Abstract
Mental simulations are often focused on a goal in the future or a problem to be solved. Recent neuroimaging studies have associated mental simulations of the future with default network activity, but the simulations in these studies were not typically directed toward achieving a particular goal. Goal-directed simulation requires cognitive control to maintain information, make decisions, and coordinate abstract action sequences. Therefore, it should recruit not only the default network, but also executive regions. To investigate whether default network and executive regions can be coactive in the context of goal-directed simulation, we designed a problem-solving task in which participants simulated solving several specific problems in imaginary scenarios while in the MRI scanner. We analyzed brain activity during simulation relative to a semantic elaboration task and found that goal-directed simulation engaged core regions of the default network and executive dorsolateral prefrontal cortex. A functional connectivity analysis with posterior cingulate and dorsolateral prefrontal cortex seeds revealed that activity in these regions was coupled throughout the goal-directed simulation period and associated with a distributed network of other default and executive regions, including medial prefrontal cortex, medial temporal, and parietal regions.
Keywords: Default mode, cognitive control, fMRI
1. Introduction
We spend a significant part of our day engaged in mental simulations, which are often focused on a particular goal or a problem to be solved in the future (Gollwitzer, 1999). Whether we imagine what to have for lunch, how to spend the next holiday, or how to resolve a dispute with a friend, these everyday simulations are both internally focused and goal-directed. Recent neuroimaging studies have shown that imagining, or simulating, future events, like remembering past events, is associated with activity in the default network (for reviews, see Buckner et al., 2008; Schacter et al., 2007; Spreng et al., 2009).
However, the simulation of future events in these studies was not directed toward achieving a particular goal, even though simulations in everyday life tend to be geared toward a future goal state or a solution to a problem (Schacter et al., 2008). For example, in typical experimental paradigms, participants imagine an event that might plausibly occur in the future in response to cue words or phrases (e.g., Addis et al., 2007; Andrews-Hanna, Reidler, Sepulcre, et al., 2010; Spreng & Grady, 2010; Szpunar et al., 2007, 2009), recombined elements of past experiences (Addis et al., 2009), or open-ended instructions to think about the past and future (Okuda et al., 2003). These studies have revealed that the default network is reliably engaged, as have related studies of even less constrained internally focused and self-referential mental explorations (Andrews-Hanna, Reidler, Huang, et al., 2010; Buckner & Carroll, 2007; D 'Argembeau et al., 2005), as well as studies of mind wandering (Christoff et al., 2009; Mason et al., 2007).
The set of interconnected brain regions that make up the default network include medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), medial and lateral temporal regions, and the inferior parietal lobule (Buckner et al., 2008; Gusnard & Raichle, 2001). Although the exact neurocognitive functions of all of the network components remain to be investigated, MPFC has been implicated in many tasks involving self-referential processing (Amodio & Frith, 2006; Andrews-Hanna, Reidler, Sepulcre, et al., 2010; D'Argembeau et al., 2007, 2009; Gusnard et al., 2001; Heatherton et al., 2006). PCC has been observed to be the critical connector hub to all regions of the default network and is hypothesized to be crucial to its functional integration (Hagman et al., 2008).
Despite its involvement in various cognitive processes, the default network is perhaps better known for decreases in activity during tasks that demand external attention (Gusnard & Raichle, 2001; Shulman et al., 1997). Activity of the default network has been described as anticorrelated with a “task-positive” network of brain regions whose activity increases during tasks that demand externally focused attention (Fox et al., 2005; Fransson, 2005; Greicius et al., 2003). This task-positive network is comprised of dorsolateral prefrontal cortex (DLPFC), the frontal eye fields, inferior precentral sulcus, middle temporal motion complex, and the superior parietal lobule (Fox et al., 2005; Toro et al., 2008). Anticorrelations between default and task-positive network regions have typically been found in resting state functional connectivity analyses as opposed to connectivity analyses based on task-evoked activity.
There has been an ongoing debate over whether task-positive and default network regions are truly anticorrelated (Fox et al., 2009), or whether negative correlations between default and task-positive network regions could be attributed to global signal regression (Murphy et al., 2009). Using a novel statistical approach that adjusted for whole-brain correlations but avoided global time-course regression, a recent study selected DLPFC, which had been the most active region during a working memory task, and medial frontal cortex, a default network area that had been the most deactivated during said task, and found that DLPFC and medial frontal cortex were the most antagonistic regions of the two networks during rest (Hampson et al., 2010). Although the exact interpretation of negative correlations remains controversial, the existing evidence of anticorrelations between the default and the task-positive network (e.g., Fox et al., 2005, 2009; Fransson, 2005; Greicius et al., 2003) raises the question of whether it is possible for brain regions from both networks to be coactive, or whether the competitive relationship between the networks prevents any coactivation of both networks or of select regions within those networks.
Goal-directed simulation directed at solving a problem is not only a prospective, self-referential process (e.g., Addis, et al., 2007), but also requires cognitive control of information. For example, when simulating a future event in which the goal is to resolve a dispute with a friend, it is not sufficient simply to imagine a future scene involving the friend. One must also retrieve and integrate what one knows about a friend's likely response, what kinds of approaches have worked in similar situations, how to most effectively raise and deal with sensitive issues, and so forth.
Goal-directed simulation of this kind is a cognitive process that may be supported by regions of both the default and the task-positive network, particularly regions of the task positive network involved in cognitive control. When we formulate a plan to solve a problem in the future, we need to keep in mind any information that might be relevant to the problem and its solution, make decisions as to how to proceed, and envision a sequence of actions that could lead to a solution. These types of cognitive processes have been researched in the context of executive function and cognitive control (Badre & D ' Esposito, 2009; Botvinick et al., 2001; Norman & Shallice, 1980) and have been associated with activations in regions pertaining to the task-positive network, such as the lateral prefrontal cortex (Miller & Cohen, 2001; Koechlin et al., 2003; Rushworth et al., 2002). In particular, the mid-DLPFC (Brodman area 9/46) appears to be critical for tasks that require encoding action sequences, coordinating actions in relation to internal goals, and maintaining abstract sequential movement plans (Badre & D ' Esposito, 2009; Badre et al., 2009), thus rendering it likely that DLPFC is engaged during problem-solving simulations. The aforementioned studies of executive function and cognitive control have typically examined executive demands associated with the manipulation and control of impersonal information, such as visuospatial patterns. Few studies, however, have examined problem-solving processes using real-world scenarios as task stimuli.
