Abstract
Secretagogin, a recently cloned member of the EF-hand family of calcium binding proteins, was localized in the mouse, rat, and rabbit retina using immunofluorescence immunohistochemistry. Secretagogin is expressed in sub-populations of ON and OFF cone bipolar cells; however, no immunoreactivity was observed in rod bipolar cells in any of these species. Using subtype-specific markers and mice expressing green fluorescent protein (GFP) within specific cell classes, we found that secretagogin is expressed in Types 2, 3, 4, 5, 6 and possibly Type 8 cone bipolar cells in the mouse retina. The expression pattern in the rat retina differs slightly with expression in cone bipolar cell Types 2, 5, 6, 7, and 8. Evaluation of secretagogin in the developing mouse retina revealed expression as early as postnatal day 6, with OFF cone bipolar cells showing secretagogin expression prior to the ON cone bipolar cells. Secretagogin is a useful marker of cone bipolar cells for studying alterations in bipolar cell morphology during development and degeneration. Further work will be necessary to elucidate the functional role of this protein in bipolar cells.
INDEXING TERMS: development, rat, mouse, rabbit
Bipolar cells are the second-order neurons in the visual system and convey glutamatergic excitatory signals to the retinal ganglion cells. The total anatomical diversity of bipolar cell subclasses has been enumerated, at least for the mouse retina (Wässle et al., 2009). The 10 or so morphological classes can be split roughly equally into two broad classes, the ON-bipolar cells, which are depolarized by light, and the OFF-bipolar cells, which are hyperpolarized by light. The axon terminals of these two groups terminate in the inner and outer half of the inner plexiform layer, respectively (Famiglietti and Kolb, 1976). While it is clear that the two groups represent the anatomical substrate for the parallel ON and OFF visual pathways, the functional significance of the diversity within these two pathways is still unknown.
One hypothesis is that the different subchannels might mediate signals at different temporal bandwidths (DeVries, 2000), and previous work has indicated that some bipolar cells respond more transiently than others (Euler et al., 1996; Berntson and Taylor, 2000; DeVries, 2000; Puthussery et al., 2009). Moreover, it is evident that increased intracellular calcium produces more transient responses in the ON-type rod-bipolar cells (Berntson et al., 2004) and, therefore, intracellular calcium dynamics may be an important contributor to temporal tuning in the different cell types. A goal of the work described here is to elucidate the molecular basis for such differing calcium sensitivity, and to this end we have identified the localization pattern of a recently cloned calcium binding protein, secretagogin, within subpopulations of bipolar cells in the mammalian retina.
Secretagogin is a member of the EF-hand (E-helix-loop-F-helix-hand) superfamily of calcium-binding proteins, which was cloned from pancreatic islets of Langerhans and neuroendocrine cells (Wagner et al., 2000). The EF-hand superfamily represents a functionally diverse group of proteins that are ubiquitously expressed in mammalian tissue and are involved in intracellular calcium signaling and homeostasis. Antibodies raised against other such calcium-binding proteins, including calretinin, calbindin, and parvalbumin, have been used to localize these proteins to discrete subpopulations of neurons in the mammalian retina. Secretagogin, a hexa EF-hand calcium-binding protein, shows high sequence homology with two of these proteins, calretinin and calbindin (Wagner et al., 2000). Although initially thought to be restricted to neuroendocrine cells (Wagner et al., 2000), it is now evident that secretagogin is expressed in numerous brain regions, with particularly abundant expression in neurons of the pituitary gland and cerebellum (Gartner et al., 2001).
Although the gene expression of secretagogin has been reported in cone bipolar cells of the mouse retina (Kim et al., 2008), no evidence for protein expression has been demonstrated to date. We describe here, for the first time, the expression pattern of the secretagogin protein in the mammalian retina, with particular emphasis on the rodent retina.
