Abstract
“Use it or lose it” is a popular adage often associated with use-dependent enhancement of cognitive abilities. Much research has focused on understanding exactly how the brain changes as a function of experience. Such experience-dependent plasticity involves both structural and functional alterations that contribute to adaptive behaviors, such as learning and memory, as well as maladaptive behaviors, including anxiety disorders, phobias, and posttraumatic stress disorder. With the advancing age of our population, understanding how use-dependent plasticity changes across the lifespan may also help to promote healthy brain aging. A common misconception is that such experience-dependent plasticity (e.g., associative learning) is synonymous with synaptic plasticity. Other forms of plasticity also play a critical role in shaping adaptive changes within the nervous system, including intrinsic plasticity – a change in the intrinsic excitability of a neuron. Intrinsic plasticity can result from a change in the number, distribution or activity of various ion channels located throughout the neuron. Here, we review evidence that intrinsic plasticity is an important and evolutionarily conserved neural correlate of learning. Intrinsic plasticity acts as a metaplasticity mechanism by lowering the threshold for synaptic changes. Thus, learning-related intrinsic changes can facilitate future synaptic plasticity and learning. Such intrinsic changes can impact the allocation of a memory trace within a brain structure, and when compromised, can contribute to cognitive decline during the aging process. This unique role of intrinsic excitability can provide insight into how memories are formed and, more interestingly, how neurons that participate in a memory trace are selected. Most importantly, modulation of intrinsic excitability can allow for regulation of learning ability – this can prevent or provide treatment for cognitive decline not only in patients with clinical disorders but also in the aging population.
Keywords: learning, memory, metaplasticity, intrinsic excitability, aging, memory modulation, memory allocation
1. Introduction
Neural pathways are plastic and continuously changing in response to internal and external stimuli. These changes can occur at synaptic as well as non-synaptic sites throughout the neuron. The non-synaptic (intrinsic) plasticity can be described as a change in the intrinsic excitability of the neuron and is independent of changes in synaptic transmission. Intrinsic plasticity has been examined in numerous animal models using a wide variety of learning paradigms. Many of these changes are learning-specific and require the same pathways as the substrate of synaptic and behavioral plasticity. Furthermore, intrinsic changes may impact future learning, indicating the involvement of a metaplasticity mechanism. Metaplasticity develops as a result of a series of time-dependent events. That is, an initial priming event first induces physiological or biochemical changes in neurons or synapses that can modulate plasticity induced by a subsequent event (e.g. low- or high-frequency stimulation, or learning, see Abraham and Bear, 1996). In this review, we will briefly examine several forms and basic mechanisms involved in intrinsic plasticity, followed by a discussion of the reciprocal interactions between intrinsic excitability and memory formation. Special emphasis will be placed on recent studies that support the role of intrinsic plasticity in modulation of the strength and allocation of new memories and how this ability is altered during aging. Evidence from these studies will be used to establish intrinsic plasticity as a metaplasticity mechanism that influences memory formation.
1.1. Plasticity: Forms and functions
Neural plasticity can serve a multitude of functions (Kim and Linden, 2007). First, plasticity could be homeostatic in nature, resulting in restoration of overall firing rates or excitability within a network (Turrigiano and Nelson, 2004). Second, it could be mnemonic, in that it contributes to or forms the basis of the memory trace or engram (Sigurdsson, Doyere, Cain, and LeDoux, 2007). Third, it could serve as a metaplasticity mechanism – a higher-order plasticity that affects lower-order synaptic or intrinsic plasticity (Abraham, 2008). Such metaplastic changes could serve to regulate future experience-dependent plasticity and thus impact behavioral plasticity.
In addition to serving many functions, neural plasticity can be achieved in remarkably diverse ways (Marder and Goaillard, 2006). A considerable proportion of these plastic mechanisms affect non-synaptic or intrinsic properties of neurons. Intrinsic plasticity is not only observed following a variety of behavioral paradigms, but it is phylogenetically conserved (see section 2), which highlights its role in behavioral plasticity. Although synaptic plasticity has received much attention as a mechanism for memory formation (Mayford, Siegelbaum, and Kandel, 2012), it has become increasingly clear that an exclusively synaptic model for memory storage is unlikely and that intrinsic plasticity also plays a critical role in learning and memory (Frick and Johnston, 2005; Song, Detert, Sehgal, and Moyer, 2012; Zhang and Linden, 2003).
Intrinsic plasticity can result from changes in the number or activation of various ion channels. Based on the location of these ion channels, intrinsic plasticity could be local (i.e., limited to a small portion of the dendrite) or global (i.e., somatic, including larger portions of proximal dendrites, thus impacting input from many synapses). Here, we provide a brief description how plasticity of various intrinsic properties affects flow of information within a neuron by following the course of synaptic inputs from the dendrite to the axon terminal.
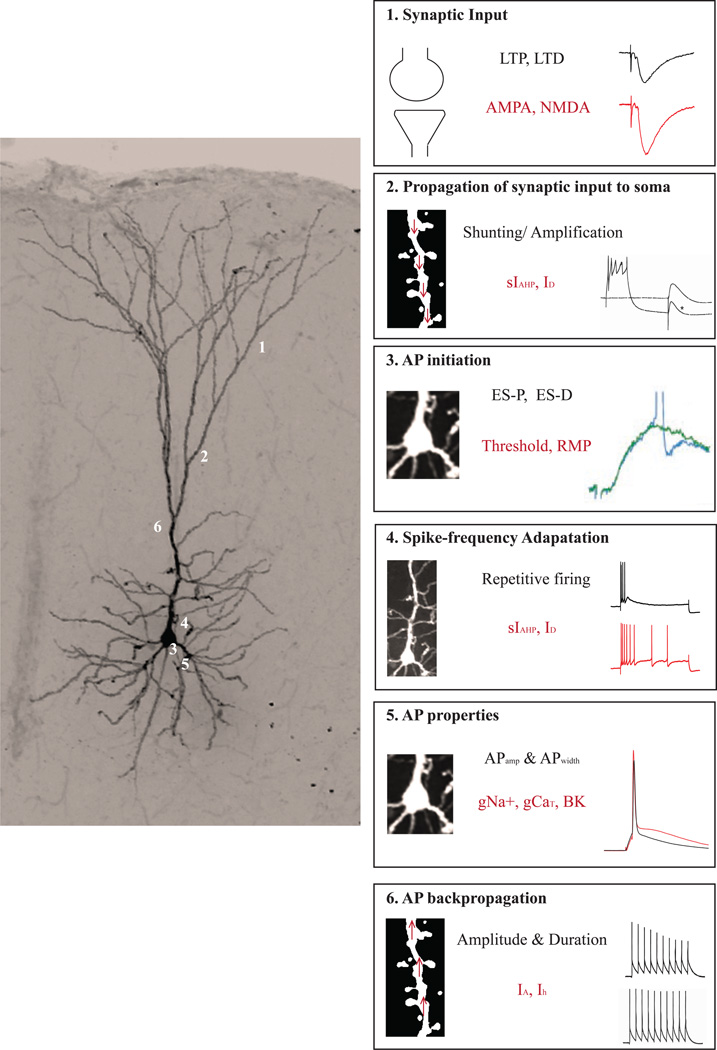
Intrinsic plasticity (dendritic or somatic) has been linked to modulation of synaptic plasticity and vice versa. Modulation of dendritic intrinsic excitability can regulate the throughput of synaptic transmission in various ways (see Figure 1). First, it can have consequences for the dendritic integration processes that influence degradation of synaptic signals (see Figure 1, Panel 2; also see Spruston, 2008, for an excellent review of how dendritic properties can affect synaptic integration in pyramidal neurons). For example, in hippocampal CA1 pyramidal neurons, repetitive firing activates the slow afterhyperpolarization current (sIAHP, see Section 1.2 for a description of the AHP, Hotson and Prince, 1980; Lancaster and Adams, 1986; Storm, 1989), which hyperpolarizes the somatic and proximal dendritic membrane potential (Sah and Bekkers, 1996). Interestingly, activation of the sIAHP reduces the amplitude of EPSPs arising from stimulation of the apical dendritic tree (Sah and Bekkers, 1996). Furthermore, inhibition of the sIAHP reduces the threshold for LTP induction in CA1 neurons (Cohen, Coussens, Raymond, and Abraham, 1999; Sah and Bekkers, 1996). Thus, the sIAHP can act as an adjustable gain control mechanism, influencing the ability of synaptic signals from dendrites to reach the soma. Similar effects have been observed in the amygdala as well as the medial prefrontal cortex following modulation of the sIAHP (Faber, Delaney, and Sah, 2005; Power, Bocklisch, Curby, and Sah, 2011; Zaitsev and Anwyl, 2012). Thus, intrinsic plasticity can alter the integration of synaptic inputs, which in turn impacts action potential generation, and neuronal output.
Figure 1. Synaptic and intrinsic properties shape neuronal information processing.
