Abstract
Introduction
Increasing numbers of people are undergoing bariatric surgery, of which approximately half are women in their child-bearing years. However, information on the long-term effects of maternal bariatric surgery in their children is lacking. Furthermore, since bariatric surgery is performed to reduce body weight, clinical studies have not been able to differentiate between benefits to the child due to maternal body weight loss versus other maternal postoperative metabolic changes. Therefore, we used the UCD-T2DM rat model of type 2 diabetes to test the hypothesis that maternal ileal interposition (IT) surgery would confer beneficial metabolic effects in offspring, independent of effects on maternal body weight.
Materials and Methods
IT surgery was performed on 2-month old prediabetic female UCD-T2DM rats. Females were bred 3 weeks after surgery and male pups were studied longitudinally.
Results
Maternal IT surgery resulted in decreased body weight in offspring compared with sham offspring (P<0.05). IT offspring exhibited improvements of glucose-stimulated insulin secretion and nutrient-stimulated GLP-2 secretion (P<0.05). Fasting plasma unconjugated bile acid concentrations were 4-fold lower in IT offspring compared with sham offspring at two months of age (P<0.001).
Conclusion
Overall, maternal IT surgery confers modest improvements of body weight and improves insulin secretion and nutrient-stimulated GLP-2 secretion in offspring in the UCD-T2DM rat model of type 2 diabetes, indicating that this is a useful model for investigating the weight-independent metabolic effects of maternal bariatric surgery.
Introduction
With the increased worldwide prevalence of obesity and type 2 diabetes, the development of therapeutic strategies to prevent or delay the onset of diabetes in predisposed individuals has become increasingly urgent [1]. In particular, the prevalence of obesity in women of reproductive age in the U.S. is greater than 30% and the prevalence of obesity among children and adolescents has increased by by more than 11% between 1994 and 2000 [2]. Several clinical studies have demonstrated that maternal obesity during gestation increases the risk of developing obesity and type 2 diabetes in children [3,4]. Bariatric surgery is currently the most effective long-term treatment for obesity and often results in rapid resolution of type 2 diabetes [5,6]. Thus, there are increasing numbers of people undergoing bariatric surgery, of whom approximately half are women in their child bearing years [7]. Specifically, more than 150,000 women of reproductive age underwent bariatric surgery between 2005–2008 [7]. Epidemiological data suggest that the risk of maternal and neonatal complications, such as gestational diabetes and low birth weight, are reduced in mothers who have undergone bariatric surgery prior to pregnancy [7]. Clinical studies report decreased incidence of obesity, improved insulin sensitivity and decreased circulating lipids and markers of inflammation in children born to mothers after bariatric surgery compared with siblings born to the same mothers prior to bariatric surgery, providing a potentially valuable model for the identification and development of new preventative and therapeutic strategies for addressing metabolic diseases [8,9].
One important question that has not been addressed is the impact of post-operative maternal metabolic changes on the child, independent of maternal body weight loss. The negative impact of obesity during gestation and the positive impact of weight loss prior to pregnancy have been supported by multiple studies [2,4,7,10,11]. Since bariatric surgery is primarily performed to reduce body weight, clinical studies have not been able to differentiate between benefits to the child due to maternal body weight loss versus maternal post-operative metabolic and endocrine changes.
Roux-en-Y gastric bypass (RYGB) surgery is one of the most common clinically performed bariatric surgeries [12]. RYGB involves several post-operative alterations in normal GI anatomy and function, such as reduction in gastric volume, bypass of the proximal small intestine and increased flux of incompletely absorbed nutrients into the distal small intestine. In contrast, ileal interposition (IT) surgery only involves movement of a segment of distal small intestine into the proximal jejunum [13]. Similar to RYGB, IT surgery results in increased circulating bile acid concentrations and increased nutrient-stimulated glucagon like peptide-1 (GLP-1) secretion [14,15]. Unlike RYGB, IT surgery does not decrease food intake or result in a loss of body weight. Therefore, IT surgery provides an ideal surgical model for studying the effects of post-operative maternal metabolic changes on the offspring, independent of changes of maternal body weight.