MATERIALS AND METHODS
Animals and tissue preparation
Retinas were isolated from adult and postnatal aged (P2–P16) C57BL/6 mice, adult Sprague–Dawley rats and adult pigmented rabbits. To identify the Type 7 bipolar cell, we used animals that express green fluorescent protein (GFP; for abbreviations used in figures, see Table 1) under the gustducin promoter (Wong et al., 1999; Huang et al., 2003). These retinas were kindly provided by S. Haverkamp (Frankfurt, Germany). Animals had access to standard chow ad libitum and were maintained on a 12-hour light/dark cycle. All animal procedures were in accordance with the National Institutes of Health guidelines and were approved by the Oregon Health and Science University Animal Care and Use Committee. Mice (>P14) were anesthetized by intraperitoneal injection of sodium pentobarbital (0.1 mL, 100 mg/mL) and then euthanized by cervical dislocation. Younger mouse pups were euthanized by decapitation. Rats and rabbits were anesthetized by intramuscular injection of ketamine/xylazine (rabbit: 60:10 mg/kg; rat: 100:5 mg/kg) and euthanized by intravenous (rabbit) or intraperitoneal (rat) overdose of sodium pentobarbital. For immunohistochemistry, eyes were enucleated, the anterior segment and vitreous removed, and posterior eyecups were placed in fixative. For Western blotting, mouse cerebellar tissue was isolated in addition to the retinas for subsequent processing.
TABLE 1.
Abbreviations Used in Figures
| CalR | Calretinin |
| CB | Cerebellum |
| GCL | Ganglion cell layer |
| Gus-GFP | Gustducin-green fluorescent protein |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| OPL | Outer plexiform layer |
| P | Postnatal |
| PKC-α | Protein kinase C-α |
| Ret | Retina |
| S | Stratum |
| SCGN | Secretagogin |
| Syt2 | Synaptotagmin 2 |
| VGLUT1 | Vesicular glutamate transporter 1 |
Immunohistochemistry
Posterior eyecups were fixed for 30 minutes in 4% paraformaldehyde (PF) in 0.1 M phosphate buffer (PB; pH, 7.4). The tissue was cryoprotected in graded sucrose solutions (10%, 20%, 30%), embedded in TissueTek O.C.T. cryosectioning medium (Sakura Finetek, Torrance, CA), and vertically sectioned at 14 μm. Nonspecific binding sites were blocked with an incubation buffer (IB) containing 3% normal horse serum, 0.3% Triton X-100, and 0.025% NaN3 in 0.1 M phosphate-buffered saline (PBS; pH 7.4) for 10 minutes. Primary antibodies were diluted in IB and applied to tissues overnight at 4°C. Secondary antibodies were conjugated to Alexa Fluor 488, 594 or 647 (Molecular Probes, Eugene, OR) and were raised in donkey with the exception of the anti-guinea pig secondary antibodies, which were raised in goat. These antibodies were applied to sections for 1 hour at 25°C (1:800 in IB). For double or triple labeling experiments, primary antibodies were mixed together for incubation. When using primary antibodies raised in goat (calretinin, GFP) with primary antibodies raised in guinea pig (VGLUT1), secondary antibodies were applied in a sequential process to prevent binding of the donkey antigoat secondary antibodies to the goat-anti-guinea pig secondary antibody. For all other double and triple labeling experiments, sections were incubated in a mixture of secondary antibodies. An anti-GFP antibody was used to enhance the fluorescent signal in the gustducin-GFP mouse. A list of the primary antibodies used in this study is shown in Table 2.
TABLE 2.