Left panel depicts a medial prefrontal cortical neuron filled with biocytin during whole-cell patch clamp recording and imaged using confocal microscopy (Olympus FV1200). Numbers 1, 2, 3, 4, 5 and 6 refer to the boxes in the right panel. (1) A vast majority of neuronal input originates on the dendritic spines with smaller contributions from synapses that are made on the dendrites, soma and axons. Synaptic inputs can undergo bidirectional plasticity in the form of LTP and LTD by modulation of AMPA and NMDA receptor-mediated transmission. (2) Propagation of the synaptically generated signal (EPSP) depends upon the active and passive dendritic properties including ionic conductances that contribute to the afterhyperpolarization (AHP). (3) Once the signal reaches the soma, neuronal output is determined based on factors like AP initiation threshold, resting membrane potential, etc…, which in turn rely on ion channels within the soma. (4) Bidirectional plasticity impacting the coupling of EPSPs to spikes is referred to as ES-P and ES-D. The number of APs generated following sustained stimulation (spike frequency adaptation) can also code relevant information, and relies upon K+ conductances, including those that underlie the AHP. (5) In addition, properties like amplitude and duration of APs can also modulate pre- and postsynaptic aspects of neuronal processing like neurotransmitter release and bAPs. (6) The magnitude and travel distance of bAPs can be influenced by IA currents, which can ultimately modulate Ca2+ influx into the dendritic compartment. Abbreviations: long term potentiation, LTP; long term depression, LTD; excitatory postsynaptic potential, EPSP; afterhyperpolarization, AHP; action potential, AP; EPSP-to-Spike coupling potentiation, ES-P; EPSP-to-Spike coupling potentiation, ES-D; backpropagating APs, bAPs. Electrophysiological traces in boxes 2, 3, 5 and 6 were adapted from Sah and Bekkers (1996), Daoudal et al. (2002), Deng et al. (2013) and Tsubokawa et al. (2000) respectively, with permission.
Better transmission of synaptic inputs to the soma is evident as an enhanced ability of an EPSP to generate an action potential (AP), referred to as EPSP-to-spike (ES) coupling or ES potentiation (Bliss and Lomo, 1973). As shown in Figure 1, Panel 3, ES coupling can undergo bidirectional plasticity following induction of long-term potentiation or depotentiation (Daoudal, Hanada, and Debanne, 2002). Furthermore, environmental enrichment can also enhance ES coupling (Malik and Chattarji, 2012). Although ES plasticity can result from changes in the balance between inhibitory and excitatory synaptic drive, changes in neuronal intrinsic excitability also contribute to ES plasticity (see Daoudal and Debanne, 2003, for review). Thus, intrinsic plasticity in the form of changes in the active properties of dendrites can shape synaptic signals significantly, and thus impact ES coupling.
Once the synaptic inputs reach the soma, various intrinsic factors can contribute to AP initiation, including modulation of AP threshold or local membrane potential. In addition to the all-or-none firing of an AP, efficient relay of neuronal information may require repetitive AP firing (see Figure 1, Panel 4). For example, in working memory tasks such persistent neuronal firing is critical for maintaining representations across time, and reduced excitability in the form of greater spike frequency adaptation may limit working memory performance (Durstewitz, Seamans, and Sejnowski, 2000).
Single AP characteristics also contribute to neuronal excitability (see Figure 1, Panel 5). AP amplitude and half-width are plastic intrinsic properties (Varela, Wang, Christianson, Maier, and Cooper, 2012) that can influence the duration and extent of Ca2+ influx at the presynaptic terminal (Deng, Rotman, Blundon, Cho, Cui, Cavalli, Zakharenko, and Klyachko, 2013). In addition, when a neuron fires an action potential, the AP can backpropagate into portions of the dendritic tree, which can be influenced by changes in local dendritic excitability (see Figure 1, Panel 6; Frick, Magee, and Johnston, 2004). Such backpropagating APs (bAPs) are associated with Ca2+ influx into the dendritic compartments (Larkum, Zhu, and Sakmann, 1999) and are important for LTP induction (Sjostrom and Hausser, 2006). Moreover, LTP induction enhances local dendritic excitability through modulation of A-type K+ channels and results in an input-specific increase in bAP amplitude (Frick et al., 2004). Thus, APs and bAPs represent yet another example of how intrinsic neuronal excitability is closely associated with synaptic throughput and plasticity in the brain.
1.2. Mechanisms of intrinsic plasticity: Change beyond the synapse
While many neuronal components are involved in intrinsic plasticity (see Section 1.1.), the current review largely focuses on plasticity of the afterhyperpolarization (AHP) and spike frequency adaptation (as discussed in Section 2). The AHP is a hyperpolarizing current that follows a burst of action potentials and limits action potential firing (Hotson and Prince, 1980). Spike frequency adaptation refers to the process by which the instantaneous firing of a neuron gradually slows over time in response to sustained excitation (e.g. see Madison and Nicoll, 1984). In CA1 neurons, spike frequency adaptation is heavily influenced by the AHP (although other currents are also involved). When the AHP is small, spike frequency adaptation is also reduced, meaning that a sustained depolarization can now evoke more action potentials.
The AHP is influenced by several underlying currents mediated by Ca2+-activated K+ channels. There are several phases of the AHP, including fast, medium, and slow AHP (for an excellent review see Storm, 1990). These are evoked as a result of action potential-elicited K+ currents, including: 1) a voltage- and Ca2+-dependent current (IC); 2) a voltage-dependent, muscarine-sensitive current (IM); 3) a Ca2+-dependent and apamin-sensitive current (IAHP); and 4) a Ca2+-dependent apamin-insensitive current (sIAHP; Gasparini and DiFrancesco, 1999; Sah, 1996; Stocker, Krause, and Pedarzani, 1999; Storm, 1989). The fast AHP is modulated by changes in IC; the medium AHP is modulated by changes in IC, IM, and the apamin-sensitive IAHP; the slow AHP is modulated by changes in the apamin-insensitive sIAHP (Gasparini and DiFrancesco, 1999; Sah, 1996; Stocker et al., 1999; Storm, 1989). Although learning-related modulation is possible for all three phases of the AHP (e.g. see Matthews, Linardakis, and Disterhoft, 2009; Matthews, Weible, Shah, and Disterhoft, 2008; Santini, Quirk, and Porter, 2008 for learning-realted changes in fast AHP), the current review will focus mostly on learning-related changes in the medium and slow AHP for two reasons: (1) learning-related changes in slow AHP are more extensively described, and (2) the authors believe that a description of learning-related slow AHP changes is adequate to support the central hypothesis of this review.
2. Intrinsic excitability: A substrate for learning
Learning involves a change in behavior in response to environmental stimuli, and it depends critically on plasticity within the nervous system. Learning-related changes include modulation of synaptic and non-synaptic (intrinsic) ion channels and receptors, dendritic branching, spine density, and plasticity through genetic and epigenetic mechanisms (Bosch and Hayashi, 2012; Mayford et al., 2012; Zovkic, Guzman-Karlsson, and Sweatt, 2013). This section summarizes the learning-related changes in the intrinsic firing properties of neurons. Intrinsic excitability changes following learning have been demonstrated in various brain regions and are often accompanied by a reduction in the AHP, a decrease in spike frequency adaptation, which leads to an increase in neuronal firing. Finally, evidence that links aging-related deficits in learning with failure to modulate intrinsic excitability is presented.
2.1. Early inroads
Early demonstrations of behaviorally induced intrinsic plasticity were observed in both invertebrate and vertebrate preparations (Alkon, 1979; Disterhoft, Coulter, and Alkon, 1986; Woody and Black-Cleworth, 1973). For example, Woody and colleagues classically conditioned cats to associate an auditory click with a glabella tap, which normally evokes an eyeblink and nose-twitch response. Following behavioral training, a reduction in rheobase (minimum current required to elicit an AP) is observed for cells projecting to the musculature involved in the learned blink-plus-twitch response (Woody and Black-Cleworth, 1973). Likewise, early research in the nudibranch Hermissenda crassicornis demonstrated that repeated pairings of light with rotation lead to reduced phototaxis (Alkon, 1974). Interestingly, following learning, excitability of the Hermissenda type B photoreceptor cell was increased with a concomitant decrease in outward K+ currents (IA and a Ca2+-sensitive K+ current; Alkon, 1979). This line of research was then extended by observations of reduced AHPs in rabbit hippocampal neurons following eyeblink conditioning (Disterhoft et al., 1986). These landmark studies were followed by a range of studies using both vertebrate and invertebrate preparations to investigate the role of intrinsic plasticity in learning. Although there is an extensive literature on learning-induced intrinsic plasticity in invertebrates (see Mozzachiodi and Byrne, 2010 for a review), for the sake of brevity, the rest of this review will focus on vertebrate studies.
2.2. Learning-related intrinsic plasticity: Vertebrate studies
Pavlovian conditioning is a powerful model system for studying the neural correlates of associative learning in a wide range of invertebrate and vertebrate preparations (Freeman and Steinmetz, 2011; Hawkins, Kandel, and Bailey, 2006; Johansen, Cain, Ostroff, and LeDoux, 2011). Conditioning involves pairing the presentation of a neutral conditioned stimulus (CS) with presentation of an unconditioned stimulus (US), which elicits an unconditioned response (UR). As learning occurs, when the CS is presented a conditioned response (CR) emerges, which can be observed in the absence of the US. There are two basic Pavlovian conditioning paradigms: delay and trace. In delay conditioning, the CS turns on and remains on while the US is presented. There is a “delay” between onset of the CS and onset of the US, which can influence how quickly learning occurs (Thompson, and Disterhoft, 1996). In trace conditioning, onset and offset of the CS is followed by a stimulus-free trace interval prior to onset of the US. This trace interval requires the animal to maintain a memory “trace” of the CS since it is no longer present during the US. Interestingly, these variations in the temporal relationship between the CS and US influence which brain structures/circuits are ultimately required for learning to occur.
