Introduction
The putative ‘red blood cell (RBC) hypothermic storage lesion’ is the focus of intense interest and investigation in transfusion medicine . Owing to detrimental changes taking place during a period of up to 42 days, the RBC ability to sustain normal functionality after transfusion can become compromised. While our understanding of the RBC storage lesion has been advanced by a myriad of historical and more recent studies1-10, the clinical significance of transfusing “old blood” remains unclear. In this issue of Transfusion, Yu et al11, discuss the possible outcomes of transfusing stored versus fresh mouse RBCs into syngeneic susceptible hosts, that is mice with diabetes. They demonstrate that transfusion of stored RBCs or supernatants from stored RBCs can induce systemic hypertension and vasoconstriction in mice suffering endothelial dysfunction (diabetic mice), and that transfusion-induced vasoconstriction can be prevented by nitric oxide (NO) inhalation or oxidation of supernatants from stored RBCs. They correlate the hypertensive effects with hemolysis of stored RBCs and NO scavenging by cell-free hemoglobin.
Yu and colleagues’ study supports recent mechanisms for the putative RBC storage lesion related to red cell hemolysis and inactivation of endothelial nitric oxide1,3,12,13 that may in part contribute to transfusion-related multi-organ injury, particularly in the setting of massive transfusion. Their work highlights the interaction between the age of banked blood and the health of the blood recipient (susceptible host), as well as characterizes the potential therapeutic use of NO to ameliorate severe outcomes in patients suffering from endothelial dysfunction caused by transfused blood hemolysis.
Defining the storage lesion: How old is “old blood”?
While there is no consensus on what time defines blood as new or old, a number of studies suggest that major changes occur between 7-14 days of storage. During this period there is a depletion of vital metabolites, particularly, 2,3-diphosphoglycerate (2,3-DPG)14. Interestingly, the average age of RBC units transfused in the US during 2009 was 18.2 days15 meaning that the average unit is transfused at storage duration less than half of its maximum shelf life (42 days). Correspondingly, most retrospective and prospective clinical studies, which evaluate the outcomes of stored RBC transfusion in different patient populations, define “old blood” as greater than 14 days or 21 days, whereas only a small number of studies have evaluated the consequences of transfusing RBC units nearing the maximum shelf life of 42 days (for review see Triulzi and Yazer14).
In addition to ongoing human clinical trials, several animal models have clarified some of the mechanisms associated with adverse reactions to transfusion of “old blood”3,7,11. Such studies simulate transfusion of fresh versus old RBCs (mostly mouse erythrocytes but also human) into syngeneic recipients, and evaluate different aspects of hemolytic response, including nitric oxide scavenging by cell free hemoglobin, hypertension, tissue injury, infections, and inflammation. An advantage of these animal models is the blood recipient severity of illness and the age and volume of transfused blood can be perfectly controlled. An important limitation of these studies is that the definition of old blood is typically the maximum allowable period of time equivalent to 42-day storage of human RBCs. Using this worst-case scenario does not reflect the typical practice used in North American hospitals. The progression of multicenter randomized clinical trials, such as the Red Cell Storage Duration Study (RECESS) and Age of Blood Evaluation (ABLE), may provide a more accurate definition of what should be considered as “old blood” in current practice.
Does transfusion into a susceptible host result in a “double-hit”?
Yu and colleagues’ study11 reveals that syngeneic transfusion of stored mouse RBCs (14-day storage that is equivalent to 42 day storage of human RBCs) can induce systemic hypertension and vasoconstriction in mice suffering endothelial dysfunction (diabetic mice), whereas such responses are not evident in wild type (healthy) mice or mice fed with high fat diet. Yu et al, findings support a “double-hit hypothesis” where adverse reactions to “old blood” transfusion are correlated with the existence of certain pathological conditions in the host. For instance, Hod and colleagues have shown that the presence of sepsis in mice can significantly exacerbate inflammatory reaction to “old blood” transfusion, possibly via enhanced bacterial growth due to consumption of RBC-derived free iron7.