Primary Antibodies Used in This Study
| Antigen | Host | Source | Cat. No./Lot No. | Dilution | Antigen |
|---|---|---|---|---|---|
| Calretinin | Goat | Millipore, Billerica, MA | AB1550/LV1486604 | 1:1,000 | Rat calretinin |
| GFP | Goat | Rockland, Gilbertsville, PA | 600-101-215/23153 | 1:1,000 | GFP fusion protein corresponding to the full length amino acid sequence (246aa) derived from Aequorea victoria |
| PKC (clone MC5) | Mouse | Sigma, St Louis, MO. | P5704/097K4854 | 1:400 | Amino acids 296-317 of PKC |
| Recoverin (Rec-1) | Rat | Gift from Dr. G. Adamus, Casey Eye Institute, Portland, OR | 1:200 | Purified recoverin (Adamus and Amundson, 1996) | |
| Secretagogin | Rabbit | Gift from Dr Ludwig Wagner, University of Vienna, Austria | 1:5,000 | Recombinant human secretagogin (Wagner et al., 2000) | |
| Synaptotagmin 2 (ZNP-1) | Mouse | Zebrafish International Resource Center, OR | ZNP-1/Prep date 02/10/09 | 1:50 | 1–5 day zebrafish embryo |
| VGLUT1 | Guinea Pig | Millipore, Billerica, MA | AB5905/LV1439669 | 1:5,000 | Synthetic peptide (aa 542-560 rat VGLUT1) |
Antibody characterization
The goat-anti-calretinin antibody recognizes an ≈30 kDa band on Western blots of rat brain extracts (Winsky et al., 1996). This antibody stains subtypes of amacrine cells, including cholinergic amacrine cells in mouse (Haverkamp and Wässle, 2000) and rat retina (Gabriel and Witkovsky, 1998) and the staining pattern observed here was consistent with these reports. The goat-anti-GFP recognizes a 33 kDa band on Western blots corresponding to the weight of the recombinant GFP protein (manufacturer’s data sheet). No staining was observed when this antibody was used on sections from wildtype mouse retina (data not shown). The mouse-anti-protein kinase C-α(PKC-α) antibody recognizes an 80 kDa polypeptide of PKC in bovine brain lysates (manufacturer’s data sheet). PKC is a well-established marker of rod bipolar cells in the rat (Greferath et al., 1990) and mouse retina (Haverkamp et al., 2003), and the same pattern of staining was observed herein. The rat-anti-Rec1 antibody recognizes a 23 kDa band on Western blots of purified bovine recoverin (Adamus and Amundson, 1996). Recoverin is a marker of Type 2 and Type 8 bipolar cells in the rat retina (Euler and Wässle, 1995; Chun et al., 1999) and a marker of Type 2 cells in the mouse retina (Haverkamp et al., 2003). The staining pattern observed herein was in accordance with these previous reports. The rabbit-anti-secretagogin antibody recognizes a 32 kDa band in retina and cerebellar lysates which corresponds to the weight of the recombinant secretagogin protein (Wagner et al., 2000). The mouse-anti-synaptotagmin 2 antibody (also known as ZNP-1) recognizes a 60 kDa protein on Western blots of mouse cerebellum and synaptosomes (Fox and Sanes, 2007). It has been previously shown to stain both Type 2 (Fox and Sanes, 2007, Wässle et al., 2009) and Type 6 (Wässle et al., 2009) bipolar cells in the mouse retina and the staining pattern seen here was consistent with these reports. The guinea pig-anti-VGLUT1 recognizes a 62 kDa band on Western blots of rat brain lysates (manufacturers data sheet). This antibody stains photoreceptor and bipolar cell terminals in the rat (Johnson et al., 2003) and mouse (Johnson et al., 2003; Sherry et al., 2003) retina and the pattern of staining obtained here was consistent with these reports.
Microscopy and imaging
Confocal images were acquired on a Zeiss LSM 510 confocal microscope with a 63×/1.40 oil-immersion objective. Images shown herein are from a single image plane (optical section thickness 0.41 μm) unless otherwise indicated.
Further image processing was confined to adjustment in the brightness, contrast, and sharpness of images with Adobe Photoshop CS (San Jose, CA). In all cases, such procedures were applied equally across the whole image.
Western blotting
The specificity of the polyclonal secretagogin antibody was assessed by Western blotting of mouse retina and cerebellum. Tissues were briefly sonicated in a 4-mM HEPES buffer containing 320 mM sucrose (pH 8.5) and a protease inhibitor cocktail (EMD Chemicals, Gibbstown, NJ, #539131). Homogenates were centrifuged at 13,000g for 1 minute. The resultant supernatant was then centrifuged at 13,000g for 15 minutes and the resultant pellet was resuspended yielding a crude membrane fraction. Samples were denatured in a buffer containing sodium dodecyl sulfate (SDS) and 2-mercaptoethanol, separated on a 12.5% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blocked in 5% w/v skim milk powder and 0.05% (v/v) Tween-20 in 0.1 M Tris-buffered saline (TBS, pH 7.4) for 2 hours at 25°C. The secretagogin primary antibody was diluted at 1:5,000 in the same buffer and applied overnight at 4°C. After washing, the membrane was incubated in a horseradish peroxidase (HRP)-conjugated donkey antirabbit secondary antibody (1:15,000, Amersham/GE Healthcare, Piscat-away, NJ) for 2 hours at 25°C. An enhanced chemiluminescence kit (Pierce, Rockford, IL) was used for detection.