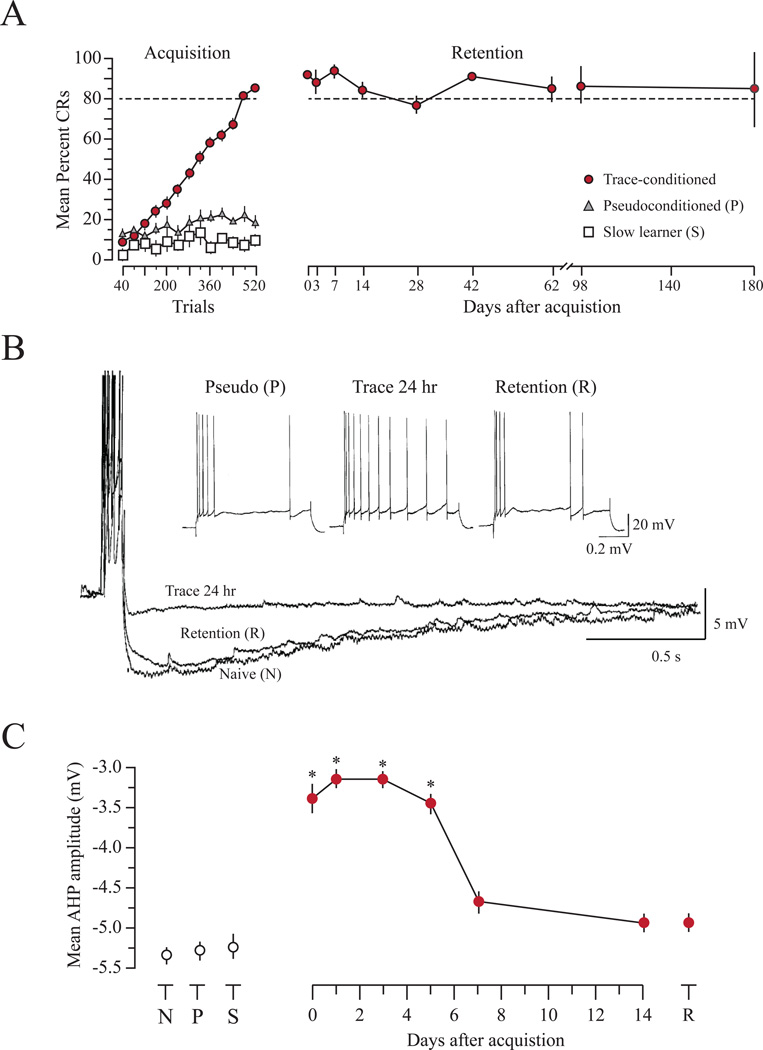
Eyeblink conditioning has been one of the most widely used model systems to study basic mechanisms of learning and memory (for review, see Christian and Thompson, 2003). While acquisition of both delay and trace paradigms involves core brainstem-cerebellar circuitry (Weiss and Disterhoft, 2011), acquisition of the trace paradigm also requires higher brain regions, including hippocampus (Moyer, Deyo, and Disterhoft, 1990; Solomon, Vander Schaaf, Thompson, and Weisz, 1986). Numerous studies have demonstrated that acquisition of eyeblink conditioning involves alterations to intrinsic neuronal excitability within these circuits. The first seminal report was from a study by John Disterhoft and Dan Alkon, where they demonstrated that the postburst AHP was significantly reduced in CA1 neurons following delay eyeblink conditioning (Disterhoft et al., 1986). This represented an increase in the intrinsic excitability since the AHP reductions were observed in the absence of synaptic activity (Coulter, Lo Turco, Kubota, Disterhoft, Moore, and Alkon, 1989). Similar changes have also been observed in hippocampal CA1 and CA3 neurons following trace eyeblink conditioning (de Jonge, Black, Deyo, and Disterhoft, 1990; Moyer, Thompson, and Disterhoft, 1996; Oh, McKay, Power, and Disterhoft, 2009; Thompson, Moyer, and Disterhoft, 1996). These changes are typically evident as reductions in the sAHP as well as spike frequency adaptation (see Figure 2). Such changes are not only learning-specific (i.e. they are not observed in pseudoconditioned controls), but they are also transient, suggesting that they may be important for acquisition and consolidation of the learned response (Moyer et al., 1996; Thompson et al., 1996). Although the vast majority of these in vitro studies have been performed using hippocampal brain slices, comparable findings have been reported within the cerebellum where the intrinsic excitability of Purkinje neurons is increased for up to 1 month following training (Schreurs, Gusev, Tomsic, Alkon, and Shi, 1998).
Figure 2. Learning-related intrinsic plasticity in hippocampal neurons is transient while memory persists.
A, The left panel (ACQUISITION) shows the normalized learning curves for trace conditioned, pseudoconditioned and slow-learning rabbits. Trace-conditioned rabbits reach criterion performance (>80% correct responses; dashed line) in ~ 7 sessions and maintain behavioral performance over an extended period of time, right panel (RETENTION). The pseudoconditioned and the slow-learning rabbits never reach criterion. B, Representative traces demonstrating reductions in the postburst AHP of a CA1 neuron from a trace-conditioned rabbit 24hr after initial learning (Trace 24 hr) relative to a neuron from a naive rabbit (Naive) and a rabbit that received an additional training session 14 d later (Retention). Inset: Examples of typical spike frequency adaptation responses in CA1 pyramidal cells from Trace 24 hr and Retention rabbits along with a rabbit 24 hr after pseudoconditioning (Pseudo). C, Learning-related reductions of the AHP amplitude were transient, lasting ~5 days. These changes are learning-specific and are not observed in naive (N), pseudoconditioned (P), or slow-learning (S) rabbits. * p < 0.001. Adapted fromMoyer et al. (1996) with permission.
Pavlovian fear conditioning has also been extensively used to study the neurobiology of emotional learning (LeDoux, 2000). Here a neutral CS, such as a tone or an odor is paired with an aversive US, such as a footshock. While delay and trace fear conditioning paradigms require the amygdala for acquisition and expression of the conditioned fear memory (for review see Davis, 2004; LeDoux, 2000; Otto, Cousens, and Herzog, 2000), trace fear conditioning also requires other higher brain regions, including hippocampus (Bangasser, Waxler, Santollo, and Shors, 2006; Kesner, Hunsaker, and Gilbert, 2005; Quinn, Oommen, Morrison, and Fanselow, 2002; Suh, Rivest, Nakashiba, Tominaga, and Tonegawa, 2011). As with eyeblink studies, trace fear conditioning also enhances the intrinsic excitability of CA1 pyramidal neurons (Kaczorowski and Disterhoft, 2009; McKay, Matthews, Oliveira, and Disterhoft, 2009; Song et al., 2012). These changes in excitability are evident as reductions in the sAHP and reduced spike frequency adaptation. Furthermore, following conditioning the behavioral performance (percent freezing) is significantly correlated with the size of the post-burst AHP in conditioned rats, but not in pseudoconditioned rats, suggesting the enhancement in intrinsic excitability is learning-specific (Song et al., 2012).
Intrinsic plasticity is also important for extinction, which is a form of learning in which repeated presentations of the CS (in the absence of the US) leads to a decrease in the CR. Extinction of conditioned fear requires a variety of structures, including the amygdala, hippocampus, and medial prefrontal cortex (mPFC, Sierra-Mercado, Padilla-Coreano, and Quirk, 2011). Within mPFC, an increase in infralimbic cortex (IL) activity is critical for inhibiting conditioned fear responses and facilitating extinction memory (Burgos-Robles, Vidal-Gonzalez, Santini, and Quirk, 2007; Chang and Maren, 2011; Milad and Quirk, 2002; Milad, Vidal-Gonzalez, and Quirk, 2004; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, and Quirk, 2006). Recent in vitro studies suggest that fear conditioning decreases the intrinsic excitability of IL neurons whereas extinction reverses this effect (Santini et al., 2008). Although the circuitry is more complicated, it is clear that acquisition and extinction can modulate the intrinsic excitability of mPFC neurons in a bidirectional manner.
Synaptic plasticity within the lateral amygdala (LA) is critical for fear learning (McKernan and Shinnick-Gallagher, 1997; Rogan, Staubli, and LeDoux, 1997; Rumpel, LeDoux, Zador, and Malinow, 2005). Following fear conditioning, LA neurons are not only more responsive (fire more action potentials) to the CS (Maren, 2000; Quirk, Armony, and LeDoux, 1997; Quirk, Repa, and LeDoux, 1995; Repa, Muller, Apergis, Desrochers, Zhou, and LeDoux, 2001), but they also discharge more synchronously (Pare and Collins, 2000). These changes may involve both synaptic and intrinsic plasticity. Rosenkranz and Grace (2002) investigated the effect of olfactory fear conditioning on LA neurons in anesthetized rats and found that odor-elicited post synaptic potentials were significantly enhanced. Furthermore, an enhancement of intrinsic excitability was also observed – higher input resistance, reduced rheobase, and a shortening of the rise and decay of membrane time constants (Rosenkranz and Grace, 2002). Thus, these studies suggest that both synaptic and intrinsic plasticity accompany acquisition of fear conditioning in LA neurons.
Despite the early evidence for the presence of intrinsic plasticity within the LA, learning-related intrinsic plasticity in LA has received little attention. A recent study has examined the time course of intrinsic plasticity following fear conditioning in LA neurons using in vitro recordings (Sehgal, Girgis, and Moyer, 2012). Fear conditioning leads to a reduction in the sAHP and spike frequency adaptation 24 hrs following conditioning. However, when tested immediately post-conditioning, the sAHP was reduced but spike-frequency adaptation remained unchanged, suggesting a possible dissociation between the learning-related AHP reduction and spike-frequency adaptation with learning (Sehgal et al., 2012). Such a dissociation between the time course of learning-related changes in the sAHP and spike frequency adaptation is consistent with other studies (e.g. Motanis, Maroun, and Barkai, 2012; Moyer et al., 1996) indicating the sAHP may regulate spike frequency adaptation in concert with other ionic conductances.
