductal hyperplasia or carcinoma in situ may preferentially benefit from such preventive strategies. This murine study was designed to provide preclinical data on efficacy and biomarker expression during early stages of HER-2/neu(+), ERPR(−) mammary carcinogenesis prior to onset of invasive malignancy so as to guide our efforts to develop dietary strategies of breast cancer prevention for high risk women.
Atypia has emerged as a histological biomarker of higher breast cancer risk in women and similarly as a predictive marker for carcinogenesis in the murine model. Dietary n-3 PUFAs appear to exert effects on early events of HER-2/neu mammary carcinogenesis as the impact on atypia scores was present at each study time point of 25, 30 and 35 weeks of age. The grade of atypia did not differ significantly with increasing age over this 10 week window, consistent with the development of focal tumors sporadically over a long latent period in the MMTV-HER-2/neu mouse model utilized for the study [7]. Indeed, atypical hyperplasia in women represents a marker of increased risk of developing breast cancer over an extended time frame and not necessarily at a specific site [22]. Only fourteen mice progressed to mammary cancer during the treatment period as detected by monitoring and histopathologic evaluation at necropsy, yet more than two thirds of the tumor-bearing mice (10/14) were found in the corn oil-fed group. This result reinforces our previous demonstration of significantly increased tumor latency in mice fed menhaden oil versus corn oil enriched diets over an extended period to the point of palpable tumors or 15 months of age [9]. Thus, the results of the present study suggest that the very earliest steps in mammary carcinogenesis are sensitive to dietary lipid patterns.
Breast cancer is a heterogeneous disease of many subtypes, each of which may require specific strategies for prevention. Derangement of HER-2/neu signal transduction by epigenetic and genetic damage defines one particularly aggressive form of human breast cancer. The decrease in atypical hyperplasia and cellular proliferation in MMTV-HER-2/neu mammary glands under conditions of increased n-3 relative to n-6 PUFA content suggests a particular sensitivity to dietary fatty acids. This effect did not directly influence expression of the MMTV-driven HER-2/neu gene in mammary glands based on diet or age by immunohistochemistry. The impact may be indirect, with exploratory qRT-PCR analyses showing significantly decreased COX-2 expression in mammary fat pad tissue from mice fed fish oil, a known downstream target of an activated HER-2/neu signaling pathway [18, 23, 24]. FASN is another gene under HER-2/neu regulation[17], but we did not see a significant effect of dietary PUFAs on FASN expression by qRT-PCR analyses under conditions of this study. FASN is expressed by both adipocytes and epithelial cells such that differential effects on gene expression in these cell types would be detectable only by methods using immunohistochemistry or microdissection for analysis of separate tissue components. In view of prior studies linking COX-2 expression to breast cancer risk and disease progression [25, 26], and suppression of MMTV-HER-2/neu mammary tumor development by the selective COX-2 inhibitor celecoxib [27, 28], our results suggest that the preventive effects of dietary n-3 PUFAs may involve modulation of COX-2 and bioactive lipid metabolism at early stages of mammary carcinogenesis. Indeed, a small pilot trial of n-3 fatty acid supplementation in prostate cancer patients (n=9) who underwent pre- and post-treatment tumor biopsies demonstrated decreased COX-2 expression in 4 of 7 evaluable subjects following the study intervention [29]. The predominance of adipose tissue in the mammary fat pad suggests a likely effect on COX-2 expression in this stromal compartment, and future investigations will evaluate the potential for differential inhibition of adipocyte/stromal and epithelial COX-2 expression. Our current study suggests that COX-2 warrants further exploration as a relevant biomarker in human studies of dietary fatty acid patterns that modulate mammary tissue response during early stages of carcinogenesis, with implications for women with premalignant conditions such as atypical ductal or lobular hyperplasia in which COX-2 overexpression can occur [26].
Mice in our study were carefully monitored for energy intake and growth. Although isocaloric diets were prepared, the MMTV-HER-2/neu mice fed fish oil were slightly heavier by about 2 grams compared to those eating corn oil based diets. Food consumption estimated by food disappearance from the feeding apparatus was similar for the two groups, but we suspect that dispersal and wastage of food by mice might have masked slight differences such that a difference in palatability led to slightly greater intake of the fish oil diets. Given the role of energy intake in promoting mammary cancer [30], the inhibitory effects of fish oil enriched diets might have been even more dramatic if food intake was controlled to exacting levels. Interestingly, Cleary et al showed that diet induced obesity did not affect tumor latency, incidence, metastasis and burden in the FVB/N MMTV-HER-2/neu transgenic mouse model, suggesting that the development of hormone receptor negative breast cancer was not susceptible to this risk factor [31]. Thus, we conclude that the slightly greater growth of the fish oil fed mice either reduced the benefits of dietary n-3 PUFAs or had little effect.