RESULTS
Characterization of the secretagogin antibody
We performed Western blotting on tissue homogenates from mouse retina and cerebellum to confirm the specificity of the polyclonal rabbit anti-secretagogin antibody. We detected a prominent band at 32 kDa in both tissues (Fig. 1), which corresponds to the predicted molecular weight of the secretagogin protein (Wagner et al., 2000; Gartner et al., 2001). These data, when taken together with previous evidence for gene expression of secretagogin in mouse cone bipolar cells (CBCs) (Kim et al., 2008), provide strong evidence that the secretagogin antibody recognizes the correct protein in the retina.
Figure 1.
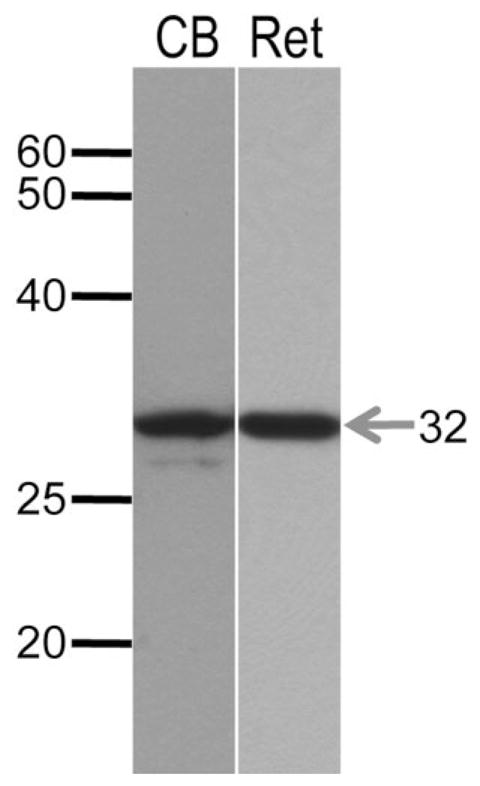
Western blot of secretagogin in retina and cerebellum. Western blotting of crude membrane fractions from homogenates of retina (Ret) and cerebellum (CB, positive control) probed with secretagogin polyclonal antibody. A prominent band is evident at ≈32 kDa in both samples, consistent with the expected molecular weight of the secretagogin protein. An additional weaker band is observed at ≈30 kDa in the cerebellum.
Localization of secretagogin in the mammalian retina
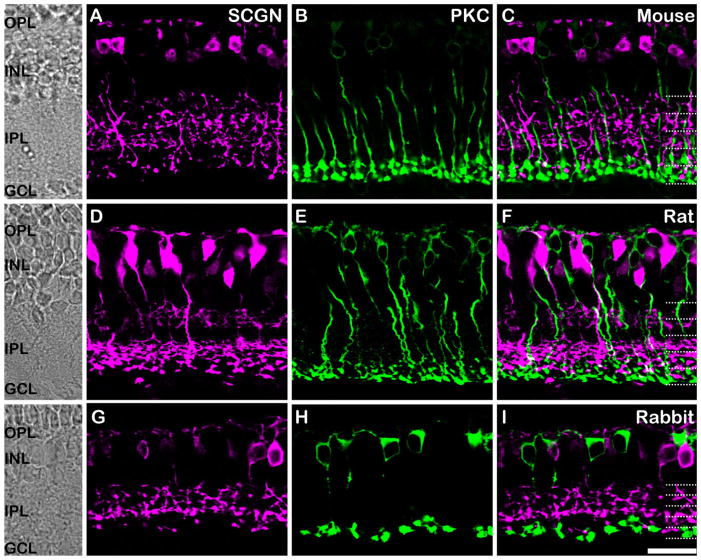
We evaluated the immunohistochemical localization of secretagogin in vertical sections of retina from the mouse, rat, and rabbit (Fig. 2). The IPL can be subdivided into five equal strata. The OFF sublamina of the IPL is comprised of the outer 2 strata, S1 and S2, while the ON sublamina is comprised of the inner three strata, S3–S5 (Hartveit, 1997; McGillem and Dacheux, 2001; Ghosh et al., 2004). In all species, secretagogin immunoreactivity was seen in the dendrites, cytoplasm, axon, and axon terminals of bipolar cells with axon terminals stratifying in both sublaminae of the IPL. Thus, secretagogin is expressed by both ON- and OFF-type bipolar cells. Double labeling for secretagogin and the rod bipolar cell (RBC) marker, PKC-α, revealed that secretagogin was not expressed in RBCs in any of the species tested, as evidenced by the complete absence of any overlap between the green and magenta in Figure 2C,F,I. The pattern of secretagogin immunoreactivity appeared to be largely conserved among species, in contrast to the expression of other closely related calcium-binding proteins that show variable cellular localization patterns in different mammalian species. For example, calbindin is expressed in horizontal and amacrine cells in the mouse retina (Pochet et al., 1991), while in the primate retina it is expressed in some cone photoreceptors (Haley et al., 1995), H2 horizontal cells (Wässle et al., 2000), and DB3 bipolar cells (Grünert et al., 1994).