In addition to LA, the basolateral nucleus of amygdala (BLA) is also critical for fear memory retrieval (Anglada-Figueroa and Quirk, 2005; Kwapis, Jarome, Schiff, and Helmstetter, 2011). Interestingly, the intrinsic excitability of BLA neurons was reduced following olfactory fear conditioning (Motanis et al., 2012). This effect was evident as an enhancement in spike frequency adaptation, but was independent of sAHP modulations. This raises the interesting possibility that within the amygdala there appears to exist sub-region specific, differential modulation of intrinsic neuronal excitability.
Of course, learning-related modulation of intrinsic excitability is not limited to classical conditioning paradigms, as operant conditioning can also modulate intrinsic excitability. A series of studies from Edi Barkai’s group utilized an operant olfactory discrimination paradigm, which required an animal to differentiate a pair of odors in order to receive a reward. Rats acquire this type of learning in a few days, and learning to differentiate one pair of odors facilitates learning to differentiate other pairs of odors – a phenomenon called “rule learning” (Saar, Grossman, and Barkai, 1998; 1999). Rule learning enhances intrinsic excitability in the piriform cortex (Saar et al., 1998), BLA (Motanis et al., 2012) and hippocampus (Zelcer, Cohen, Richter-Levin, Lebiosn, Grossberger, and Barkai, 2006). In these brain regions, the neurons display transient reductions in both the sAHP and spike-frequency adaptation after learning.
To summarize, intrinsic plasticity in the vertebrate brain is learning-specific, transient, and widespread – meaning it is observed in many different brain regions. In some cases these intrinsic changes are known to last anywhere from hours to days, or even weeks (Brons and Woody, 1980; Moyer et al., 1996; Saar et al., 1998; Schreurs et al., 1998). Given that these changes are correlated with learning (Song et al., 2012), such plasticity is an important predictor of learning-induced behavioral plasticity.
2.3. Aging and aberrant intrinsic excitability
Aging is associated with reduced cognitive ability. Furthermore, cognitive decline in the elderly can be viewed along a continuum and can be present even in the absence of neurological disorders like Alzheimer’s disease (AD). Moreover, the effect of normal aging (in absence of disorders) varies between individuals – some elderly individuals suffer more pronounced cognitive deficits whereas others are relatively unimpaired (Deary, Corley, Gow, Harris, Houlihan, Marioni, Penke, Rafnsson, and Starr, 2009). Normal aging leads to a decline in many brain functions but the two brain regions consistently implicated in these functional alterations are the medial temporal lobe (MTL) and the prefrontal cortex (PFC; Burke and Barnes, 2006; also reviewed later).
Hippocampal involvement in a variety of learning and memory paradigms is well documented (for a review, see Squire, 2004). Although aged rodents typically perform worse than young rodents on many hippocampus-dependent tasks, aged animals can be divided into aged impaired (AI) or aged unimpaired (AU) based on their performance (Gallagher, Burwell, and Burchinal, 1993). Heterogeneity in acquisition of Morris water maze (MWM) in aged rodents is associated with differences in CA1 neuronal excitability. The AI rats display greater sAHP and spike frequency adaptation relative to young and AU rats, and the sAHP amplitude was correlated with behavioral performance (Tombaugh, Rowe, and Rose, 2005). Interestingly, in this study the electrophysiological recordings were performed weeks (~2–4 weeks) after MWM training indicating these changes may not be learning-related and may reflect basal differences in CA1 excitability. Thus, it is possible that pre-learning differences in the intrinsic excitability of neurons contribute to aging-related cognitive decline and could explain the heterogeneity observed in cognitive performance during the aging process. Alternatively, it is also possible that intrinsic excitability changes following MWM last longer than those seen following acquisition of trace eyeblink conditioning.
Normal aging also leads to pronounced deficits in acquisition of hippocampus-dependent trace eyeblink and fear conditioning tasks. These deficits are observed in aged rodents (Kishimoto, Suzuki, Kawahara, and Kirino, 2001; Knuttinen, Gamelli, Weiss, Power, and Disterhoft, 2001; Moyer and Brown, 2006), rabbits (Deyo, Straube, and Disterhoft, 1989; Moyer, Power, Thompson, and Disterhoft, 2000; Solomon and Groccia-Ellison, 1996; Thompson et al., 1996), and humans (Finkbiner and Woodruff-Pak, 1991). In addition to forming associations between the CS and the US, animals also form associations between the conditioning context and the US. Such contextual fear associations require hippocampal function (Kim and Fanselow, 1992) and are impaired in aged rodents (Kaczorowski and Disterhoft, 2009; Moyer and Brown, 2006). Rodents, especially aging rodents, display heterogeneity in the acquisition of trace and context conditioning. Animals (young, middle-aged, or aged) that acquire context or trace conditioning have enhanced CA1 intrinsic excitability relative to age-matched control and slow learning animals (Kaczorowski and Disterhoft, 2009; Moyer et al., 2000; Song et al., 2012). These data indicate that reduced intrinsic excitability (pre- and post-learning) may be an important predictor of cognitive decline. It is interesting to note that animals that learn well have significantly smaller AHPs than animals that learn poorly. This is seen not only in adult animals but also aged animals where the AHP is significantly larger. This bidirectional modulation of AHP suggests that it is an important intrinsic mechanism influencing behavioral plasticity.
PFC function is also impaired during normal aging. The PFC is critical for working memory and executive function (Funahashi, Bruce, and Goldman-Rakic, 1993; Mair, Burk, and Porter, 1998). Impairments in learning working memory tasks, such as the Delayed non-matching-to-sample (DNMS) are observed across species with aging (Dunnett, Evenden, and Iversen, 1988; Lyons-Warren, Lillie, and Hershey, 2004; Moss, Killiany, Lai, Rosene, and Herndon, 1997; Moss, Rosene, and Peters, 1988). In primates, working memory tasks are dependent upon alterations in AP firing rates in dorsolateral PFC (Goldman-Rakic, 1995) and normal aging results in changes in the intrinsic properties of primate dorsolateral PFC neurons. Specifically, aging increases input resistance, decreases AP amplitude and fall time, and increases AP firing rate in layer II/III dorsolateral PFC neurons (Chang and Maren, 2011). Furthermore, in aged monkeys, performance on DNMS has a U-shaped quadratic relationship with the firing rate of layer II/III dorsolateral PFC neurons, where either low or very high firing rates predict poor performance (Chang, Rosene, Killiany, Mangiamele, and Luebke, 2005). Interestingly, modulation of excitability of layer III dorsolateral PFC neurons by inhibiting cAMP signaling, HCN channels or KCNQ channels restores persistent firing during the delay period and leads to improved performance of DNMS task (Wang, Gamo, Yang, Jin, Wang, Laubach, Mazer, Lee, and Arnsten, 2011). These data indicate that subregion-specific alterations in intrinsic properties may underlie learning deficits, and that modulation of excitability can ameliorate aging-related cognitive decline.
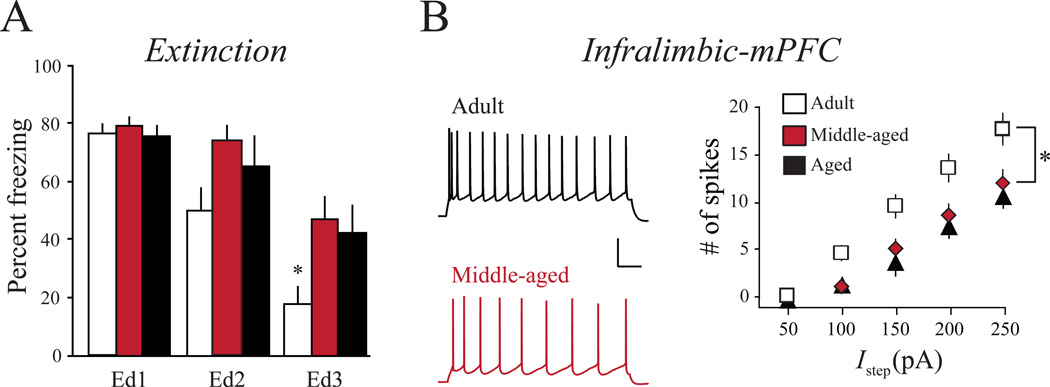
PFC subregions are also critical for cognitive flexibility (Oualian and Gisquet-Verrier, 2010). One example of cognitive flexibility is behavioral extinction, which is the learned inhibition of a behavioral response as a result of a change in stimulus contingencies (for a review of extinction, see Milad and Quirk, 2012). As previously mentioned in Section 2.1, PFC (particularly IL) is critical for extinction of a conditioned fear response. Moyer and colleagues studied the effects of normal aging on extinction of trace fear conditioning (Kaczorowski, Davis, and Moyer, 2012). They found that both middle-aged and aged rats were significantly impaired, as evidenced by continued freezing following the CS (see Figure 3A). The emergence of these aging-related extinction deficits paralleled a significant decrease in the intrinsic excitability in IL regular spiking neurons (see Figure 3B), and an increase in the intrinsic excitability of PL burst spiking neurons. The association between extinction deficits and low intrinsic excitability in IL neurons is significant, because similar findings have been reported in human studies. In humans, extinction deficits are thought to underlie a variety of disorders, including posttraumatic stress disorders (PTSD). PTSD patients have decreased metabolism in ventromedial PFC (vmPFC) as compared to control subjects (Bremner, Narayan, Staib, Southwick, McGlashan, and Charney, 1999; Bremner, Staib, Kaloupek, Southwick, Soufer, and Charney, 1999; Shin, Wright, Cannistraro, Wedig, McMullin, Martis, Macklin, Lasko, Cavanagh, Krangel, Orr, Pitman, Whalen, and Rauch, 2005). Hence, modulation of intrinsic excitability within IL could strengthen extinction learning in aging populations and also potentially benefit patients at risk for or suffering from PTSD.