Given the diverse mental processes that are integral to problem-solving simulations, we expect their neural basis to be composed of regions from both the default and the task-positive network. However, such a hypothesis would be incongruent with the anticorrelation of the two networks. One recent study has provided evidence for the coactivation of areas of the default and the task-positive network (Spreng et al., 2010). Spreng and colleagues revealed that while participants silently formulated plans for their personal future in order to attain goals, the default network was coactive with regions associated with cognitive control and executive function, including DLPFC.
To investigate whether the default network and executive regions can also be coactive in the context of goal-directed problem-solving simulations, we designed a problem-solving task based on an earlier cognitive paradigm (Patalano & Seifert, 1997), in which participants were asked to imagine themselves (i.e., simulate) actively solving a problem. Participants were presented with imaginary scenarios, in which they needed to solve several specific problems and were given cues that could aid formulating a plan to solve each problem. We hypothesized that, relative to a semantic elaboration task, participants ' problem-solving simulations would recruit core regions of the default network and executive regions. We predicted co-activation of PCC because of its central role in default mode processes (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Buckner et al., 2009; Hagman et al., 2008) and of DLPFC because of its involvement in coordinating and maintaining action sequences and goals (Badre & D'Esposito, 2009; Botvinick et al., 2001; Norman & Shallice, 1980). Further, we expected activity of these default and executive regions to be coupled as a functional network during goal-directed simulation.
2. Material and methods
2.1 Participants
Twenty-nine healthy young adults (mean age = 22.4 years, SD = 3.1; 16 women) gave written consent and participated in the experiment in accordance with the guidelines set forth by the Committee on the Use of Human Subjects in Research at Harvard University and the Human Subjects Research Committee at Massachusetts General Hospital. All participants had normal or corrected-to-normal vision, reported no history of neurological or psychiatric conditions, and were right-handed native English speakers.
2.2 Materials and procedure
The simulation task was designed to present participants with problems to which they could easily relate and which they could imagine solving themselves in the future. To perform the simulation task, participants read and then kept in mind a scenario and an associated problem while imagining themselves in the setting specified in the scenario, solving the problem. We based our task on a scenario from a cognitive paradigm (Patalano & Seifert, 1997) in which an individual is asked to imagine being left alone in a friend's dorm room, and to solve a number of problems that then arise. We expanded the number of scenarios in the original paradigm from one to six: being left alone in a friend's dormitory room, volunteering in a local retirement community, navigating a new neighborhood, organizing a camping trip, house-sitting for family-friends, and planning a class research project. We devised ten unique problems that an individual could encounter in each scenario. However, each participant was only presented with five of the ten problems that were associated with each scenario, whereby fourteen participants were randomly assigned one set of five problems, and the remaining fifteen participants simulated the other set of five problems.
In order to simulate solving the problems pertaining to each scenario, participants were given cues that could aid formulating a plan to solve each problem. One such scenario took place in a friend's dormitory room, where participants imagined being left alone. As one of the problems, participants imagined trying on the friend's class ring and being unable to remove it. Soap was suggested as a possible object that could help them solve the problem, and participants simulated removing the class ring using soap. Thus, subjects imagined both the problem solving process and the resulting solution to the problem. By providing participants with a word for an object (referred to as “object-word”) that allowed them to solve each problem in a scenario, we sought increased experimental control over the specific content of their mental simulations.
As a comparison condition we used a semantic elaboration task for which participants silently generated semantically associated words. Since the future simulation task was not based on problems that participants actually faced in their everyday lives, there was a possibility that participants relied mostly on semantic rather than episodic forms of knowledge when imagining themselves in the scenarios. To investigate whether goal-directed simulation in our paradigm is merely a form of semantic elaboration, the control task was designed to engage regions of the semantic network (Binder et al., 2009). Participants were asked to silently generate words that were semantically associated with a cue word. Cue words were counterbalanced across subjects.
Prior to scanning, participants became familiar with both experimental tasks by completing one trial of each condition on a laptop computer. In an event-related design in the scanner participants were presented with three runs that were self-paced except for fixed simulation, association, and rating periods. Each run consisted of ten trials of each condition (30 total per condition); the order of conditions was counterbalanced across participants. Within a run, subjects were presented with two blocks of five simulations trials that alternated with two blocks of five association trials. For the simulation task, participants read a short scenario and performed a button press to indicate when they had finished reading. They were then presented with the description of a problem that could occur in the context of the given scenario, and with the object-word that could be used to solve this problem. Participants pressed a button to advance to the subsequent screen, which instructed them to imagine themselves in the scenario faced with the problem and interacting with the object in order to solve the problem. This simulation period was fixed and lasted 7.5 seconds. Following the simulation period, participants rated the vividness of their simulation on a scale from one to four, with four being most vivid. See Figure 1 for the sequence of stimuli participants saw in the scanner. For each run, participants read two scenarios that were each followed by five associated problems.
Figure 1.
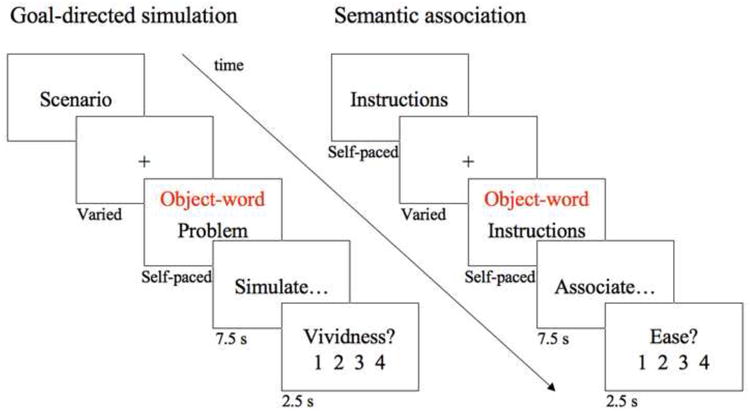
Task stimuli. Sequence of fMRI stimuli for each task condition.