Figure 2.
Localization of secretagogin in cone bipolar cells of the mammalian retina. Vertical sections of mouse (top panels), rat (middle panels), and rabbit (bottom panels) retina immunolabeled for secretagogin (SCGN; magenta) and the rod bipolar cell marker protein kinase C-α (PKC-α; green). The retinal layers are indicated on transmitted light photomicrographs (left column) and the IPL is divided into five equal strata (dotted lines). A–C: Mouse retina. Secretagogin is expressed in the dendrites, soma, and axon terminals of bipolar cells in the mouse retina. Double labeling with PKC-α (B) shows no overlap of staining, suggesting the absence of secretagogin in rod bipolar cells (C). D–F: Rat retina. Secretagogin (D) is expressed in the dendrites, soma, and axon terminals of bipolar cells stratifying at all levels of the IPL. Double labeling with PKC-α (E) shows no overlap of staining (F). G–I: Rabbit retina. A similar pattern of secretagogin labeling is observed in the rabbit retina (G), and like in rat and mouse, no PKC-α staining is evident in the secretagogin-positive bipolar cells. Scale bar = 20 μm.
Secretagogin is expressed in a subset of cone bipolar cells of the mouse retina
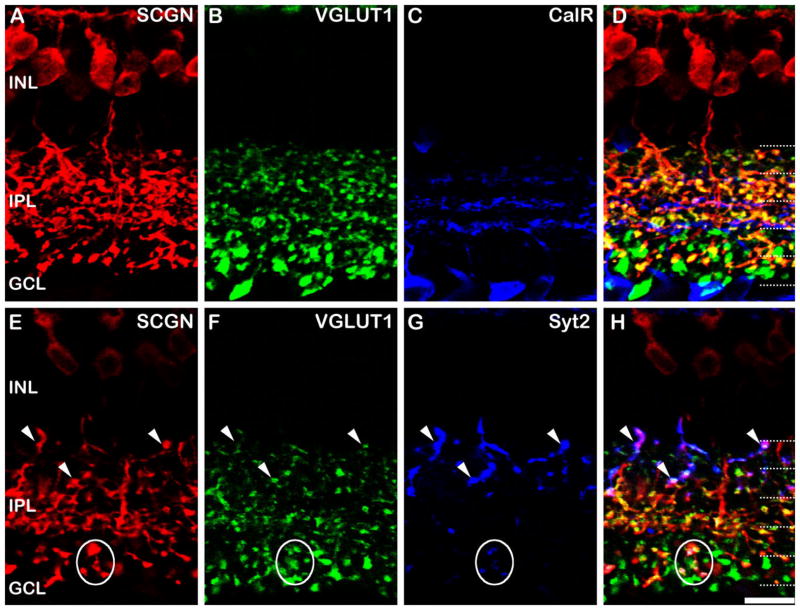
In order to evaluate whether secretagogin expression was present in all or a subset of CBCs (Fig. 3), we used a vesicular glutamate transporter 1 (VGLUT1) antibody, which labels all glutamatergic bipolar cell terminals (Johnson et al., 2003; Sherry et al., 2003). We triple-labeled for VGLUT1, calretinin, and secretagogin to assess whether any of the CBC axon terminals were spared of secretagogin staining (Fig. 3A–D). Calretinin (CalR) was included as a marker to delineate the sublaminae of the IPL (Fig. 3C); the central band of calretinin staining divides sublamina a from sublamina b, while the more distal and proximal bands correspond to the plexus of dendrites from the OFF and ON starburst amacrine cells, respectively. As far as we could tell, all the VGLUT1-positive axon terminals in S2 and S3 of the IPL (Fig. 3B) also showed labeling for secretagogin (Fig. 3A); however, there was a subset of axon terminals in S1 that did not, as is evident by the pure green profiles in Figure 3D. Additional pure green secretagogin-negative terminals can be localized to S4/5, since they lie below the innermost band of CalR labeling (Fig. 3C), which delineates S3 from S4. We sought to determine the identity of the unlabeled bipolar cell terminals in S1, S4, and S5 using subtype-specific bipolar cell markers.