Figure 3. Normal aging leads to aberrant intrinsic plasticity.
A, Aging results in extinction deficits. Mean freezing on the first trial of extinction day 1 does not differ as a function of age indicating no differences in acquisition and consolidation of trace fear conditioning across age-groups. Adult rats exhibit a significant decrease in freezing across 3 days of extinction indicating successful extinction relative to both middle-aged and aged rats. (Ed1, Ed2 and Ed3 refer to percent freezing on the first trial of extinction days 1, 2 and 3 respectively). B, Plot and representative traces illustrate that the mean number of spikes (excitability) evoked by increasing current steps decreases in infralimbic cortex regular spiking neurons from middle-aged and aged rats compared to adult rats. Scale 20mV, 100ms. * p < 0.05. Adapted fromKaczorowski et al. (2012) with permission.
In order to understand how neuronal activity is altered during normal aging, it is important to identify the underlying ionic conductances and relate these not only to changes in neuronal activity but also to changes in cognitive function. A number of studies have indicated that aberrant function of potassium channels as well as intracellular calcium homeostasis significantly contributes to aging-related cognitive decline. Given the wealth of data suggesting aging-related deficits in calcium regulation (for reviews see Disterhoft, Thompson, Moyer, and Mogul, 1996; Thibault, Gant, and Landfield, 2007; Toescu and Verkhratsky, 2007), it is not surprising that slow AHP (sAHP), largely mediated by a Ca2+-dependent K+ current (Alger and Nicoll, 1980), is enhanced in aging animals (Kumar and Foster, 2002; Landfield and Pitler, 1984; Moyer and Disterhoft, 1994; Moyer, Thompson, Black, and Disterhoft, 1992). In aged animals, an enhanced sIAHP is correlated with an enhanced sAHP and impaired learning ability (Power, Wu, Sametsky, Oh, and Disterhoft, 2002). Other K+ channels that contribute to the AHP in hippocampal neurons include K+ channels containing auxiliary Kvβ1.1 subunit (Giese, Storm, Reuter, Fedorov, Shao, Leicher, Pongs, and Silva, 1998) and small-conductance Ca2+-activated K+ channel type 2 (SK2 and SK3; see Stocker, 2004 for review). Aged mice that lack Kvβ1.1 subunits have increased hippocampal neuronal excitability, exhibit a smaller AHP, lower LTP induction threshold, and improved spatial learning relative to age-matched controls (Murphy, Fedorov, Giese, Ohno, Friedman, Chen, and Silva, 2004). Elevated expression of SK3 in the hippocampus is also correlated with impairment of LTP, and trace fear conditioning in aged mice (Blank, Nijholt, Kye, Radulovic, and Spiess, 2003). Transgenic mice that overexpress SK2 channels also demonstrated a higher LTP induction threshold and impairment in learning both hippocampus- and amygdala-dependent tasks (Hammond, Bond, Strassmaier, Ngo-Anh, Adelman, Maylie, and Stackman, 2006). Thus, aberrant intrinsic plasticity may emerge during normal aging, as a result of alterations in the numbers, distributions, or modulation of K+ channels that underlie the AHP and impact neuronal excitability during learning.
3. Implications of intrinsic plasticity on learning
3.1. Does intrinsic plasticity encode memory?
As described above, changes in neuronal excitability are learning-specific since they are observed in animals that learned, but not in pseudoconditioned controls or animals that failed to learn (Moyer et al., 1996; Oh, Kuo, Wu, Sametsky, and Disterhoft, 2003; Song et al., 2012). These changes are not restricted to adult animals, as learning-related AHP reductions are also observed in middle-aged and aged animals (Kaczorowski and Disterhoft, 2009; Moyer et al., 2000). While these data suggest that intrinsic plasticity underlies memory formation, a mnemonic role for intrinsic plasticity as a mechanism for maintaining a long-term memory seems unlikely for two reasons. First, these changes are transient (lasting only a few days) whereas behavioral expression of the memory can last for weeks, months, or even years. Within the hippocampus, enhanced intrinsic excitability of CA1 neurons is no longer evident 7 days following acquisition of trace eyeblink conditioning (see Figure 2), even though behavioral expression of the memory is evident for at least 6 months (Moyer et al., 1996). It is worth mentioning though that in some cases learning-related intrinsic plasticity can be persistent and hence, may very well underlie certain long-term memories (Brons and Woody, 1980; Schreurs et al., 1998). However, the majority of studies that have looked at the time course of learning-induced intrinsic plasticity have found that these changes are short-lived (Motanis et al., 2012; Moyer et al., 1996; Saar et al., 1998; Thompson et al., 1996; Zelcer et al., 2006). Second, global intrinsic plasticity is not synapse-specific, which limits its information storage capacity. It is unlikely that such plasticity would underlie a memory trace without quickly saturating the capacity for new memory formation (Moyer et al., 1996; Zhang and Linden, 2003). Thus, although intrinsic plasticity is learning-specific, its transient and global nature suggests that it is not likely to code for the memory itself.
An alternate explanation suggests that within the hippocampus the time course of enhanced intrinsic excitability reflects a period of time when these memories are undergoing memory consolidation (Moyer et al., 1996; Thompson et al., 1996). Over time, as the memory trace is transferred to higher cortical structures (Kim, Clark, and Thompson, 1995; Nadel and Moscovitch, 1997), transient intrinsic plasticity could facilitate the consolidation of memory from hippocampus to higher cortical structures, such as prefrontal cortex (Wierzynski, Lubenov, Gu, and Siapas, 2009). Such a system-level consolidation process requires reactivation and replay of memories (Girardeau, Benchenane, Wiener, Buzsaki, and Zugaro, 2009; Ji and Wilson, 2007), and enhanced excitability could facilitate these processes by lowering neuronal spike firing requirements. Thus, transient enhancement of excitability may facilitate processes that allow successful memory formation without directly encoding the memory (discussed further in Sections 3.2. and 3.3.).
In order to demonstrate a clear functional role for intrinsic plasticity, it is important to determine the necessity and sufficiency of intrinsic plasticity. This is still poorly understood. It is clear that in order to advance our understanding of exactly how intrinsic plasticity contributes to cognitive functions, including learning and memory, it is important to understand how intrinsic plasticity influences synaptic and behavioral changes. That synaptic and intrinsic plasticity also share similar signaling pathways presents unique complications to understanding how these processes interact to influence behavioral plasticity.
3.2. Interactions between synaptic and intrinsic plasticity: Chicken or the egg?
Canadian psychologist Donald Hebb postulated that changes in connection strength could be the cellular mechanism for learning and memory (Hebb, 1949). Since then, synaptic plasticity has been demonstrated in vitro and in vivo in a variety of brain regions (for review, see Lynch, 2004). Numerous experiments not only suggest that synaptic plasticity shares some of the same cellular and molecular pathways as does learning, but they also suggest that blocking synaptic plasticity impairs learning (e.g., Gruart and Delgado-Garcia, 2007; Morris, Anderson, Lynch, and Baudry, 1986; Whitlock, Heynen, Shuler, and Bear, 2006).
Synaptic plasticity is often accompanied by intrinsic plasticity. Stimulation protocols that induce synaptic plasticity also modulate intrinsic excitability. For example, LTP induction in hippocampal CA1 neurons leads to ES potentiation (Bliss and Lomo, 1973; Daoudal et al., 2002), increases local dendritic excitability, and facilitates bAPs (Frick et al., 2004). These changes are input specific, and are NMDA receptor-dependent (Daoudal et al., 2002; Frick et al., 2004). In addition, learning can facilitate synaptic transmission as well as intrinsic excitability in hippocampus and piriform cortex (Moyer et al., 1996; Power, Thompson, Moyer, and Disterhoft, 1997; Saar et al., 1998; Saar, Grossman, and Barkai, 2002). Hence, synaptic and intrinsic plasticity can be induced by the same stimuli (e.g., learning or in vitro stimulation). Synaptic and intrinsic plasticity are also mediated by the same intracellular signaling pathways. For example, both types of plasticity depend on the activation of NMDARs and some intracellular cascades such as PKA, PKC, and CAMKII (Daoudal and Debanne, 2003). However, it is unclear which form of plasticity comes first, whether learning-related intrinsic plasticity facilitates learning-related synaptic plasticity, or whether both are induced in parallel. This remains an open question, which will warrant additional research.