We have previously demonstrated that IT surgery in polygenic obese UCD-T2DM rats results in a 4 month delay (equivalent to 10 years in a human lifespan) in the onset of type 2 diabetes, independent of changes of body weight [14]. The UCD-T2DM rat model develops adult-onset polygenic obesity, insulin resistance and hyperglycemia, without a defect in leptin signaling, in both genders, making this model ideal for the study of the metabolic effects of maternal bariatric surgery on type 2 diabetes risk [16]. Thus, the purpose of the current study was to perform an initial investigation of the hypothesis that maternal IT surgery would confer beneficial metabolic effects in offspring, independent of effects on maternal body weight.
Materials and Methods
Diets and Animals
UCD-T2DM rats were individually housed in hanging wire cages in the animal facility in the Department of Nutrition at the University of California, Davis and maintained on a 14:10 hour light-dark cycle. Weight-matched female UCD-T2DM rats underwent IT or sham surgery (n=4 per group) at 2 months of age with an average body weight of 232 ± 5g. Female siblings were studied with one being sham-operated and the other being IT-operated. Three weeks after surgery females were mated to unoperated male siblings. Similar to our previous study in UCD-T2DM rats [14], body weight did not differ between IT and sham-operated dams (Table 1). Litters were culled such that the number of pups per litter within each cohort were matched in order to control for nutrient intake during the nursing period. After weaning, the male offspring were studied, resulting in an average of 4 ± 1 offspring studied per litter. All animals received ground rodent chow (no. 5012, Ralston Purina, Belmont, CA). Food intake and body weight were measured three times a week. Monthly fasting blood samples were collected and an oral glucose tolerance test (OGTT) was performed at 4 months of age. The experimental protocols were approved by the UC Davis Institutional Animal Care and Use Committee.
Table 1.
Dam Body Weight
| Months After Surgery | Sham Body Weight (g) | IT Body Weight (g) |
|---|---|---|
| 1 | 285 ± 4 | 279 ± 7 |
| 3 | 384 ± 14 | 382 ± 3 |
| 4 | 397 ± 14 | 405 ± 3 |
| 7 | 430 ± 23 | 456 ± 19 |
| 10 | 439 ± 35 | 453 ± 25 |
Values expressed as mean ± SEM. n=4.
Ileal Interposition Surgery
IT surgery was performed as previously described [14]. Rats were placed on a liquid diet (Boost®, Novartis, Minneapolis, MN) 4 days prior to surgery and for 7 days post-surgery and received enrofloxacin (20 mg/kg/d, s.c.) before and after surgery. Anesthesia was induced and maintained with isoflurane (2–5%). A midline abdominal incision was made and a 10 cm segment of distal small intestine 5–10 cm proximal to the ileocecal valve was isolated and transected. An anastomosis was made with the remaining ends of the ileum using 7-0 PDS suture (Ethicon®). Next, a transection was made 5–10 cm distal to the ligament of Treitz. The isolated ileal segment was then inserted isoperistaltically. Sham-operated animals were treated in the same manner as the IT group. Sham surgeries were performed by making transections in the same locations as in the IT-operated animals and bowel were reattached by anastomosis in their original position.
Hormone and Metabolite Measurements
Fasting (13 hr) plasma samples were collected from offspring starting at 2 months of age up to 6 months of age into EDTA treated tubes. Plasma glucose, cholesterol and triglycerides (TG) were measured using enzymatic colorimetric assays (Thermo DMA Louisville, CO; L-type TG H kit). Insulin was measured with a rat specific RIA (Millipore, St. Charles, MO).