Figure 3.
Secretagogin in OFF bipolar cells of the mouse retina. Vertical sections of wildtype mouse retina labeled for secretagogin (SCGN), VGLUT1, and various cell markers. The overlaid triple labels are shown in the right-hand column. A–D: Vertical sections of mouse retina labeled for secretagogin (SCGN; A), VGLUT1 (B), and calretinin (CalR; C). All secretagogin labeled processes in the IPL were also labeled for VGLUT1 (D, yellow terminals), confirming their identity as bipolar cells. However, a subset of VGLUT1 stained bipolar cells in S1, S4, and S5 are not labeled for secretagogin (D, green terminals). Calretinin labeling allows accurate identification of sub strata in the IPL (dotted lines). E–H: Another section labeled for secretagogin (E), VGLUT1 (F), and synaptotagmin 2 (Syt2, G), a marker of Type 2 OFF cone bipolar cells and Type 6 ON cone bipolar cells. The axon terminals of the Type 2 cells (arrowheads) and Type 6 cells (ovals) are also labeled for secretagogin. Dotted lines indicate the five IPL strata. Scale bar = 10 μm.
Previous studies indicate that only two types of bipolar cells have axon terminals that are predominately restricted to S1 of the IPL: the Type 1 and Type 2 OFF cone bipolar cells (Ghosh et al., 2004). The Type 2 bipolar cell axon terminals can be specifically labeled using an antibody directed against synaptotagmin 2 (Syt2; Fox and Sanes, 2007; Wässle et al., 2009). Therefore, to determine whether the secretagogin-negative terminals were Type 2 bipolar cells, we triple-labeled for Syt2, VGLUT1, and secretagogin (Fig. 3E–H). The staining clearly shows that the Syt2 containing Type 2 bipolar cells also label for secretagogin (white arrowheads, Fig. 3E–H) and, therefore, we conclude that the Type 1 bipolar cells, the only alternative, must account for the OFF CBC terminals in S1 that are devoid of secretagogin labeling.
This leaves the secretagogin-negative axon terminals of ON CBCs within S4/5 unaccounted for. There are four candidate bipolar cell types: Types 6–9. We can examine Type 6 ON CBC bipolar cells using the Syt2 antibody, since, in addition to labeling the Type 2 bipolar cell, Syt2 also weakly stains the axon terminals of the Type 6 (Wässle et al., 2009). According to the classification proposed by Ghosh et al. (2004), the axon terminals of these cells are somewhat diffusely stratified in S3–S5. Although the terminals are sparse (Fig. 3G), it is clear that the Syt2 staining in Type 6 cells are also labeled with secretagogin (white circles, Fig. 3E–H); therefore, Type 6 cells cannot account for the secretagogin-negative terminals in the ON-sublamina.