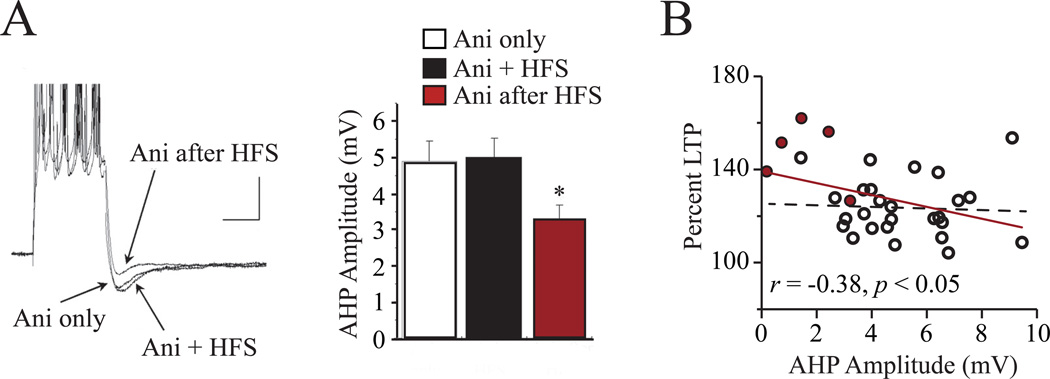
Several lines of evidence suggest that intrinsic plasticity can facilitate synaptic plasticity. Drugs or other treatments that reduce the AHP (and thus enhance intrinsic excitability) also facilitate the induction of LTP (Cohen and Abraham, 1996; Cohen et al., 1999; Sah and Bekkers, 1996). Such facilitation is also evident in absence of any changes in baseline synaptic transmission, indicating they do not result from better synaptic signal propagation alone. Furthermore, enhancement of synaptic plasticity can be achieved by increasing intrinsic excitability via downregulating transient A-type K+ channels (Chen, Yuan, Zhao, Birnbaum, Frick, Jung, Schwarz, Sweatt, and Johnston, 2006; Hoffman and Johnston, 1998), blocking of SK2 channels with BDNF or apamin (Kramar, Lin, Lin, Arai, Gall, and Lynch, 2004). Finally, recent studies suggest that intrinsic plasticity can be induced independent of synaptic plasticity, indicating that intrinsic plasticity is generated before synaptic plasticity. For example, Barkai and colleagues (Cohen-Matsliah, Motanis, Rosenblum, and Barkai, 2010) demonstrated that a high frequency synaptic stimulation (e.g., 20 stimuli at 50 Hz) although not sufficient to induce LTP at 1 hour following stimulation, was capable of reducing the post-burst AHP in CA1 pyramidal neurons 3–6 hours later (see Figure 3A). Thus, intrinsic plasticity, particularly AHP changes, can facilitate synaptic plasticity and may be a metaplasticity mechanism.
That synaptic plasticity occurs after or in the presence of intrinsic plasticity (Saar et al., 1998; 1999; 2002; Saar, Reuveni, and Barkai, 2012; Song et al., 2012) is consistent with the hypothesis that intrinsic plasticity could lead to synaptic plasticity. Although a relationship between intrinsic and synaptic plasticity has been well documented, a significant correlation between the two forms of plasticity following behavioral training was not reported until recently (Song et al., 2012). Moyer and colleagues examined the effect of trace fear conditioning on both intrinsic excitability (intracellular recordings) and synaptic plasticity (field recordings) of hippocampal CA1 neurons from the same animals. Both intrinsic excitability (size of AHP) and synaptic plasticity (percent LTP) were significantly correlated with behavioral performance (percent time spent freezing). Interestingly, intrinsic excitability and synaptic plasticity were significantly correlated with each other in good learners (Figure 3B). Thus, the data suggests that synaptic stimulation can lead to long term changes in intrinsic excitability (see Figure 3A) and learning-related changes in intrinsic plasticity predict the strength of subsequent LTP induction (see Figure 3B). It is possible that synaptic stimulation during learning results in intrinsic plasticity, and the magnitude of this plasticity predicts future synaptic plasticity, however, this still remains to be proven.
3.3. Metaplasticity: Change begets change
Consistent with a role for intrinsic plasticity in memory consolidation, learning-specific changes in intrinsic neuronal excitability can also serve a metaplasticity function. Metaplasticity refers to the higher-order plasticity that affects synaptic or intrinsic plasticity. Plasticity of intrinsic excitability is one such mechanism that could underlie metaplasticity (see Abraham, 2008, for discussion of other mechanisms implicated in metaplasticity induction).
The duration of learning-induced enhancements of intrinsic excitability also overlaps with a time period of enhanced learning (Saar et al., 1998; Zelcer et al., 2006). Using an olfactory discrimination paradigm where water-deprived rats learn to choose a particular odor for a water reward, discrimination between the first odor pair takes ~7 days whereas learning to discriminate subsequent pairs occurs more rapidly (~ 1 day). This phenomenon is called “rule learning”. Following acquisition of rule learning, intrinsic excitability of piriform cortical neurons is enhanced for up to 3 days and returns to baseline levels by 5 days. Remarkably, if training is suspended after acquisition of rule learning, rats display better discrimination for 1–2 additional days, which corresponds to the aforementioned period of enhanced excitability (Saar et al., 1998). Thus, the period of enhanced excitability of piriform cortex neurons matches the period during which rats display enhanced learning abilities.
Learning-induced intrinsic plasticity within a specific structure can also facilitate the acquisition of a different learning task dependent on that structure. Olfactory learning results in transient enhancement of hippocampal intrinsic excitability. During this period of enhanced neuronal excitability, acquisition of the hippocampus-dependent Morris water maze task is facilitated (Zelcer et al., 2006). Thus, intrinsic plasticity may be a mechanism that facilitates acquisition of new learning. It should be noted, however, that learning-related enhancement of learning has not been universally observed in all reported studies. For example, simultaneous but not consecutive training on two hippocampus-dependent tasks, trace eyeblink conditioning and MWM, facilitates acquisition of the trace eyeblink but not the water maze task (Kuo, Lee, and Disterhoft, 2006). These data indicate that learning-induced facilitation of learning may depend on additional factors, including the nature and timing of the learning paradigms. Establishing the contingencies that allow for learning-induced facilitation of learning should be an exciting new avenue for memory researchers.
If modulation of intrinsic excitability affects learning, might it also explain individual differences or heterogeneity in learning ability? Or is the ability to modulate intrinsic excitability an index of intelligence? On more than one occasion, rodents classified as “fast-learners” or “good learners” have been demonstrated to have greater learning-induced enhancement of intrinsic excitability (Cohen-Matsliah, Rosenblum, and Barkai, 2009; Song et al., 2012). For example, rats display heterogeneity in their ability to discriminate between odors in a simple maze (Cohen-Matsliah et al., 2009). Intrinsic excitability of piriform cortex neurons in fast performers (i.e., rats that display maximum efficacy right away on exposure to the maze) is greater relative to those from control rats. In contrast, piriform cortex neurons from slow performers are less excitable than control neurons. These differences are observed early on (12 h following maze learning) and subside as the performance of slow and fast learners converge. Furthermore, these performance differences are maintained on a complex olfactory discrimination maze (Cohen-Matsliah et al., 2009). Thus, fast performers appear to modulate intrinsic excitability sooner (12h) than slow performers (3 days). Similarly, following trace fear conditioning, CA1 neurons from rats classified as good learners had higher intrinsic excitability than those from poor learners or pseudoconditioned rats (Song et al., 2012). These data indicate that individual differences in learning ability could reflect a differential capacity to modulate intrinsic neuronal excitability.
If intrinsic excitability regulates the strength of learning, then it stands to reason that interventions capable of reducing the postburst AHP or otherwise enhancing intrinsic excitability should enhance learning and vice versa (also see Disterhoft and Oh, 2006). Indeed, early studies demonstrated that nimodipine, an L-type Ca2+ channel blocker, enhanced hippocampal intrinsic excitability by reducing the postburst AHP (Moyer et al., 1992), and facilitated the acquisition of trace eye-blink conditioning in aging rabbits (Deyo et al., 1989). More recently, administration of the SK2 channel agonist NS309 was shown to not only increase the size of the medium postburst AHP, but also impair the ability of rats to learn trace eyeblink conditioning (McKay, Oh, Galvez, Burgdorf, Kroes, Weiss, Adelman, Moskal, and Disterhoft, 2012), suggesting that direct enhancement of the AHP can negatively impact cognitive function. In addition, β-adrenergic receptor antagonists that modulate the AHP in LA neurons (Faber and Sah, 2002) block acquisition as well as reconsolidation of fear conditioning (Bush, Caparosa, Gekker, and Ledoux, 2010; Debiec and Ledoux, 2004; Muravieva and Alberini, 2010). Modulation of CREB expression within LA enhances both intrinsic excitability (Zhou, Won, Karlsson, Zhou, Rogerson, Balaji, Neve, Poirazi, and Silva, 2009) and fear learning (Han, Kushner, Yiu, Cole, Matynia, Brown, Neve, Guzowski, Silva, and Josselyn, 2007). Furthermore, enhanced noradrenergic and cholinergic transmission decreases the sAHP, increases spike firing, and enhances mPFC-dependent learning (Mueller, Porter, and Quirk, 2008; Santini and Porter, 2010; Santini, Sepulveda-Orengo, and Porter, 2012). Taken together, these studies support a significant role for intrinsic plasticity in modulation of cognitive function.
Intrinsic plasticity could also be responsible for priming effects observed in various memory paradigms. For example, a single training trial may not [on its own] be sufficient to elicit a memory of the trial, however a subsequent trial may then allow for memory formation in a time-dependent manner. While a single weak fear conditioning trial does not result in long-term fear memory, a second conditioning trial within a circumscribed window of time allows for long-term fear memory. This priming effect is dependent on PKA signaling within the amygdala following the initial trial (Parsons and Davis, 2012). As PKA signaling can also underlie learning-induced intrinsic plasticity (Oh et al., 2009), it is likely that increased intrinsic excitability following the first trial can underlie facilitated acquisition following the second trial. Similar results have been observed, for example, in Aplysia for sensitization of a defensive reflex (Philips, Tzvetkova, and Carew, 2007). Thus, mechanisms that modulate intrinsic plasticity could also regulate efficacy of learning in spaced training paradigms.