Oral Glucose Tolerance Test
At 4 months of age an OGTT was performed on offspring. Animals were fasted overnight and then received a 50% dextrose solution (1 g/kg BW) by oral gavage. Blood was collected from the tail and placed in tubes containing EDTA, aprotinin and a DPP-IV inhibitor. Plasma glucose and insulin were measured as described above. Total GLP-1 was measured by sandwich electrochemiluminescence immunoassay (Meso Scale Discovery; Gaithersburg, MA). Total GLP-2 was measured by ELISA (Millipore, St. Charles, MO).
Monthly fasting plasma bile acid profiles
Monthly bile acid profiles were analyzed as previously described [17,18]. An internal standard mixture of (D4-cholate, D4-β-muricholate, D4-a-muricholate, D4-chenodeoxycholate, D4-deoxycholate, D4-hyodeoxycholate, D4-ursodeoxycholate, D4–lithocholate) and their tauroconjugated and glycoconjugated counterparts was prepared in acetonitrile. Eluted bile salts were detected by negative ion, electrospray mass spectrometry on a linear trap LTQ mass spectrometer (Thermo-Finnigan, San Jose, CA) using a data-dependent stage of detection triggering a qualitative 0 m/z neutral loss scan at 27% normalized collision energy. Peak areas from initial scans of individual bile salts at 514.3, 498.3, 464.3, 447.3, 411.3, 407.3, 395.3, 393.3 and 391.3 m/z were integrated and response factors were defined by peak area ratios of analytes to that of internal, deuterated standards. The response factors were read against those obtained from standard curves in surrogate matrix and molar levels of serum or plasma levels were interpolated from standard curves. Response factors for all samples were comprised of peak area ratios of non-labeled salts normalized to the stable-labeled counterparts. Concentrations were interpolated by linear regression from curves of known standards.
Statistics and Data Analyses
All male offspring from each litter were enrolled into study, resulting in a total of 21 male IT offspring and 18 male sham offspring being studied. However, in all data sets, the average of the data points from animals in the same litter was used for statistical analysis and presentation of the data, resulting in an n of 4 per group. This is because the experimental variable being tested in this study is the impact of surgery in the dam and presumably on the in utero and pre-weaning environment on the offspring from each litter. All statistical analyses were performed using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA). Data were analyzed using two-factor repeated measures ANOVA or Student’s t-test where appropriate. Data are presented as mean ± SEM. Differences were considered significant at P<0.05.
Results
Maternal IT surgery reduces body weight in offspring
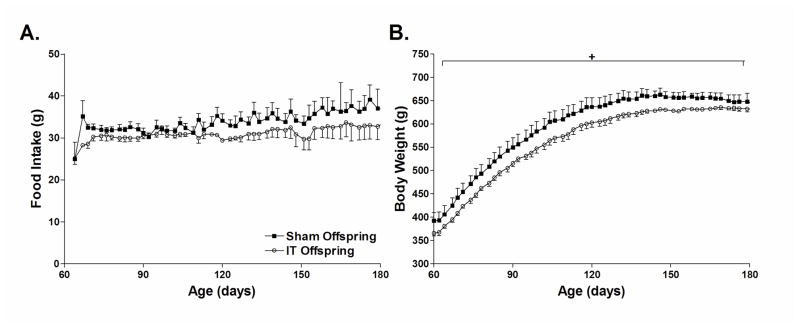
Food intake did not differ between groups by two-factor repeated measures ANOVA (Figure 1A). However, cumulative food intake between 4 to 6 months of age was 10% lower in IT offspring compared with sham offspring (Cumulative food intake: Sham Offspring = 2,064 ± 112 g, IT Offspring = 1,840 ± 100 g; P<0.05). Body weight was significantly lower in IT offspring compared with sham offspring at as early as 1 month of age (Sham Offspring = 105 ± 3 g; IT Offspring = 96 ± 3 g, P<0.05). Body weight continued to be approximately 5% lower in IT offspring from 2 months of age to 6 months of age (BW at 2 months of age: Sham offspring = 379 ± 4 g, IT offspring = 358 ± 4 g; P<0.05) (Figure 1B).