The first screen of the semantic association condition displayed the task instructions; analogous to the simulation task, participants advanced to the next screen by pressing a button. Participants viewed the object-word and pressed a button once they were ready to commence the association generation period, which was fixed and lasted 7.5 seconds. They then had 2.5 seconds to rate on a scale of one to four the ease with which they were able to generate semantic associates for the object-word (four being the easiest). Within each run, participants performed two blocks of five association tasks.
All experimental trials were intermixed with varying periods of fixation trials, with fixation intervals ranging from one to four TRs (M=2.17, SD=0.04). The end of each run had six TRs of fixation. None of the object-words presented in the simulation and association task were repeated across tasks within participants, and object-words were randomized and counterbalanced across participants. All visual stimuli were presented in black on a white background using an Apple MacBook computer (Apple Computers) that ran PsyScope × B51 (Cohen et al., 1993).
2.3 fMRI data collection
Participants were scanned using a 3-Tesla Tim Trio system (Siemens) with a 12-channel phased-array head coil. High-resolution three-dimensional T1-weighted images were acquired as anatomical scans [repetition time (TR), 2530 ms; echo time (TE), 3.44 ms; flip angle (FA), 7°; 1.0 mm3 isotropic voxels]. Functional data were collected using a gradient-echo echo-planar pulse sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (TR, 2500ms; TE, 30ms; FA, 90°; 3 × 3 × 3 mm3 voxels; 36 axial slices parallel to plane of the anterior commissure-posterior commissure; 0.5 mm gap between slices). Head motion was restricted using a pillow and two padded clamps. Participants held a button box in their left hand, and earplugs were provided to attenuate scanner noise. Visual stimuli were projected onto a screen positioned at the head of the magnet bore, which was reflected in a mirror on top of the head coil.
2.4 fMRI data
2.4.1 Preprocessing
We used SPM8 (Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks, Sherborn, MA) to preprocess and analyze the fMRI data. We excluded the first four volumes of each run to avoid potential T1-equilibration effects and performed slice-timing corrections to the fifth slice. To remove systematic differences and movement-induced variance between sessions, images were realigned. Images were normalized to the Montreal Neurological Institute (MNI) EPI template (voxel size = 3 × 3 × 3 mm3) and smoothed using a 6 mm full-width at half maximum (FWHM) Gaussian kernel. A high-pass filter with a cutoff value of 128 seconds was applied to the images to account for low-frequency drifts.
2.4.2 Task contrast analysis
For each participant we generated a general linear model (GLM) using SPM8 that was comprised of task effects, a mean and linear drift for each of the three functional runs, and six motion parameters. Task effects were modeled with the canonical hemodynamic response function, its temporal derivative, and its dispersion derivative (Friston et al., 1998) and included the following cognitive events: reading a scenario, reading an associated problem, reading instructions for the association task, as well as simulating and associating, which were each combined with their respective rating period. Simulation and association periods were combined with their rating periods to avoid regressing out relevant activation, as these periods always occurred subsequently without interspersed fixation. The resulting parameter estimates and tcontrast images of the conditions of interest at each voxel were then submitted to a second-level, random-effects analysis to create mean t-images. To identify neural activity associated with the goal-directed simulation distinct from semantic elaboration, we performed the direct whole-brain contrast with p < .001 uncorrected and a required cluster size of k > 20. We identified peak MNI coordinates of active regions based on the results of an automated peak-search algorithm. According to the same parameters, we also compared each condition of interest to fixation.
Specific regions of interest (ROIs) were generated by creating an 8 mm-radius sphere around peak coordinates that emerged from the whole-brain contrast. Parameter estimates for each ROI and condition were plotted to explore the underlying signal behind the whole-brain contrast results.
2.5 Task-related functional connectivity
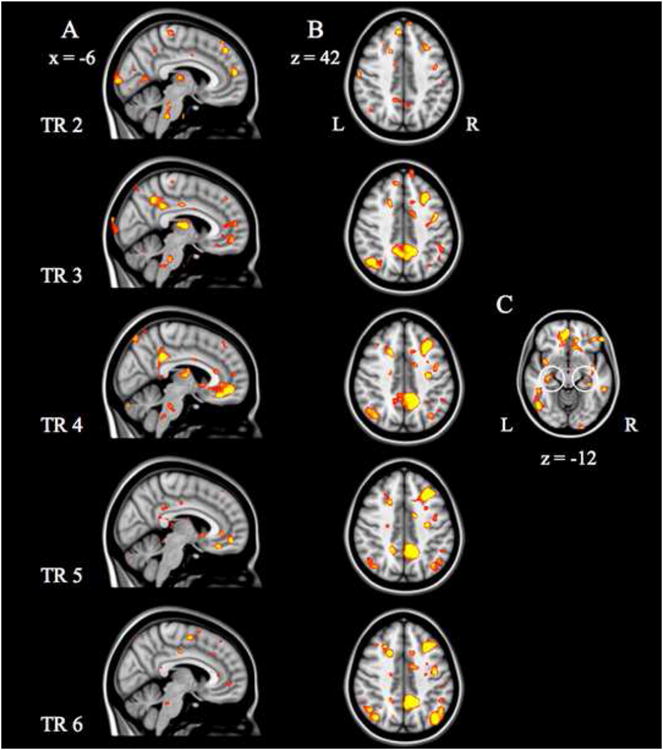
In order to test the hypothesis that PCC and right and left DLPFC would behave as a functional network during simulation, we conducted a task-related functional connectivity analysis using seed partial least squares (PLS; Burianova, McIntosh & Grady, 2010; McIntosh, 1999; McIntosh, Chau & Protzner, 2004). Seed PLS is a data-driven, multivariate functional connectivity analysis technique used to investigate the relationship between the activity of a set of seed regions and the activity in the rest of the brain. BOLD signal values from PCC (9, -50, 39; see Table 1), right DLPFC (33, 25, 45) and left DLPFC (-33, 31, 48), and their 26 neighborhood voxels were extracted and averaged from the third, fourth, and fifth TR post simulation trial onset. In order to assess the pattern of covariance between the three seed regions for the simulation condition, which indicates whether these regions are part of a functional network, the activity of the seeds was not averaged together. The activity of each seed was correlated with the activity in all other brain voxels across the five TRs, across participants. These correlations were then combined into a matrix and decomposed with singular value decomposition. This analysis resulted in a set of orthogonal latent variables which consists of A) a “singular value”: the amount of covariance accounted for by the latent variable, B) a “singular profile”: the pattern of covariance for the seed region with the rest of the brain (Figure 3), and C) a “singular image”: the voxelwise pattern of brain regions that covary with the seed activity across the five-TR trial (Figure 4). The significance of the pattern of connectivity was determined by permutation testing, which randomly reorders the data matrix and calculates the singular values for a new set of latent variables for each reordering. Each newly computed singular value of a latent variable is then compared to the original latent variable, resulting in a probability of the permuted values that exceeds the original value. We conducted five hundred permutations.