Our experience also indicates the need for critical monitoring of diets prepared by commercial suppliers. The fish oil diets contained TBHQ at 0.0042% instead of 0.0023% due to manufacturer error that was discovered only after the study concluded. TBHQ is an additive to prevent oxidation of PUFAs during diet preparation, shipping, storage and feeding [11]. We doubt that this difference affected the study outcomes as this amount of TBHQ was several orders of magnitude lower than doses used in rodent studies evaluating the chemopreventive and carcinogenic properties of TBHQ and its metabolites which typically ranged from 0.4 to 1% [32, 33]. Gonzalez et al reported attenuation of the suppressive effects of fish oil on mammary tumorigenesis by supplementation of fish oil diets with 0.4% TBHQ and vitamin E [34]. Furthermore, our study findings at early stages of mammary carcinogenesis are concordant with our prior demonstration of decreased mammary gland atypia and tumorigenesis in mice fed diets prepared to exact specifications [9].
Taken together, these findings support the hypothesis that dietary n-3 PUFAs elicit anticancer effects at early premalignant stages and may provide a potent nutritional strategy for the prevention of HER-2/neu(+), ERPR(−) breast cancer subtypes. As suggested by emerging human studies, hormone insensitive disease may prove uniquely susceptible to dietary interventions such as n-3 PUFAs. For example, a dietary pattern of higher intake of fruits, vegetables, whole grains, low fat dairy products, fish and poultry appears associated with lower risk for developing ER(−) cancer in postmenopausal women [35]. The Women’s Intervention Nutrition Study (WINS) findings also indicate beneficial effects of a low fat diet for the subset of survivors with ER(−) breast cancers [36]. A role for dietary modulation of risk for breast cancer with HER-2/neu overexpression is supported by the ORDET study of 8,984 women in Varese Province, Italy, which demonstrated that diets high in raw vegetables and olive oil were protective against HER-2/neu(+) breast cancer at medium and high levels of intake, with preventive benefit for the HER-2/neu(+) subtype with also medium intake of cooked vegetables, rice, poultry, fish [37]. The present data provides further evidence for potential dietary modulation of specific breast cancer subtypes and warrants further investigation in prospective human trials of breast cancer prevention.
Acknowledgments
This research was supported by NIH R21 CA116024 to LDY and funding from the Molecular Carcinogenesis and Chemoprevention Program of The Ohio State University Comprehensive Cancer Center. We gratefully acknowledge the technical assistance of Kimberly Carter for tissue processing and Susie Jones for immunohistochemistry.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochimica Biophysica Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 4.van de Vijver MJ, Peterse JL, Mooi WJ, et al. Neu-protein overexpression in breast cancer: association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. New England Journal of Medicine. 1988;319:1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 5.Tsuda H, Hirohashi S. Multiple developmental pathways of highly aggressive breast cancers disclosed by comparison of histological grades and c-erbB-2 expression patterns in both the non-invasive and invasive portions. Pathology International. 1998;48:518–525. doi: 10.1111/j.1440-1827.1998.tb03943.x. [DOI] [PubMed] [Google Scholar]
- 6.Stark A, Hulka BS, Joens S, et al. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. Journal of Clinical Oncology. 2000;18:267–274. doi: 10.1200/JCO.2000.18.2.267. [DOI] [PubMed] [Google Scholar]
- 7.Guy CT, Webster MA, Schaller M, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proceedings of the National Academy of Science, USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Carlo E, Diodoro MG, Boggio K, et al. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Laboratory Investigation. 1999;79:1261–1269. [PubMed] [Google Scholar]
- 9.Yee LD, Young DC, Rosol TJ, et al. Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARγ ligand rosiglitazone. Journal of Nutrition. 2005;135:983–988. doi: 10.1093/jn/135.5.983. [DOI] [PubMed] [Google Scholar]
- 10.Cardiff RD, Anver MR, Gusterson BA, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 11.Reeves PG, Nielsen FH, Fahey GCJ. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of Nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 12.Yee LD, Lester JL, Cole RM, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. American Journal of Clinical Nutrition. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee LD, Williams N, Wen P, et al. Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clinical Cancer Research. 2007;13:246–252. doi: 10.1158/1078-0432.CCR-06-1947. [DOI] [PubMed] [Google Scholar]