Figure 1.
Food intake (A) and body weight (B) in sham and IT offspring. *P<0.05 by two-factor repeated measures ANOVA.
Maternal IT surgery does not affect fasting plasma glucose and insulin concentrations in offspring
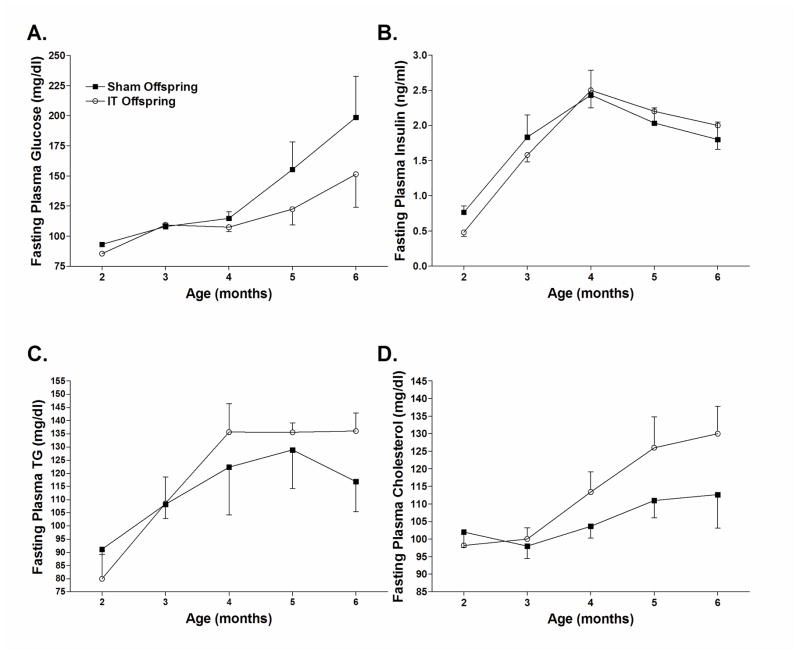
Fasting plasma glucose concentrations tended to be lower in IT offspring compared with sham offspring at 2, 5 and 6 months of age (Figure 2A). This was significant by Student’s t-test at 2 months of age (P<0.05) but was not significant by two-factor repeated measures ANOVA. Fasting plasma insulin concentrations were 40% lower in IT offspring compared with sham offspring at 2 months of age (Figure 2B). This was also significant by Student’s t-test (P<0.05), but not by two-factor repeated measures ANOVA. Surprisingly, IT offspring exhibited a trend for increased circulating TG and cholesterol concentrations (Figure 2C and D).
Figure 2.
Fasting plasma glucose (A), insulin (B), triglyceride (C) and cholesterol (D) concentrations in sham and IT offspring.