A “double-hit” hypothesis for risk of transfusion of aged stored blood raises fundamental questions regarding the actual risk associated with transfusion of “old blood” including which pathological situations are expected to promote transfusion injury? Which patient populations are at high risk and which, if any, are likely to tolerate” old blood”? These questions also raise ethical issues, such as possible discrimination among patient populations, where some are given higher priority to fresher RBC units while other are subjected to possible risks associated with older RBC units. Risk assessment would require comprehensive studies to characterize the response of different patient populations to old blood transfusion. One approach can use animal models to compare the 24-hour clearance rate of stored RBCs in vivo under various pathological situations such as anemia, sepsis or endothelial dysfunction. If proven relevant, RBC storage duration may become a significant factor that cannot be overlooked in the case of transfusing susceptible hosts.
Mechanisms for the “double-hit”: Red cell hemolysis in a hostile environment?
While our primary concern during transfusion is the well being of the patient, we tend to disregard the fact that the patient’s circulation can be a hostile environment for transfused RBCs. In other words, intravascular hemolysis of transfused RBCs may be induced by factors related to the patient rather than the RBCs. Oxidative stress and inflammation have been associated with several pathological conditions including obesity, insulin resistance, hypertension, and coronary artery disease16,17. Elevated levels of reactive oxygen species in the patient’s circulation can compromise RBC membrane integrity via oxidation leading to changes in rheological properties and possibly hemolysis. Likewise, increased expression of endothelial adhesion molecules, related to systemic inflammatory or oxidative stress, can increase red cell-endothelial shear and hemolysis18. In addition, drug treatments could affect the survival of transfused RBCs in the circulation, as some xenobiotics, such as phenylhydrazine or aniline, are redox active agents that are capable of inducing hemolysis through oxidative denaturation of the hemoglobin19,20.
Interestingly, Holtom and colleagues18 have demonstrated enhanced formation of RBC-derived membrane microparticles in response to incubation of fresh whole blood with inflamed endothelium under flow. These studies revealed the capacity of inflamed endothelial cells to induce membrane damage in fresh RBCs. Prolonged hypothermic storage may render RBCs even more susceptible to membrane injury under similar conditions, and may explain the higher rate of clearance of stored RBCs from the circulation. RBC-derived microparticles can promote transfusion injuries as hemoglobin entrapped within can enhance nitric oxide scavenging leading to vasoconstriction3. Other mechanisms are related to pro-inflammatory effects and interactions with platelets21.
Evaluating hemolytic propensity: Are all red cell donors the same?
The current selection criteria for blood donors have significantly reduced the risk of transmitting infectious diseases or inducing immune responses in patients requiring blood transfusion. Other regulations ensure that RBC units with greater than 1 % hemolysis will be discarded, thereby protecting patients from hemolysis-mediated transfusion injury. Despite the regulations and selection of what are believed to be “healthy donors”, our research group and others have observed that specific donor RBC units are more likely to hemolyze during storage while others hardly hemolyze at all (for instance, Donadee et al3, reported free heme concentrations in the range of 55 μmol/L to 100 μmol/L in RBC units stored for 39 days). This interesting phenomenon has motivated a new field of research in transfusion medicine that is investigating the genetic and molecular basis of donor red blood cell hemolysis22,23.
For example, it is now apparent that the donor’s gender may relate to the propensity of RBCs to hemolyze during storage and under various stress conditions. Raval and colleagues24 have demonstrated that premenopausal female gender is associated with less RBC hemolysis during experimental mechanical stress. We have reported gender differences in human and mouse models where female RBCs hemolyzed less than males in response to osmotic stress (human and mouse), mechanical stress (human), and oxidative stress (mouse)22,23. These studies suggest that female sex hormones may enhance membrane integrity by protecting against mechanical24 or osmotic stress25. Other studies in progress evaluate whether having a genetic trait associated with hemolytic disorders (i.e. donors who are heterozygous to sickle cell disease, thalassemia, or glucose-6-phosphate dehydrogenase (G6PD) deficiency) renders RBCs more susceptible to storage hemolysis. Since most countries do not screen blood donors for such traits, a large number of donors with no history of disease are not aware that they are carriers of hemolytic disorders.