Table 1.
Peak regions of activation for problem-solving simulation > semantic association. Lat = Laterality, B = Bilateral, L = Left, R = Right, BA = Brodman's Area, AMG = Amygdala, IPL = Inferior parietal lobule, ITS = Inferior temporal sulcus, ITG = Inferior temporal gyrus, MOG = Middle occipital gyrus, MTG = Middle temporal gyrus, PostCG = Postcentral gyrus, PCu = Precuneus, SMG = Supramarginal gyrus, SPL = Superior parietal lobule, TPJ = Temporoparietal junction. Locations of the maxima are reported in the stereotaxic coordinates of MNI space.
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Lat | Region | BA | x | y | z | t |
| R | TPJ | 39 | 48 | −65 | 24 | 6.81 |
| L | IPL | 19 | −36 | −83 | 39 | 6.37 |
| B | PCC | 31 | 9 | −50 | 39 | 5.73 |
| R | DLPFC | 9/46 | 33 | 25 | 45 | 5.53 |
| R | MTG/ITS | 21 | 48 | −11 | −21 | 5.5 |
| B | PCu | 7 | −9 | −56 | 63 | 5.45 |
| L | DLPFC | 9/46 | −33 | 31 | 48 | 4.97 |
| L | PCC | 31 | −9 | −31 | 41 | 4.85 |
| L | SMG | 40 | −57 | −23 | 36 | 4.62 |
| R | SMG | 40 | 63 | −23 | 30 | 4.43 |
| L | PCG | 3/4 | −39 | −32 | 54 | 4.41 |
| R | ITG | 37 | 48 | −59 | −9 | 4.11 |
| R | MPFC | 9 | 7 | 49 | 28 | 4.1 |
| R | AMG | 24 | −2 | −21 | 3.83 | |
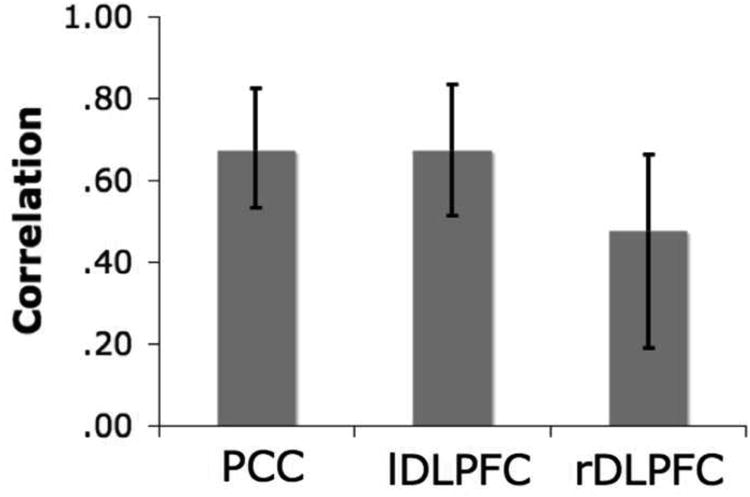
Figure 3.
Correlations of activity in the PCC, lDLPFC, and rDLPFC seeds with their respective brain scores show how activity in the three seeds covaries with activity in the entire network. Error bars represent 95% confidence intervals based on the bootstrap, which indicate no differences in the pattern of connectivity between the three seed regions.
Figure 4.
Regions of the distributed functional network in the seed PLS analysis, including A. PCC and MPFC, B. DLPFC and PCC, and C. HC development across TRs. Activity in PCC and DLPFC are coupled across all five TRs. This functional network included the additional recruitment of MPFC and parietal regions. Recruitment of the hippocampus was restricted to the fourth TR (circled in C).
In a second, independent step, the reliability of the associated brain voxel salience (or weight) from the singular image was determined by bootstrap resampling with replacement, using 100 iterations, to estimate the standard errors for each voxel (Efron & Tibshirani, 1985). For each voxel, the salience/standard error ratio, or bootstrap ratio (BSR), was calculated. Peak voxels with a BSR greater than 3.23 were considered reliable and approximate a p < .001. Clusters containing at least 20 reliable voxels were extracted, and a local maximum for each cluster was defined as the voxel with a BSR higher than any other voxel in a 2 cm cube centered on that voxel. In seed PLS, the singular profile correlation values represent the relationship between activity in each seed region and the whole brain pattern identified in the analysis. If PCC were anticorrelated with DLPFC, the activity of these regions and their functional connectivity would be dissociated. However, if PCC and DLPFC behaved as a functional network, their activity would covary together and be functionally connected with a distributed network of brain regions involved in goal-directed simulation. As summary measures of each subject's expression of each latent variable, we calculated “brain scores” by multiplying the BOLD signal in each voxel by each voxel's salience and summing across all brain voxels for each participant. We calculated the correlation between these brain scores and the seed values to assess the relation between the whole brain pattern and activity in the three seed regions. Based on the mean brain scores and the bootstrap, we calculated 95% confidence intervals, which can determine similarities and differences in correlation magnitude between the seed activity and the covarying brain activity, depending on whether or not the confidence intervals overlap.