Maternal IT surgery improves insulin secretion and nutrient-stimulated GLP-2 secretion in offspring
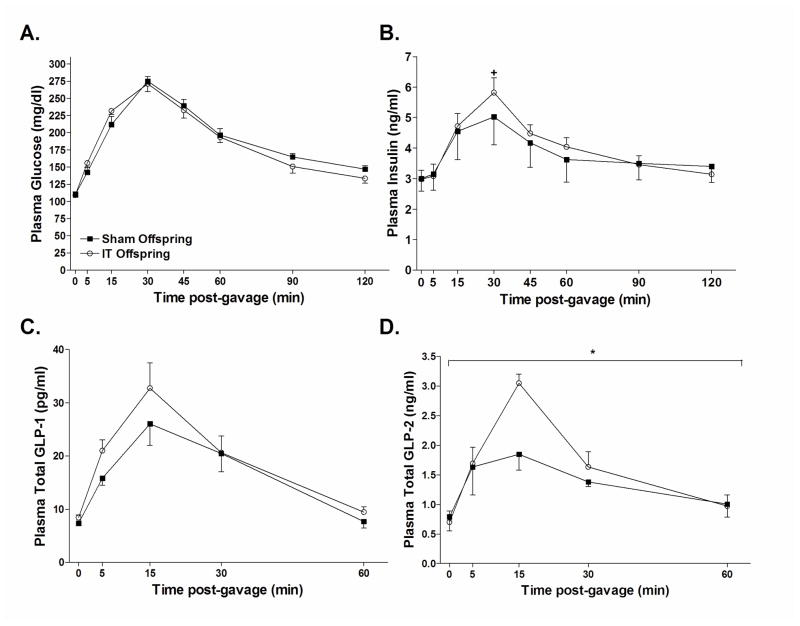
In order to further investigate the metabolic effects of maternal IT surgery, an OGTT was performed at 4 months of age. The glucose and insulin area under the curve (AUC) during the OGTT did not differ between groups (Glucose AUC: Sham offspring = 23,274 ± 775 mg/dl × 120 min, IT offspring = 22,775 ± 900 mg/dl × 120 min; Insulin AUC: Sham offspring = 464 ± 82 ng/ml × 120 min, IT offspring = 486 ± 36 ng/ml × 120 min) (Figure 3A–B). However, the percent increase of plasma insulin concentrations from fasting to peak values at 30 minutes after the glucose gavage was ~30% higher in IT offspring compared with sham offspring, indicative of an improvement of glucose-stimulated insulin secretion in IT offspring (P<0.05) (Figure 3B). Plasma total GLP-1 excursions tended to be increased in IT offspring compared with sham offspring; however, this did not reach significance (GLP-1 AUC: Sham offspring = 1,038 ± 149 pg/ml × 60 min, IT offspring = 1,194 ± 150 pg/ml × 60 min) (Figure 3C). GLP-2 concentrations at 15 minutes following the glucose gavage were 40% higher in IT offspring and the GLP-2 AUC was 23% higher in IT offspring compared with sham offspring (GLP-2 AUC: Sham offspring = 85 ± 6 pg/ml × 60 min, IT offspring = 104 ± 8 pg/ml × 60 min; P<0.05) (Figure 3D).
Figure 3.
Plasma glucose (A), insulin (B), GLP-1 (C) and GLP-2 (D) concentrations during an oral glucose tolerance test. Animals were fasted overnight and received 1 g/kg body weight dose of dextrose by oral gavage. +P<0.05 for the percent change of plasma insulin concentrations from baseline to peak values compared with sham offspring by Student’s t-test. *P<0.05 for the AUC compared with sham offspring by Student’s t-test.
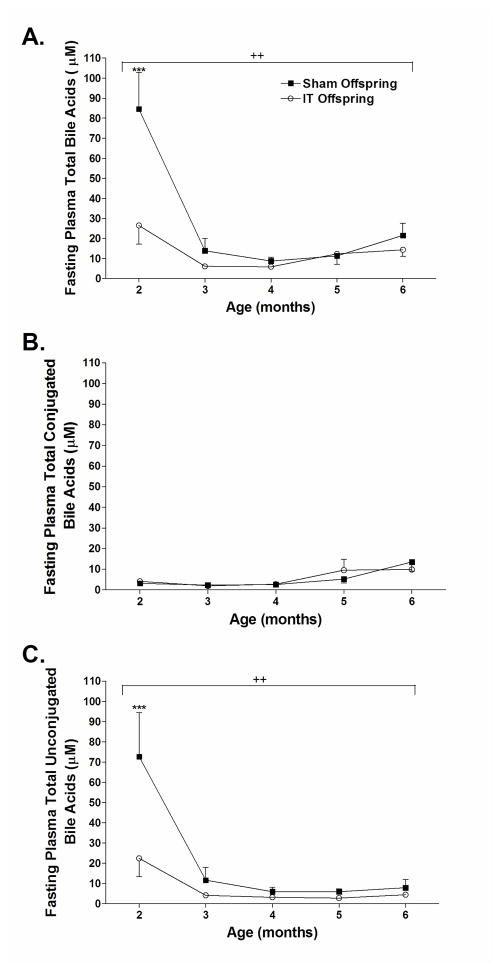
Maternal IT surgery decreases circulating unconjugated bile acid concentrations in offspring
Circulating bile acid concentrations have been reported to be elevated after several types of bariatric surgery including RYGB, IT and vertical sleeve gastrectomy [14,19,20,21]. These increases of circulating bile acids have been proposed to contribute to the metabolic benefits observed after bariatric surgery [19,20]. Therefore, we assessed circulating bile acid profiles over time in sham and IT offspring. Interestingly, total circulating bile acid concentrations were 80% lower in IT offspring compared with sham offspring at two months of age (Figure 4A) (P<0.001). Bile acid concentrations then dropped in sham offspring to levels similar to IT offspring starting at 3 months of age. Total plasma conjugated bile acid concentrations did not differ between groups (Figure 4B), but total plasma unconjugated bile acid concentrations were only 25% of that in IT offspring, mimicking the same pattern seen in total circulating bile acid concentrations over time (P<0.001) (Figure 4C).