In addition to genetic background, RBC characteristics can be affected by the donor’s lifestyle. A recent survey of over one million blood donors in the US during the years 2007-2008 revealed that 25 % of donors were obese (body mass index ≥ 30.0 kg/m2)26. The correlation between obesity and hemolytic propensity is yet to be determined,although the high risk of cardiovascular disease and oxidative stress associated with obesity could potentially compromise RBC functionality. Understanding the molecular mechanisms of hemolysis could improve the process of donor screening and may reduce storage hemolysis, although subsequent studies should clarify whether these observations are clinically significant.
Inhaled nitric oxide: NO more storage problems?
Recently, Donadee et al,3 provided insight to the critical role that hemolysis plays in promoting hypertension via NO scavenging by cell-free hemoglobin and red cell microparticles. Yu and colleagues’ study11 supports these finding by showing that the hypertensive reactions to transfusion of “old blood” are correlated with increased levels hemolysis, and accumulation of free or microparticle-encapsulated hemoglobin in the supernatant. They further showed that hemolysis-mediated hypertension could be prevented by treating hypertensive animals with inhaled NO. Similarly, studies by Roback and colleagues27 have demonstrated the ability of stored RBCs (28-42 days) to antagonize NO-mediated vasodilation in rat aortic ring models. The correlation between hemolysis-mediated NO depletion and transfusion injuries, particularly in patients suffering endothelial dysfunction, has encouraged the investigation of methods to improve NO bioavailability in the circulation. For example, Hu and colleagues28 have demonstrated that enhancing NO-dependent signaling via Sildenafil treatment in mice suffering pulmonary arterial hypertension (PAH) reduced platelet activation, thrombosis and even mortality, which were induced by acute hemolysis and NO depletion by free hemoglobin.
The potential use of NO for therapeutics has gained growing attention over the last decade with myriad human and animal model studies demonstrating the positive effects of NO treatments in various pathological situations (for reviews see Lundberg et al,29,30). Administration of NO precursors, nitrite and nitrate, or NO gas inhalation has been proven effective in protecting against ischemia reperfusion injury, stomach ulcers, myocardial infarction, and bacterial infection in mice and other animal models30. In regard to transfusion, the use of NO therapeutics for restoring normal NO signaling and blood flow after transfusion could be highly relevant, as demonstrated by Yu et al,11.
Further Thoughts
In this editorial we highlight new insights into the significance of red cell donor genetics, red cell storage time, the susceptible host, and the number of units transfused that may all interact to mediate adverse reactions in critically ill patients. These new studies also highlight the use of NO therapeutics, specifically inhaled NO gas, to improve NO bioavailability. Despite signigicant progress, numerous issues require further investigation, such as better definition of a storage duration associated with higher risks of transfusion injuries, characterization of patient populations likely to be susceptible to transfusion of “old blood”, and which genetic factors contribute to hemolytic propensity. Fortunately, several large research projects, such as RECESS and ABLE, have been established to address the issue of RBC storage duration and transfusion injuries. Additionally, a growing number of studies are aimed at characterizing the molecular and genetic basis of hemolysis. Future resolution of these fundamental issues could improve the process of donor screening, and reduce storage hemolysis as well as transfusion-related injuries.
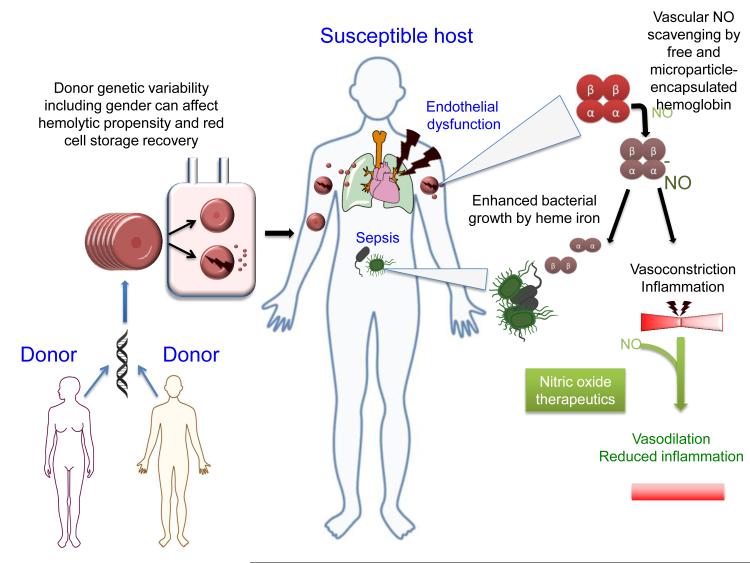
Figure 1.