3. Results
3.1 Behavioral findings
The behavioral ratings of vividness and ease, which we collected after each simulation and association period, confirmed that participants complied with the task. Participants were able to imagine solving problems with moderate vividness (mean vividness = 1.82, SD = .39) and did not judge semantic associations to be too easy (mean ease = 1.77, SD = .29). Participants provided a rating for almost all trials (6.73% missing responses).
3.2 fMRI results
3.2.1 GLM results
The simulation task, relative to semantic association, engaged PCC, the right middle temporal gyrus, right MPFC, the right temporo-parietal junction, and the left inferior parietal lobule. These regions are consistent with the default network (Buckner et al., 2008; Fox et al., 2005; Raichle et al., 2001; Table 1; Figure 2A). Additional activity was observed during goal-directed simulation in bilateral DLPFC (Figure 2B), regions associated with cognitive control and working memory (Badre & D ' Esposito, 2009; D ' Esposito et al., 1995; Vincent et al., 2008). The ROI parameter estimates of PCC (9 -50 28), MPFC (7 49 28), and right (33 25 45) and left DLPFC (-33 31 48) illustrate that these regions were more strongly engaged or less deactivated during simulation than during association (Figure 2C). Relative to simulation, semantic association was associated with bilateral anterior insula, lingual gyrus, left medial superior frontal gyrus, and inferior frontal gyrus activity (Table 2). PCC, MPFC, and right and left DLPFC were deactivated during association relative to fixation (Figure 2C). Semantic network regions such as alfO and LG were more active during association compared to fixation (Supplemental Figure 2; Supplemental Table 2), whereas the contrast of simulation over fixation revealed activations in lDLPFC and frontal areas (Supplemental Figure 1; Supplemental Table 1).
Figure 2.
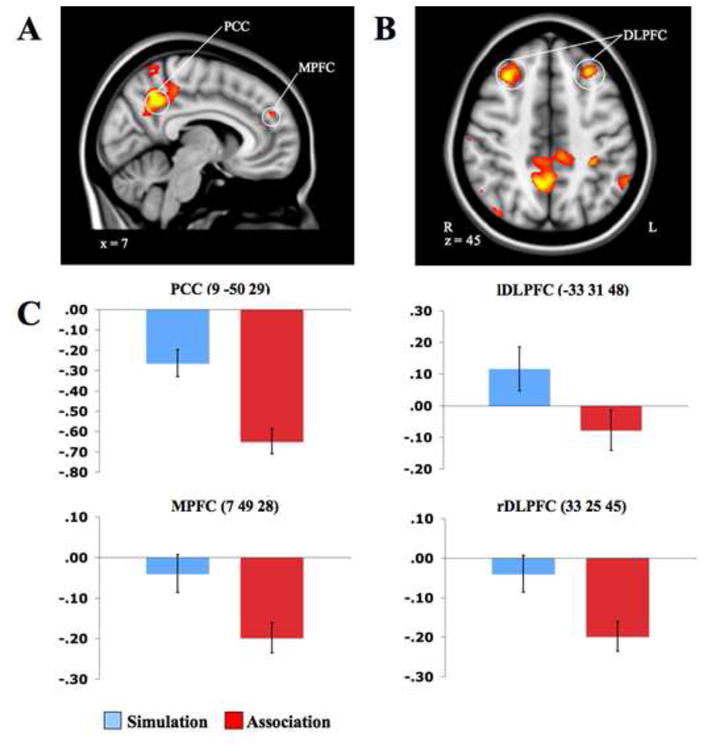
Problem-solving simulation > semantic association. Activations in regions of the default network and regions of executive function, including A. PCC and MPFC, and B. bilateral DLPFC. DLPFC, MPFC, and PCC parameter estimates for each condition are displayed in C. On the Y-axis is the mean parameter estimate, and error bars are standard error of the mean (SEM).
Table 2.
Peak regions of activation for semantic association > problem-solving simulation. aIfO = Anterior insula/frontal operculum, IFG = Inferior frontal gyrus, LG = Lingual gyrus, MSFG = Medial superior frontal gyrus, PreCG = Precentral gyrus.
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Lat | Region | BA | x | y | z | t |
| L | aIfO | −33 | 19 | 0 | 7.24 | |
| B | MSFG | 6 | −6 | 16 | 51 | 7.21 |
| L | IFG | 9 | −51 | 19 | 33 | 6.33 |
| L | PreCG | 4 | −45 | −8 | 60 | 4.98 |
| R | LG | 18 | 18 | −89 | −3 | 4.85 |
| L | LG | 18 | −6 | −86 | −6 | 4.85 |
| R | aIfO | 33 | 25 | 0 | 4.36 | |
3.2.2 Task-related functional connectivity
The seed PLS analysis revealed a significant pattern of task-related functional connectivity with one significant latent variable (p = .002, accounting for 62.72% of the covariance). During simulation, measures of overall brain activity were significantly correlated with activity in the three seed regions, across participants. All three seed regions reliably covaried together during simulation, See Figure 3. PCC and DLPFC seed regions were functionally associated with this distributed network across the entire five-TR trial. This distributed functional network included MPFC, lateral parietal regions, lateral temporal regions, and superior frontal regions. The medial temporal lobes were also functionally recruited, but only in the first three timepoints of the trial. See Table 3 and Figure 4 for associated regions at each TR.
Table 3.
Regions functionally connected with PCC and DLPFC during goal-directed simulation for each TR. AG = Angular gyrus, ATL = Anterior temporal lobe, CALG = Calcarine gyrus, CT = Cerebellar tonsil, FP = Frontal pole, HC = Hippocampus, IOG = Inferior occipital gyrus, LOG = Lateral orbital gyrus, MFG = Middle frontal gyrus, MOG = Middle occipital gyrus, MTOG = Middle temporo-occipital gyrus, OP = Occipital pole, PC = Pyramis of the cerebellum, PHG = Parahippocampal gyrus, RSC = Retrosplenial cortex, SMA = Supplementary motor area, STG = Superior temporal gyrus, STS = Superior temporal sulcus, Thal = Thalamus, VMPFC = Ventromedial prefrontal cortex.