Figure 4.
Fasting plasma total bile acid (A), total conjugated bile acid (B) and total unconjugated bile acid (C) concentrations in sham and IT offspring. ++P<0.01 by two-way ANOVA, ***P<0.001 by Bonferroni’s post-test.
Conclusions
Overall, there is little information on the short and long term effects of maternal bariatric surgery on offspring. Furthermore, most of the available data are confounded by the potential effects of maternal weight loss after bariatric surgery. This is the first study to evaluate the effect of the metabolic changes induced by only one component of Roux-en-Y gastric bypass surgery (moving the distal small intestine proximally) on offspring. This approach allows us to investigate the metabolic effects of maternal distal intestinal stimulation with incompletely absorbed nutrients on offspring without confounding differences in maternal body weight. In this initial study, we found that maternal IT surgery imparts reductions of body weight and improvements of insulin secretion and nutrient-stimulated GLP-2 secretion, independent of differences in maternal body weight.
Maternal IT surgery resulted in significant decreases of body weight in offspring. However, food intake only differed significantly between groups when comparing cumulative food intake between 4 to 6 months of age. This suggests that IT offspring may have increased energy expenditure compared with sham offspring. These results are consistent with previous human clinical studies. Children born after maternal biliopancreatic surgery exhibit lower birth weights and a lower incidence of obesity [8,9]. However, in these previous studies, substantial maternal body weight loss occurred prior to pregnancy. Thus, this is the first study to provide data that suggest that the metabolic effects of maternal bariatric surgery alone may be sufficient to produce decreases of body weight in offspring.
Maternal IT surgery also resulted in a modest improvement glucose-stimulated insulin secretion. Previous studies have demonstrated that the intrauterine environment plays a crucial role in the development of the pancreatic islets. In particular, previous work by Sandovici et al. demonstrated that exposure to suboptimal nutrition during early development of chow-fed Wistar rats results in development of diabetes and is related to epigenetic silencing of the enhancer region of the promoter for the HNF-a gene, which is required for β-cell proliferation [22].