Further support for intrinsic plasticity as a metaplasticity mechanism comes from observations that certain environmental conditions enhance learning ability as well as alter intrinsic excitability. Indeed, environmental enrichment is not only capable of enhancing learning (Greenough, Fulcher, Yuwiler, and Geller, 1970), but it also enhances intrinsic excitability and E-S potentiation in hippocampal CA1 neurons (Malik and Chattarji, 2012). Thus, factors such as environmental conditions, behavioral history, as well as pharmacological manipulations that influence learning also impact intrinsic excitability and vice versa, indicating that intrinsic plasticity is an important modulator of memory acquisition and strength.
4. New horizons
4.1. Engram: Unraveling the enigma
A fundamental question in the search for the engram is “How are neurons selected for memory formation?” For example, although about 70% of LA neurons receive sensory inputs (Romanski, Clugnet, Bordi, and LeDoux, 1993), only ~20–30% of LA neurons display learning-related synaptic plasticity that is critical for fear conditioning memory (Rumpel et al., 2005). Therefore, only a small proportion of the neurons within LA, a structure critical for fear memory are encoding the memory. How are these neurons that are incorporated into the memory trace different from the other neurons that are left out?
It is possible that as per Lashley’s law of equipotentiality (Lashley, 1929), all neurons within a structure are equally capable of being incorporated into the memory trace. This supports the probabilistic view of memory formation where all neurons within a structure receive information necessary for memory encoding; however only a few end up coding for the memory. The alternate view supports the deterministic nature of the memory trace and implies that the neurons incorporated into a memory trace are predetermined – perhaps on the basis of their synaptic inputs (Squire, 1987).
In recent years, there has been considerable evidence to support the probabilistic view of memory formation (Han et al., 2007; Won and Silva, 2008). In a notable study, a viral vector was used to inject a wildtype or dominant negative form of CREB (cyclic AMP response element-binding protein) into either transgenic CREB-deficient or wildtype mice (Han et al., 2007). These viral transfections affected ~20% of LA neurons. Neurons with higher CREB levels were more likely to be incorporated into the memory trace than the surrounding, CREB-deficient neurons. Furthermore, selective ablation of the CREB overexpressing neurons, which were preferentially incorporated into the memory trace, abolished the fear memory (Han, Kushner, Yiu, Hsiang, Buch, Waisman, Bontempi, Neve, Frankland, and Josselyn, 2009). These data suggest that LA neurons are equally likely to be incorporated into the memory trace, but certain factors bias neurons to be preferentially recruited.
How does CREB upregulation bias memory allocation? It is well established that CREB expression can modulate neuronal excitability (Benito and Barco, 2010). Upregulation of CREB expression within a subset of LA neurons enhances their intrinsic excitability and leads to greater fear conditioning-related LTP (Zhou et al., 2009). Thus, it is likely that enhanced excitability facilitates synaptic plasticity, which could bias the allocation of new memories to a subset of neurons. Further support for the probabilistic nature of the memory trace and the role of intrinsic excitability in biasing this trace come from a study manipulating intrinsic excitability of hippocampal CA1 neurons during spatial exploration (Lee, Lin, and Lee, 2012). In this study, whole-cell patch clamp recordings were obtained from CA1 neurons while the animals explored a novel environment. Hippocampal CA1 neurons represent spatial information by environment-specific spiking activity such that these place cells fire in a particular place in the animal's environment (O'Keefe and Dostrovsky, 1971). Remarkably, increasing the excitability of a neuron by injecting a small, depolarizing current injection turns a previously silent cell into a place cell. In contrast, reducing the excitability by injecting a small hyperpolarizing current changed a place cell to a silent cell (Lee et al., 2012). These data indicate that even silent CA1 neurons receive spatial information and that this information can be uncovered by enhancement of its intrinsic excitability.
If intrinsic excitability can bias the recruitment of neurons to become part of a memory circuit, then what are the implications of learning-related intrinsic plasticity for memory allocation? Fear conditioning enhances intrinsic excitability within a subset of LA neurons (Sehgal et al., 2012). Thus, learning-related intrinsic plasticity can be restricted to a small subset of neurons within a structure. It is likely that these neurons displaying enhanced excitability are also especially likely to encode a subsequent memory trace. Keeping in mind that learning-related intrinsic plasticity is transient; such learning-related bias in memory allocation should also be transient. Interestingly, the levels of CREB, especially the phosphorylated active form of CREB, are dependent upon the previous activity of a neuron (Sheng, Thompson, and Greenberg, 1991). The activity-dependent modulation of CREB levels within neurons could determine the intrinsic excitability, and thus the probability with which these neurons are incorporated into the trace. This would mean neurons that were previously active because they were a part of the memory trace are more likely to be a part of the memory trace again.
These and many such hypotheses are as yet untested. The use of transgenic rodent models as well as optogenetic manipulations would greatly facilitate our understanding of these fundamental questions in neuroscience. These new tools in conjunction with analyses intrinsic and synaptic plasticity in single cell studies can greatly advance our understanding of not only how a memory trace is formed but also how it is updated or even degraded by experience or age.
4.2. Clinical perspective: From mice to men
Understanding how memories are formed and modulated is of significant clinical relevance. According to the US census bureau, middle-aged and aged individuals will constitute 45 percent of the US population by the year 2050, drastically increasing the socio-economic impact of aging-related cognitive decline (U.S. Census Bureau, 2004). Such aging-related cognitive decline is well-documented for hippocampus- and PFC-dependent tasks (Burke and Barnes, 2006). Furthermore, these impairments can be rescued by manipulating intrinsic excitability for hippocampal (Deyo et al., 1989; Disterhoft and Oh, 2006; Moyer et al., 1992) as well as PFC-dependent learning (Wang et al., 2011). In addition to normal aging (Chang et al., 2005; Kaczorowski et al., 2012; Moyer et al., 2000; Moyer et al., 1992), rodent models of Alzheimer’s disease also display aberrant intrinsic plasticity (Kaczorowski, Sametsky, Shah, Vassar, and Disterhoft, 2011). Modulation of intrinsic excitability could be an important factor in the search for neurobiological approaches to mitigate or prevent the onset of aging-related cognitive impairments and even rescue those deficits after they emerge.
Associative memories can predict aversive or appetitive stimuli. In some cases, such as PTSD, these memories are maladaptive and can lead to reoccurrence of the traumatic events (Mahan and Ressler, 2012). Such an abnormal fear response may arise as a result of metaplasticity, where some prior events lead to alterations in the intrinsic excitability of neurons within the fear circuit (Rosenkranz, Venheim, and Padival, 2010). In other cases, associative memories can provoke drug seeking as a result of presentation of cues previously associated with drug taking (Childress, McLellan, and O'Brien, 1986). By understanding the interplay between intrinsic excitability and behavioral plasticity, it may be possible to develop neurobiologically based treatment strategies that when combined with exposure therapy causes extinction of these abnormal associations (Myers, Carlezon, and Davis, 2011). Strengthening this extinction learning by enhancing intrinsic excitability can provide treatment for pathological forms of memory.
Finally, the old saying, an ounce of prevention is worth a pound of cure is certainly relevant here. Understanding the fundamental mechanisms that underlie memory formation may influence our ability to maximize the beneficial effects of experience-dependent plasticity and facilitate development of treatment strategies aimed at improving our quality of life.
Figure 4. Intrinsic plasticity is induced by synaptic stimulation and predicts future synaptic plasticity.
A, High frequency stimulation (HFS) that does not induce LTP can induce long-lasting reductions in the AHP. Postburst AHP in CA1 pyramidal neurons is reduced following HFS, and this reduction is dependent upon de novo protein synthesis. Anisomycin, a protein synthesis inhibitor blocks HFS-induced AHP reductions when applied during (Ani + HFS), but not after HFS (Ani after HFS). Left panel illustrates representative traces and right panel shows that the average AHP amplitude is reduced in neurons administered (Ani-after HFS) in a protein-synthesis dependent manner. * p < 0.05. Scale bars in left panel: 5 mV, 50 ms. B, Synaptic plasticity is correlated with intrinsic plasticity. Following acquisition of trace fear conditioning, intracellular and field recordings were made in the same slices. For slices from control, poor and good learner rats, the magnitude of LTP induced correlates with the AHP amplitude (red line). However, if data from good learners (red circles) are eliminated from the plot, the correlation is no longer significant (dashed line). A and B were adapted with permission from Cohen-Matsliah et al. (2009) and Song et al. (Song et al., 2012) respectively.