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Lat | Region | x | y | z | |
| TR 2 | |||||
| L | PCC | −12 | −42 | 33 | 5.45 |
| L | ITG | −48 | −51 | −21 | 8.58 |
| R | PreCG | 33 | −27 | 54 | 8.22 |
| R | IFG | 54 | 24 | 15 | 7.59 |
| L | Thal | −9 | −18 | 9 | 7.41 |
| L | MOG | −39 | −81 | 6 | 7.23 |
| R | CALG | 21 | −72 | 12 | 7.13 |
| L | MSFG | −6 | 42 | 42 | 6.95 |
| R | ATL | 66 | −9 | −9 | 6.82 |
| L | OP | −9 | −99 | 3 | 6.76 |
| R | PHG | 27 | −24 | −27 | 6.59 |
| B | MPFC | −6 | 54 | 15 | 6.46 |
| L | PreCG | −63 | −3 | 30 | 6.32 |
| L | MTG | −48 | −51 | 9 | 6.16 |
| R | RSC | 12 | −48 | 6 | 5.91 |
| L | IFG | −51 | 21 | 18 | 5.88 |
| L | IPL | −48 | −45 | 30 | 5.79 |
| L | aIfO | −42 | −15 | 9 | 5.37 |
| L | MFG | −42 | 6 | 54 | 5.31 |
| TR 3 | |||||
| B | PCC | 15 | −48 | 39 | 8.77 |
| R | DLPFC | 30 | 24 | 42 | 6.98 |
| L | DLPFC | −45 | 3 | 60 | 5.59 |
| L | ATL | −45 | 15 | −33 | 8.71 |
| R | PHG | 21 | 3 | −21 | 8.69 |
| R | MSFG | 12 | 63 | 30 | 8.46 |
| L | ITG | −48 | −54 | −21 | 7.91 |
| R | ITG | 51 | −6 | −33 | 7.72 |
| R | PC | 9 | −81 | −27 | 7.28 |
| L | Thal | −18 | −30 | 3 | 7.23 |
| L | AG | −42 | −63 | 42 | 7.13 |
| L | OP | −30 | −99 | −12 | 6.89 |
| R | Thal | 9 | −9 | 6 | 6.88 |
| L | PHG | −33 | −18 | −30 | 6.76 |
| R | MTOG | 51 | −66 | 27 | 6.65 |
| R | IPL | 57 | −39 | 57 | 6.45 |
| L | IFG | −51 | 21 | 18 | 6.40 |
| L | OP | −9 | −105 | −12 | 6.26 |
| R | IFG | 36 | 30 | 15 | 6.01 |
| L | FP | −54 | 36 | −9 | 5.98 |
| R | PreCG | 30 | −18 | 60 | 5.95 |
| L | CALG | −18 | −60 | 6 | 5.84 |
| L | MSFG | −12 | 39 | 45 | 5.62 |
| L | VMPFC | −3 | 51 | −9 | 5.47 |
| L | STG | −45 | −24 | 9 | 5.25 |
| L | PC | −30 | −75 | −33 | 5.19 |
| L | ITG | −54 | −9 | −36 | 5.01 |
| L | Brainstem | −6 | −30 | −36 | 4.91 |
| R | STG | 63 | −27 | 12 | 4.76 |
| L | MPFC | −6 | 54 | 12 | 4.38 |
| TR 4 | |||||
| B | PCC/RSC | 15 | −45 | 42 | 9.62 |
| R | DLPFC | 30 | 24 | 42 | 8.54 |
| L | DLPFC | −36 | 21 | 24 | 6.14 |
| R | FP | 39 | 36 | −15 | 8.16 |
| L | ITG | −48 | −54 | −21 | 7.95 |
| B | VMPFC | −6 | 48 | −9 | 7.89 |
| R | STG | 36 | 6 | −18 | 7.13 |
| R | ITG | 48 | −30 | −24 | 6.88 |
| R | IFG | 36 | 30 | 15 | 6.53 |
| R | AG | 36 | −66 | 45 | 5.95 |
| R | PC | 6 | −81 | −27 | 5.91 |
| R | MSFG | 12 | 60 | 30 | 5.89 |
| L | Thal | −9 | −9 | 9 | 5.82 |
| R | SPL | 21 | −63 | 69 | 5.76 |
| L | STG | −36 | 9 | −36 | 5.34 |
| R | SMG | 48 | −45 | 36 | 5.33 |
| L | SPL | −33 | −69 | 54 | 5.32 |
| L | PCu | −3 | −72 | 60 | 5.21 |
| R | Brainstem | 6 | −39 | −45 | 5.13 |
| L | aIfO | −39 | 3 | −12 | 5.07 |
| R | MOG | 48 | −72 | 27 | 4.96 |
| L | HC | −33 | −24 | −12 | 4.95 |
| R | HC | 33 | −32 | −12 | 5.56 |
| TR 5 | |||||
| R | PCC | 15 | −54 | 39 | 9.17 |
| L | PCC | −17 | −46 | 39 | 7.99 |
| R | DLPFC | 30 | 24 | 42 | 9.92 |
| R | SPL | 30 | −57 | 72 | 8.11 |
| R | PreCG | 42 | −3 | 24 | 7.60 |
| L | IOG | −48 | −72 | −18 | 7.26 |
| R | MSFG | 12 | 63 | 27 | 6.47 |
| L | VMPFC | −6 | 48 | −12 | 6.39 |
| R | PC | 33 | −72 | −36 | 6.21 |
| L | CT | −27 | −78 | −33 | 6.15 |
| R | AG | 39 | −66 | 45 | 5.85 |
| R | FP | 48 | 36 | −15 | 5.84 |
| L | ITG | −45 | −33 | −18 | 5.82 |
| R | aIfO | 36 | 3 | −15 | 5.31 |
| R | IFG | 33 | 27 | 18 | 5.28 |
| L | PHG | −36 | −12 | −27 | 5.23 |
| L | AG | −39 | −69 | 42 | 4.82 |
| R | SMG | 60 | −36 | 54 | 4.73 |
| L | ATL | −51 | 9 | −36 | 4.43 |
| TR 6 | |||||
| R | PCC | 6 | −45 | 36 | 9.60 |
| L | PCC | −18 | −48 | 36 | 7.99 |
| R | DLPFC | 30 | 21 | 42 | 9.90 |
| L | DLPFC | −18 | 15 | 42 | 8.23 |
| R | PC | 30 | −72 | −27 | 8.63 |
| R | PreCG | 33 | −30 | 54 | 7.96 |
| R | AG | 39 | −69 | 39 | 7.53 |
| R | MSFG | 12 | 63 | 30 | 7.29 |
| L | IOG | −48 | −72 | −15 | 7.27 |
| R | SMG | 48 | −45 | 36 | 6.84 |
| L | AG | −36 | −69 | 42 | 6.58 |
| R | IFG | 36 | 30 | 18 | 6.16 |
| R | SMA | 6 | 3 | 48 | 6.03 |
| L | PC | −30 | −78 | −33 | 6.02 |
| R | aIfO | 39 | −3 | 0 | 5.17 |
| L | MOG | −24 | −81 | 12 | 5.08 |
| R | SPL | 27 | −60 | 72 | 4.46 |
4. Discussion
The present study investigated the neural correlates of simulations aimed at solving a set of problems in the future. This goal-directed task involves introspective, self-referential processing, and also requires formulating a plan to solve the problem, integrating and sustaining relevant information, and maintaining an abstract sequence of steps leading to the problem's solution. Relative to semantic association, problem-solving simulations were associated with activations in MPFC, PCC, the posterior temporoparietal junction, the inferior parietal lobule, and the middle temporal gyrus, all of which are regions that make up the default network (Buckner, Andrews-Hanna, & Schacter, 2008). This finding is in line with previous research on the function of the default network, showing that it is engaged during internally focused, self-referential mental projections (Buckner & Carroll, 2007). Problem-solving simulations also engaged executive regions, including bilateral DLPFC, which is part of the task-positive network (Badre & D ' Esposito, 2009; Fox et al., 2005; Toro et al., 2008). Compared to semantic association, problem-solving simulations may have triggered more self-referential and spatial processing and required more working memory to keep in mind the scenarios and associated problems. Our seed PLS analysis confirmed that PCC and DLPFC can behave as a functional network: The seeds were functionally connected with a distributed network of regions that consisted of default network and executive regions, both of which appear to be involved in goal-directed simulation. This finding provides novel evidence of coupling between components of the default network and executive regions that have been associated with cognitive control during goal-directed mental simulation.