Post-operative increases of post-prandial GLP-1 secretion have been suggested to contribute to the metabolic improvements observed after bariatric surgery and we hypothesized that these increases may be conferred to the offspring [23,24]. GLP-1 acts to increase glucose-stimulated insulin secretion, inhibit glucagon secretion, decrease food intake and may also promote β-cell proliferation [25,26,27,28]. While there was a trend for IT offspring to exhibit increases of nutrient-stimulated GLP-1 secretion during the OGTT, this difference did not reach significance. However, nutrient-stimulated GLP-2 secretion was significantly elevated in IT offspring compared with sham offspring. GLP-2 is produced and secreted from the same gut enteroendocrine L cells as GLP-1 and acts to stimulate proliferation of intestinal cells and thus may contribute to an increase in intestinal L-cell number [29]. Nutrient-stimulated GLP-2 secretion has been shown to be elevated after IT and vertical sleeve gastrectomy surgery in rats and early after RYGB and VSG in humans [30,31,32]. Therefore, increases of nutrient-stimulated GLP-2 secretion in IT-operated dams may have been conferred to the offspring. Interestingly, Shen et al. reported in rats that feeding dietary-resistant starch to mothers results in increased circulating GLP-1 concentrations in the mothers and improved fasting plasma glucose and increased circulating GLP-1 concentrations in offspring, suggesting that increases of maternal gut hormones can be conferred to offspring, perhaps by an epigenetic mechanism(s) [33].
The trend for maternal IT surgery to increase circulating cholesterol concentrations was surprising and is in contrast to previous studies demonstrating that maternal bariatric surgery in humans results in decreases of circulating lipids in the subsequent generation [9]. These conflicting data suggest that the effect of maternal bariatric surgery to reduce circulating lipid concentrations in offspring may be related maternal body weight loss and not to the effect of post-operative maternal metabolic changes.
Post-operative increases of circulating bile acid concentrations have become a popularly cited mechanism for the metabolic benefits observed after several types of bariatric surgery [20,21,23]. Bile acids have been shown to decrease hepatic gluconeogenesis and lipogenesis and increase insulin mediated glucose disposal in adipocytes by signaling through farnesoid X receptor [34]. Bile acids have also been shown to signal through TGR5 receptors located in the distal small intestine to increase GLP-1 secretion and in TGR5 receptors on skeletal muscle and brown adipose tissue to increase energy expenditure [34,35]. Therefore, we investigated changes of circulating bile acid profiles in IT and sham offspring. IT offspring exhibited lower circulating total and unconjugated bile acid concentrations compared with sham offspring at two months of age. This difference in circulating bile acid concentrations was no longer present at 3 months of age. Therefore, circulating bile acids do not appear to play a major role in mediating the metabolic benefits of maternal IT surgery. We hypothesize that circulating bile acid concentrations were lower in IT offspring early in life possibly because enzymes regulating bile acid synthesis had been down regulated in utero due to exposure to higher maternal circulating bile acid concentrations.
In conclusion, maternal IT surgery produces several metabolic benefits, all of which are independent of maternal body weight. Maternal IT surgery reduces body weight and improves insulin secretion and nutrient-stimulated GLP-2 secretion in offspring in the UCD-T2DM rat model of type 2 diabetes. Overall, these data suggest that the metabolic effects of bariatric surgery likely confer metabolic benefits to offspring independently of changes of maternal body weight, providing a potentially valuable model for the identification and development of new preventative and therapeutic modalities for obesity and type 2 diabetes. One short coming of this study is the lack of more in-depth data collected on the dams. We are making the assumption that the dams exhibit metabolic outcomes similar to those previously observed in male UCD-T2DM rats after IT surgery [14,19]. Furthermore, these results suggest the need for additional and more comprehensive studies, including identification of epigenetic and/or other developmental factors involved in conferring metabolic effects of bariatric surgery to the next generation.
Acknowledgments
Grants: This research was supported NIH grant 1RC1DK087307-01 and the University of California, Davis Veterinary Scientist Training Program. Dr. Havel’s laboratory also received funding during the project period from NIH Grants AT-002993, AT-003545, HL-075675, HL-091333, DK-095980 and R01-HL-107256, and a Multicampus Award from the University of California, Office of the President. This research was also partly supported by NIH grant R01DK095960 to B.P.C. and P.J.H.