- 14.Berruti A, Generali D, Bertaglia V, et al. Intermediate endpoints of primary systemic therapy in breast cancer patients. J Natl Cancer Inst Monogr. 2011;2011:142–146. doi: 10.1093/jncimonographs/lgr036. [DOI] [PubMed] [Google Scholar]
- 15.Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan QJ, Kimler BF, Clark J, et al. Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer epidemiology biomarkers and prevention. 2005;14:786–789. doi: 10.1158/1055-9965.EPI-04-0239. [DOI] [PubMed] [Google Scholar]
- 17.Menendez JA, Vellon L, Mehmi I, et al. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene expression in cancer cells. Proceedings of the National Academy of Science, USA. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. Journal of Biological Chemistry. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 19.Reseland JE, Haugen F, Hollung K, et al. Reduction of leptin gene expression by dietary polyunsaturated fatty acids. Journal of Lipid Research. 2001;42:743–750. [PubMed] [Google Scholar]
- 20.Yang Z, Bagheri-Yarmand R, Balasenthil S, et al. HER2 regulation of peroxisome proliferator-activated receptor γ (PPARγ) expression and sensitivity of breast cancer cells to PPARγ ligand therapy. Clinical Cancer Research. 2003;9:3198–3203. [PubMed] [Google Scholar]
- 21.Flachs P, Mohamed-Ali V, Horakova O, et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49:394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 22.Degnim AC, Visscher DW, Berman HK, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. Journal of Clinical Onocology. 2007;25:2671–2677. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 23.Vadlamudi R, Mandal M, Adam L, et al. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999;18:305–314. doi: 10.1038/sj.onc.1202307. [DOI] [PubMed] [Google Scholar]
- 24.Benoit V, Relic B, Leval XX, et al. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004;23:1631–1635. doi: 10.1038/sj.onc.1207295. [DOI] [PubMed] [Google Scholar]
- 25.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Research. 2002;62:632–635. [PubMed] [Google Scholar]
- 26.Visscher DW, Pankratz VS, Santisteban M, et al. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. Journal of the National Cancer Institute. 2008;100:421–427. doi: 10.1093/jnci/djn036. [DOI] [PubMed] [Google Scholar]
- 27.Howe LR, Subbaramaiah K, Patel J, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Research. 2002;62:5405–5407. [PubMed] [Google Scholar]
- 28.Lanza-Jacoby S, Miller SG, Flynn J, et al. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer epidemiology biomarkers and prevention. 2003;12:1486–1491. [PubMed] [Google Scholar]
- 29.Aronson WJ, Glaspy JA, Reddy ST, et al. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283–288. doi: 10.1016/s0090-4295(01)01116-5. [DOI] [PubMed] [Google Scholar]
- 30.Clinton S. Diet, anthropometry and breast cancer: integration of experimental and epidemiologic approaches. Journal of Nutrition. 1997;127:916S–920S. doi: 10.1093/jn/127.5.916S. [DOI] [PubMed] [Google Scholar]
- 31.Pape-Ansorge KA, Grande JP, Christensen TA, et al. Effect of moderate caloric restriction and/or weight cycling on mammary tumor incidence and latency in MMTV-neu female mice. Nutrition and Cancer. 2002;44:161–168. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- 32.Program NT. National Toxicology Program Technical Report Series. 1997. NTP Toxicology and Carcinogenesis Studies of t-Butylhydroquinone (CAS No. 1948-33-0) in F344/N Rats and B6C3F(1) Mice (Feed Studies) pp. 1–326. [PubMed] [Google Scholar]
- 33.Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8:1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez MJ, Schemmel RA, Gray JI, et al. Effect of dietary fat on growth of MCF-7 and MDA-MB-231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis. 1991;12:1231–1235. doi: 10.1093/carcin/12.7.1231. [DOI] [PubMed] [Google Scholar]
- 35.Fung TT, Hu FB, Holmes MD, et al. Dietary patterns and the risk of postmenopausal breast cancer. International Journal of Cancer. 2005;116:116–121. doi: 10.1002/ijc.20999. [DOI] [PubMed] [Google Scholar]
- 36.Chlebowski RT, Blackburn GL, Elashoff RE, et al. Dietary fat reduction in postmenopausal women with primary breast cancer: Phase III Women’s Intervention Nutrition Study (WINS); American Society of Clinical Oncology Annual Meeting; 2005; Abstract 10. [Google Scholar]
- 37.Sant M, Allemani C, Sieri S, et al. Salad vegetables dietary pattern protects against HER-2-positive breast cancer: a prospective Italian study. International Journal of Cancer. 2007;121:911–914. doi: 10.1002/ijc.22714. [DOI] [PubMed] [Google Scholar]