The seed regions selected for the task-related functional connectivity analysis have been previously identified as important components of their respective networks. PCC, which remained more active throughout the goal-directed simulation task relative to semantic elaboration, connects all of the components of the default network (Hagman et al., 2008). Previous research has suggested that PCC plays an important role in spatial processing (Spreng et al., 2009; Vogt et al., 2006), specifically in generating the context in which scenarios are situated (Szpunar et al., 2009), which represents a feature of the simulation task that clearly distinguishes it from semantic elaboration. Szpunar and colleagues (2009) have argued that PCC serves to reinstate familiar contextual settings from memory and is often coactive with parahippocampal cortex, which was found to covary with PCC in the functional connectivity analysis. When participants in the present experiment were directed to imagine a friend's dormitory room, a local retirement community, a neighborhood, a camping site, and family friend's home, they may have used PCC and parahippocampal cortex to reconstruct familiar contexts for each of those settings from memory.
In addition to the recruitment of PCC, goal-directed simulations were associated with activations of bilateral DLPFC, which were also selected as seed regions. The engagement of DLPFC is most likely attributable to working memory and cognitive control demands of the simulation task, which we would expect to exceed those of the semantic association task. Regions of the prefrontal cortex have been found to support the coordination of actions and thoughts related to internal goals (Koechlin et al., 2003), and DLPFC in particular appears to play an important role in encoding action sequences, coordinating actions in relation to goals, and maintaining abstract sequential movement plans (Badre & D ' Esposito, 2009; Badre et al., 2009). A recent study found that personal goals which were difficult to construct and varied in content, time-frame, emotional valence, and level of abstractness, engaged DLPFC, indicating that DLPFC involvement may be important for generalized goal-directed projection (Packer & Cunningham, 2009). As all of these processes are relevant to problem-solving simulations, they may explain our findings of DLPFC involvement during goal-directed simulation.
MPFC may have become engaged, relative to semantic association, as participants focused on simulating themselves in a situation in the future. A series of previous experiments has associated MPFC with self-referential processing ((D 'Argembeau et al., 2007; Gutchess et al., 2007, 2010; Macrae et al., 2004), and it has been hypothesized to be important for assessing the self-relevance of any type of information or representation (Northoff et al., 2006; Schmitz & Johnson, 2007). MPFC appears to support diverse self-judgments, including judgments about one's personality traits, current mental states, and physical attributes, which are important to assess in order to decide which future actions to take (Jenkins & Mitchell, in press; Seitz et al., 2009). Self-assessment and self-control of actions are critical components of problem-solving. Involvement of MPFC in such mental processes is only enhanced if participants imagine personal as opposed to nonpersonal future goals (D ' Argembeau et al., 2009). In line with all of these previous findings, MPFC was likely engaged by the present paradigm when participants imagined themselves in each given situation, assessed the self-relevance of the provided scenarios, and possibly took into account their current mental state and personality traits while formulating a plan to solve each problem.
As part of the distributed functional network associated with PCC and DLPFC, medial temporal lobe regions were recruited early on during the simulation period. Most interestingly, our analysis showed bilateral hippocampus, which has been implicated in relational processing (e.g., Eichenbaum & Cohen, 2001) and, more specifically, the recombination and encoding of details from disparate past experiences into imagined future events (Schacter & Addis, 2007, 2009). Although the hippocampal system has most often been associated with the encoding or retrieval of memories, there is a growing body of work suggesting that it plays an important role in the ability to envision events in the future (e.g., Buckner, 2010). During the initial phase of the problem-solving simulation, the hippocampus was likely involved in constructing the simulation by helping to piece together new representations based on past experiences (e.g., a time participants tried to get a ring off a finger, were under stress, used soap, etc.) in order to formulate a solution to the problem, and to “pre-experience” the event.