Acknowledgements
This work was supported by a Research Growth Initiative from the University of Wisconsin–Milwaukee (J.R. Moyer, Jr.), NIA grant R03-AG042814 (J.R. Moyer, Jr.) and Quincy Bioscience (J.R. Moyer, Jr.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Epileptiform burst afterhyperolarization: calciumdependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alkon DL. Associative training of Hermissenda. J Gen Physiol. 1974;64:70–84. doi: 10.1085/jgp.64.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL. Voltage-dependent calcium and potassium ion conductances: a contingency mechanism for an associative learning model. Science. 1979;205:810–816. doi: 10.1126/science.223244. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. Small-conductance, Ca2+- activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci. 2003;6:911–912. doi: 10.1038/nn1101. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons JF, Woody CD. Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. J Neurophysiol. 1980;44:605–615. doi: 10.1152/jn.1980.44.3.605. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learn Mem. 2011;18:221–225. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Role of conditioning factors in the development of drug dependence. Psychiatr Clin North Am. 1986;9:413–425. [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Motanis H, Rosenblum K, Barkai E. A novel role for protein synthesis in long-term neuronal plasticity: maintaining reduced postburst afterhyperpolarization. J Neurosci. 2010;30:4338–4342. doi: 10.1523/JNEUROSCI.5005-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Rosenblum K, Barkai E. Olfactory-learning abilities are correlated with the rate by which intrinsic neuronal excitability is modulated in the piriform cortex. Eur J Neurosci. 2009;30:1339–1348. doi: 10.1111/j.1460-9568.2009.06894.x. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Abraham WC. Facilitation of long-term potentiation by prior activation of metabotropic glutamate receptors. J Neurophysiol. 1996;76:953–962. doi: 10.1152/jn.1996.76.2.953. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. J Neurophysiol. 1999;82:3139–3148. doi: 10.1152/jn.1999.82.6.3139. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Lo Turco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:14512–14517. doi: 10.1073/pnas.222546399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- de Jonge MC, Black J, Deyo RA, Disterhoft JF. Learning-induced afterhyperpolarization reductions in hippocampus are specific for cell type and potassium conductance. Exp Brain Res. 1990;80:456–462. doi: 10.1007/BF00227987. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM. Age-associated cognitive decline. Br Med Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP Regulates Neurotransmitter Release and Synaptic Information Transmission by Modulating Action Potential Duration via BK Channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease. J Physiol Paris. 2006;99:180–192. doi: 10.1016/j.jphysparis.2005.12.079. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Evenden JL, Iversen SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology (Berl) 1988;96:174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 1991;6:109–117. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol. 2005;64:100–115. doi: 10.1002/neu.20148. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gasparini S, DiFrancesco D. Action of serotonin on the hyperpolarization-activated cation current (Ih) in rat CA1 hippocampal neurons. Eur J Neurosci. 1999;11:3093–3100. doi: 10.1046/j.1460-9568.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and afterhyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Fulcher JK, Yuwiler A, Geller E. Enriched rearing and chronic electroshock: effects on brain and behavior in mice. Physiol Behav. 1970;5:371–373. doi: 10.1016/0031-9384(70)90114-9. [DOI] [PubMed] [Google Scholar]
- Gruart A, Delgado-Garcia JM. Activity-dependent changes of the hippocampal CA3-CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav. 2007;6(Suppl 1):24–31. doi: 10.1111/j.1601-183X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson JR, Prince DA. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980;43:409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, Moyer JR., Jr Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol Aging. 2012;33:1744–1757. doi: 10.1016/j.neurobiolaging.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft JF. Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learn Mem. 2009;16:362–366. doi: 10.1101/lm.1365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Sametsky E, Shah S, Vassar R, Disterhoft JF. Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer's disease. Neurobiol Aging. 2011;32:1452–1465. doi: 10.1016/j.neurobiolaging.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Gilbert PE. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav Neurosci. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12:3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Agerelated effects on eyeblink conditioning in the F344 × BN F1 hybrid rat. Neurobiol Aging. 2001;22:1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- Kuo AG, Lee G, Disterhoft JF. Simultaneous training on two hippocampusdependent tasks facilitates acquisition of trace eyeblink conditioning. Learn Mem. 2006;13:201–207. doi: 10.1101/lm.98406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learn Mem. 2011;18:728–732. doi: 10.1101/lm.023945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Brain mechanisms and intelligence; a quantitative study of injuries to the brain. Chicago, Ill.: The University of Chicago Press; 1929. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Lyons-Warren A, Lillie R, Hershey T. Short- and long-term spatial delayed response performance across the lifespan. Dev Neuropsychol. 2004;26:661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Malik R, Chattarji S. Enhanced intrinsic excitability and EPSP-spike coupling accompany enriched environment-induced facilitation of LTP in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2012;107:1366–1378. doi: 10.1152/jn.01009.2011. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur J Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci. 2009;29:4750–4755. doi: 10.1523/JNEUROSCI.0384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol. 2009;102:2763–2770. doi: 10.1152/jn.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Galvez R, Burgdorf J, Kroes RA, Weiss C, Adelman JP, Moskal JR, Disterhoft JF. Increasing SK2 channel activity impairs associative learning. J Neurophysiol. 2012;108:863–870. doi: 10.1152/jn.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch G, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18:13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9:495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. Learning-Induced Bidirectional Plasticity of Intrinsic Neuronal Excitability Reflects the Valence of the Outcome. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs394. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Brown TH. Impaired trace and contextual fear conditioning in aged rats. Behav Neurosci. 2006;120:612–624. doi: 10.1037/0735-7044.120.3.612. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eyeblink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Disterhoft JF. Nimodipine decreases calcium action potentials in rabbit hippocampal CA1 neurons in an age-dependent and concentration-dependent manner. Hippocampus. 1994;4:11–17. doi: 10.1002/hipo.450040104. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010;33:17–26. doi: 10.1016/j.tins.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muravieva EV, Alberini CM. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn Mem. 2010;17:306–313. doi: 10.1101/lm.1794710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol. 2004;14:1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF. Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol. 2003;90:2171–2179. doi: 10.1152/jn.01177.2002. [DOI] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF. Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci U S A. 2009;106:1620–1625. doi: 10.1073/pnas.0807708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Cousens G, Herzog C. Behavioral and neuropsychological foundations of olfactory fear conditioning. Behav Brain Res. 2000;110:119–128. doi: 10.1016/s0166-4328(99)00190-4. [DOI] [PubMed] [Google Scholar]
- Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learn Mem. 2010;17:654–668. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Davis M. A metaplasticity-like mechanism supports the selection of fear memories: role of protein kinase a in the amygdala. J Neurosci. 2012;32:7843–7851. doi: 10.1523/JNEUROSCI.0939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips GT, Tzvetkova EI, Carew TJ. Transient mitogen-activated protein kinase activation is confined to a narrow temporal window required for the induction of two-trial long-term memory in Aplysia. J Neurosci. 2007;27:13701–13705. doi: 10.1523/JNEUROSCI.4262-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Bocklisch C, Curby P, Sah P. Location and function of the slow afterhyperpolarization channels in the basolateral amygdala. J Neurosci. 2011;31:526–537. doi: 10.1523/JNEUROSCI.1045-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Thompson LT, Moyer JR, Jr, Disterhoft JF. Enhanced synaptic transmission in CA1 hippocampus after eyeblink conditioning. J Neurophysiol. 1997;78:1184–1187. doi: 10.1152/jn.1997.78.2.1184. [DOI] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22:7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. J Neurosci. 1999;19:8616–8622. doi: 10.1523/JNEUROSCI.19-19-08616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol. 2002;87:2358–2363. doi: 10.1152/jn.2002.87.5.2358. [DOI] [PubMed] [Google Scholar]
- Saar D, Reuveni I, Barkai E. Mechanisms underlying rule learning-induced enhancement of excitatory and inhibitory synaptic transmission. J Neurophysiol. 2012;107:1222–1229. doi: 10.1152/jn.00356.2011. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30:12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Girgis AM, Moyer JR., Jr Fear conditioning modulates intrinsic excitability of lateral amygdala neurons; Abstract submitted for 42nd annual conference of the Society for the Neuroscience; New Orleans, LA. 2012. [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Groccia-Ellison ME. Classic conditioning in aged rabbits: delay, trace, and long-delay conditioning. Behav Neurosci. 1996;110:427–435. doi: 10.1037//0735-7044.110.3.427. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Song C, Detert JA, Sehgal M, Moyer JR., Jr Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J Neurophysiol. 2012;107:3397–3408. doi: 10.1152/jn.00692.2011. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Squire LR, editor. Memory and Brain. 1st ed. Oxford University Press; 1987. [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Trace eyeblink conditioning in rabbits demonstrates heterogeneity of learning ability both between and within age groups. Neurobiol Aging. 1996;17:619–629. doi: 10.1016/0197-4580(96)00026-7. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A. The importance of being subtle: small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell. 2007;6:267–273. doi: 10.1111/j.1474-9726.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa H, Offermanns S, Simon M, Kano M. Calcium-dependent persistent facilitation of spike backpropagation in the CA1 pyramidal neurons. J Neurosci. 2000;20:4878–4884. doi: 10.1523/JNEUROSCI.20-13-04878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau P.D. U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin. 2004 [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J Neurosci. 2012;32:12848–12853. doi: 10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behav Neurosci. 2011;125:318–326. doi: 10.1037/a0023520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spiketiming relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J, Silva AJ. Molecular and cellular mechanisms of memory allocation in neuronetworks. Neurobiol Learn Mem. 2008;89:285–292. doi: 10.1016/j.nlm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CD, Black-Cleworth P. Differences in excitability of cortical neurons as a function of motor projection in conditioned cats. J Neurophysiol. 1973;36:1104–1116. doi: 10.1152/jn.1973.36.6.1104. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Anwyl R. Inhibition of the slow afterhyperpolarization restores the classical spike timing-dependent plasticity rule obeyed in layer 2/3 pyramidal cells of the prefrontal cortex. J Neurophysiol. 2012;107:205–215. doi: 10.1152/jn.00452.2011. [DOI] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex. 2006;16:460–468. doi: 10.1093/cercor/bhi125. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Guzman-Karlsson MC, Sweatt JD. Epigenetic regulation of memory formation and maintenance. Learn Mem. 2013;20:61–74. doi: 10.1101/lm.026575.112. [DOI] [PMC free article] [PubMed] [Google Scholar]