Donor genetic variation including gender can affect hemolytic propensity and red cell storage recovery. The red cell storage lesion can involve modifications in membrane structure leading to the release of microparticles and hemolysis. Massive transfusions of red cell units or transfusion into patients suffering endothelial dysfunction, infection, and other pathological situations (susceptible hosts) can exacerbate inflammation, vasoconstriction and hypertension leading to multiple organ dysfunction syndrome (MODS). Additionally, hemolysis can exacerbate sepsis by the release of iron from denatured hemoglobins. Nitric oxide therapeutics can potentially mitigate transfusion-related injuries in patients suffering endothelial dysfunction by improving NO bioavailability required for vasodilation and proper endothelial function.
Acknowledgments
Research support: Dr. Gladwin receives research support from NIH grants R01HL098032, RO1HL096973, and PO1HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
Conflict of interest: Dr. Gladwin is listed as a co-inventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Aires Pharmaceuticals on the development of a phase II proof of concept trial using inhaled nitrite for pulmonary arterial hypertension. TK has no conflict of interest.
References
- 1.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler E, Kuhl W, West C. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood. 1982;59:1141–7. [PubMed] [Google Scholar]
- 3.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrio BW, Finch CA. Erythrocyte preservation. I. The relation of the storage lesion to in vivo erythrocyte senescence. J Clin Invest. 1954;33:242–6. doi: 10.1172/JCI102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–23. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 6.Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non–transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangalmurti NS, Chatterjee S, Cheng G, Andersen E, Mohammed A, Siegel DL, Schmidt AM, Albelda SM, Lee JS. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion. 2010;50:2353–61. doi: 10.1111/j.1537-2995.2010.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparrow RL, Healey G, Patton KA, Veale MF. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci. 2006;34:15–23. doi: 10.1016/j.transci.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03473.x. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–79. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–47. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker BI, Schlumpf WK, Schulman J, Green J. Report of the US Department of Health and Human Services. The 2009 national blood collection and utilization survey report. US Department of Health and Human Services, Office of the Assistant Secretary for Health; Washington, DC: 2011. [Google Scholar]
- 16.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Holtom E, Usherwood JR, Macey MG, Lawson C. Microparticle formation after co-culture of human whole blood and umbilical artery in a novel in vitro model of flow. Cytometry Part A. 2011 doi: 10.1002/cyto.a.22010. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 19.Nagababu E, Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal. 2004;6:967–78. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 20.Winterbourn CC. Free-radical production and oxidative reactions of hemoglobin. Environ Health Perspect. 1985;64:321–30. doi: 10.1289/ehp.8564321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong Z, Cavaretta J, Qu L, Stolz DB, Triulzi D, Lee JS. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–21. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanias T, Lee J, Yazer MH, Triulzi DJ, Lippert A, Gladwin MT. Correlation between female gender and the red blood cell propensity to hemolyze under various stresses. ASH Annual Meeting Abstracts.2011. p. 2325. [Google Scholar]
- 23.Kanias T, Lee JS, Raval JS, Yazer MH, Gladwin MT. Gender differences in the hemolytic propensity of human and mouse red blood cells AABB anual meeting abstracts (Transfusion).2011. pp. 1A–236A. [Google Scholar]
- 24.Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Kameneva MV, Yazer MH. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–21. doi: 10.1111/j.1423-0410.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- 25.DeVenuto F, Wilson SM. Distribution of progesterone and Its effect on human blood during storage. Transfusion. 1976;16:107–12. doi: 10.1046/j.1537-2995.1976.16276155103.x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy EL, Schlumpf K, Wright DJ, Cable R, Roback J, Sacher R, Busch MP. BMI and obesity in US blood donors: a potential public health role for the blood centre. Public Health Nutr. 2012:1–8. doi: 10.1017/S1368980011003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roback JD. Vascular effects of the red blood cell storage lesion. Hematology Am Soc Hematol Educ Program. 2011;2011:475–9. doi: 10.1182/asheducation-2011.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116:1613–22. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr., Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–9. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]