The coupling of PCC and DLPFC during the simulation task provides additional evidence against anticorrelation between the default and the task-positive networks. In most observations of the default network, activity decreases with increasing task-positive network activation (Kelly et al., 2007) and task difficulty (McKiernan et al., 2003). There are very few studies that have reported regions of both networks to be active in parallel. Default network and executive system regions may contribute to insight during creative thinking (Kounios et al., 2006, Kounios et al., 2008; Subramaniam et al., 2009), mind wandering (Christoff et al., 2009), and autobiographical planning (Spreng et al., 2010). Spreng and colleagues showed that while participants formulated plans for their personal future, the default network (e.g., PCC, MPFC, hippocampus) was coactive with regions associated with cognitive control and executive function (e.g., DLPFC, anterior extent of the inferior parietal lobule). In another autobiographical planning task, however, planning personal and non-personal events was only supported by default network regions (D ' Argembeau et al., 2009). Differences between the autobiographical planning tasks may account for the distinct findings, as participants in the latter experiment merely imagined future events that were relevant to personal goals, whereas Spreng et al.'s participants constructed strategic plans to reach desired goal states. Because similar mental processes are required for problem-solving simulations, they may account for the recruitment of executive regions.
Simulating how to solve a problem in the future may be part of a group of cognitive processes that allow task-positive network regions to be coactive with default network regions without suppressing the contribution of either network. Problem-solving simulations and personal planning are both internally focused, self-referential, and directed toward a future goal. People engage in these mental processes frequently across the life span (Gollwitzer, 1999). Further studies will be needed to investigate the range of situations under which regions from both the default and task-positive networks can be engaged simultaneously and cooperatively. Future research will also be required to examine whether anticorrelations between default and task-positive network regions are task-dependent and whether they might differ between intrinsic and task-evoked activity. It is also yet to be determined whether methods of connectivity analysis could influence findings of anticorrelations in task-evoked analyses. Clarification of such conceptual and methodological issues should provide a stronger basis than currently exists for developing a cognitive neuroscience account of how mental simulation contributes to solving future problems.
Supplementary Material
Supplemental Table 1. Peak regions of activation for problem-solving simulation > fixation.
Supplemental Table 2. Peak regions of activation for semantic association > fixation.
Supplemental Figure 1. Problem-solving simulation > fixation, p<.01, FWE-corrected.
Supplemental Figure 2. Semantic association > fixation, p<.01, FWE-corrected.
Research Highlights.
- Mental simulations are often goal-directed and focused on a problem to be solved
- Problem-solving simulations require prospection and cognitive control
- They are supported by both default network and executive regions
- Default network and task-positive network regions behave as a functional network
Acknowledgments
We are grateful to Gagan Wig for his assistance with the initial conceptual design of the study and pilot work. We would also like to thank Amitai Shenhav for his help with SPM8 scripting. This research was supported by NIMH Grant MH060941.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J, Reidler J, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Reidler J, Sepulcre J, Poulin R, Buckner R. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nature Reviews Neuroscience. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney J, D'Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nature Neuroscience. 2009;12:515–522. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desay RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual Review of Psychology. 2010;16:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience. 2009;29:1860–74. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. NeuroImage. 2010;49:865–74. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Science U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments and Computers. 1993;25:257–271. [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laures S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: A PET study. NeuroImage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- D'Argembeau AD, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, et al. The neural basis of personal goal processing when envisioning future events. Journal of Cognitive Neuroscience. 2009;22:1701–1713. doi: 10.1162/jocn.2009.21314. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;16:279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Efron BR, Tibshirani R. The bootstrap method for assessing statistical accuracy. Behaviourmetrika. 1985;7:1–35. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated reseting state brain networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Review Neuroscience. 2001;2:4259–4264. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15:822–837. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48:211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VC, et al. Mapping the structural core of the human cerebral cortex. Public Library of Science Biology. 2008;7:1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience. doi: 10.1080/17470919.2010.507948. in press. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2007;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kuonios J, Fleck JI, Green DL, Payne L, Stevenson JL, Bowden EM, et al. The origins of insight in resting-state brain activity. Neuropsychologia. 2008;46:281–91. doi: 10.1016/j.neuropsychologia.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounios J, Frymiare JL, Bowden EM, Fleck JI, Subramaniam K, Parrish TB, et al. The prepared mind: neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychological Science. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mason M, Norton M, Van Horn J, Wegner D, Grafton S, Macrae C. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatemporal analysis of event-related fMRI data using partial least squares. NeuroImage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murphy K, Rasmus MB, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to Action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. New York: Plenum Press; 1980. pp. 1–18. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage. 2006;15:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA. Neural correlates of reflection on goal states: The role of regulatory focus and temporal distance. Social Neuroscience. 2009;4:412–425. doi: 10.1080/17470910902750186. [DOI] [PubMed] [Google Scholar]
- Patalano AL, Seifert CM. Opportunistic planning: Being reminded of pending goals. Cognitive Psychology. 1997;34:1–36. doi: 10.1006/cogp.1997.0655. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. Journal of Neurophysiology. 2002;87:2577–92. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transaction of the Royal Society of London B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society of London B. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Review Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications The Year in Cognitive Neuroscience. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31:585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Franz M, Anzari NP. Value judgments and self-control of action: The role of the medial frontal cortex. Brain Research Reviews. 2009;60:368–378. doi: 10.1016/j.brainresrev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME. Common blood flow changes across visual tasks 2: Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;8:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory-of-mind and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K, Kounios J, Parrish TB, Jung-Beeman M. A brain mechanism for facilitation of insight by positive affect. Journal of Cognitive Neuroscience. 2009;21:415–432. doi: 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Chan JC, McDermott KB. Contextual processing in episodic future thought. Cerebral Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences U S A. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cerebral Cortex. 2008;18:2553–9. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Peak regions of activation for problem-solving simulation > fixation.
Supplemental Table 2. Peak regions of activation for semantic association > fixation.
Supplemental Figure 1. Problem-solving simulation > fixation, p<.01, FWE-corrected.
Supplemental Figure 2. Semantic association > fixation, p<.01, FWE-corrected.