We thank Ruby Hsieh, Susan Bennett, Cheryl Phillips and the Meyer Hall Animal Facility for their excellent animal care. We thank Linda Jung and MSD for the use of the Sector Imager 240. We thank Philip Sipes for technical support with the bile acid analyses.
Footnotes
Disclosures: Bethany Cummings: no conflict of interest, James Graham: no conflict of interest, Kimber Stanhope: no conflict of interest, Michael Chouinard: no conflict of interest, Peter Havel: no conflict of interest.
References
- 1.Defronzo RA, Abdul-Ghani MA. Preservation of {beta}-Cell Function: The Key to Diabetes Prevention. J Clin Endocrinol Metab. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 2.Kral JG. Preventing and treating obesity in girls and young women to curb the epidemic. Obes Res. 2004;12:1539–1546. doi: 10.1038/oby.2004.193. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Jr, Liese AD, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooney K, Ozanne SE. Maternal over-nutrition and offspring obesity predisposition: targets for preventative interventions. Int J Obes (Lond) 2011;35:883–890. doi: 10.1038/ijo.2011.96. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 6.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 7.Maggard MA, Yermilov I, Li Z, Maglione M, Newberry S, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286–2296. doi: 10.1001/jama.2008.641. [DOI] [PubMed] [Google Scholar]
- 8.Kral JG, Biron S, Simard S, Hould FS, Lebel S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644–1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 9.Smith J, Cianflone K, Biron S, Hould FS, Lebel S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–4283. doi: 10.1210/jc.2009-0709. [DOI] [PubMed] [Google Scholar]
- 10.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 11.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 13.Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav. 2006;88:277–282. doi: 10.1016/j.physbeh.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446. 2446, e2431. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, et al. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1782–1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bootsma AH, Overmars H, van Rooij A, van Lint AE, Wanders RJ, et al. Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J Inherit Metab Dis. 1999;22:307–310. doi: 10.1023/a:1005543802724. [DOI] [PubMed] [Google Scholar]
- 18.Torchia EC, Labonte ED, Agellon LB. Separation and quantitation of bile acids using an isocratic solvent system for high performance liquid chromatography coupled to an evaporative light scattering detector. Anal Biochem. 2001;298:293–298. doi: 10.1006/abio.2001.5379. [DOI] [PubMed] [Google Scholar]
- 19.Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6:443–456. doi: 10.1242/dmm.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patti MEHS, Bernier R, Bianco AC, Larsen PR, Holst JJ, Mun E, Auwerx J, Goldfine AB. Gastric bypass surgery increases bile acid levels: potential contribution to improved glucose tolerance. Diabetes. 2007;S6:A379. [Google Scholar]
- 22.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A. 2011;108:5449–5454. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 24.Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15–27. doi: 10.1152/ajpregu.00038.2011. [DOI] [PubMed] [Google Scholar]
- 25.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 26.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Cummings BP, Stanhope KL, Graham JL, Baskin DG, Griffen SC, et al. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes. 2010;59:2653–2661. doi: 10.2337/db09-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoda M, Kanda Y, Hamamoto S, Tawaramoto K, Hashiramoto M, et al. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2010;54:1098–1108. doi: 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estall JL, Drucker DJ. Glucagon-like Peptide-2. Annu Rev Nutr. 2006;26:391–411. doi: 10.1146/annurev.nutr.26.061505.111223. [DOI] [PubMed] [Google Scholar]
- 30.Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, et al. Vertical Sleeve Gastrectomy Improves Glucose and Lipid Metabolism and Delays Diabetes Onset in UCD-T2DM Rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012 doi: 10.1007/s00464-012-2166-y. [DOI] [PubMed] [Google Scholar]
- 32.Thulesen J, Hartmann B, Kissow H, Jeppesen PB, Orskov C, et al. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal--jejunal transposition or small bowel resection. Dig Dis Sci. 2001;46:379–388. doi: 10.1023/a:1005572832571. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol Nutr Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]