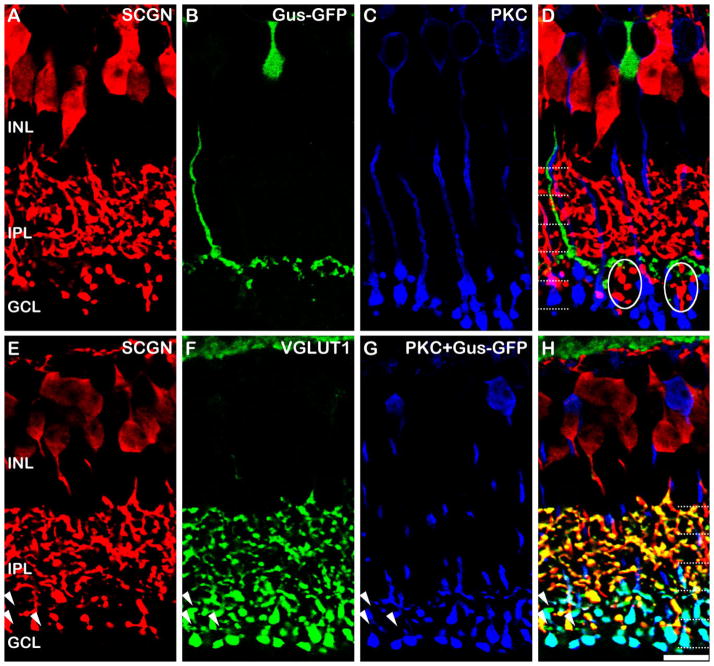
The next possibility is the Type 7 ON CBC, which is stratified in S4 of the IPL. These cells are selectively labeled in the Gus-GFP transgenic mouse, which express GFP under the gustducin promoter (Wong et al., 1999; Huang et al., 2003). We found the GFP expression in the peripheral regions of the retina to be somewhat variable, and therefore we restricted our analysis to central regions where a continuous band of GFP staining could be seen in S4 (Fig. 4B). Comparable material from peripheral retina often contained gaps within the S4 labeling, presumably where Type 7 cells were devoid of GFP expression (not shown). It should also be noted that RBCs are weakly and variably labeled in the Gus-GFP mouse, and again, we restricted our analysis to regions lacking such staining, or alternatively we adjusted the imaging gain to preferentially highlight the Type 7 cells. Figure 4A–D shows a projected image of a confocal stack (total thickness 2.05 μm) through a vertical section of the Gus-GFP retina that has been immunostained for GFP, PKC, and secretagogin. The pure green profiles in the composite image (Fig. 4D) clearly demonstrate that the Type 7 ON CBC terminals are devoid of secretagogin and therefore the Type 7 cells could comprise the secretagogin-negative bipolar cells in S4 of the IPL (Fig. 3D).
Figure 4.
Secretagogin in ON bipolar cells of the mouse retina. A–D: Vertical sections of the Gus-GFP retina labeled for secretagogin (SCGN; A), GFP (B), and PKC-α (C). Images A–C are from a projected confocal stack of 2.05 μm thickness. None of the Type 7 ON CBCs that are labeled for GFP label for secretagogin (D, green). However, some secretagogin labeled bipolar cells stratify in the region between the axon terminals of the Type 7 ON CBCs and the RBCs; these are likely to be Type 8 cells based on the level of stratification in the IPL (ovals). E–H: Vertical sections of the Gus-GFP retina labeled for secretagogin (E), VGLUT1 (F), and PKC-α/GFP (labeled with same colored secondary antibody in G). The triple overlay (H) shows that a small number of VGLUT1-positive axon terminals located at the level of the RBCs in S5 do not show SCGN labeling (arrowheads). These cells are neither RBCs nor Type 7 cells, which appear light blue in the triple label. Dotted lines indicate the five IPL strata. Scale bar = 10 μm.
If the Type 7 cells do account for all of the secretagogin-negative cells in S4/5, then the terminals in this region stained with the pan bipolar cell terminal marker, VGLUT1, should also be labeled for either secretagogin or GFP. To test for this, we labeled for secretagogin (red), GFP (blue), and VGLUT1 (green, Fig. 4E–H). To establish whether we had accounted for all of the bipolar cell terminals in the ON sublamina, we concurrently labeled the rod-bipolar cells with PKC-α (also blue). This also distinguished them from any non-Type 7 CBC terminals that might be present. The results indicate that labeling the Type 7 bipolar cells did account for the vast majority of the secretagogin-negative terminals in the ON sublamina; however, occasionally we observed a sparse number of VGLUT1-positive axon terminals in the region between the rod bipolar cell and Type 7 bipolar cell axon terminals that did not show secretagogin immunoreactivity (arrowheads, Fig. 4H). It is possible that these axon terminals are from Type 9 bipolar cells (blue cone selective bipolar), since their axon terminals are distributed in S5 and they are a sparsely distributed subtype, constituting ≈1% of the total bipolar cell population in the mouse (Haverkamp et al., 2005). Since a marker of the mouse Type 8 cell is currently lacking, it is impossible to conclude with certainty that the Type 8 cell expresses SCGN. However, dye-injection of a single cell that showed morphological features consistent with the Type 8 cell and subsequent staining with the SCGN antibody showed that this cell also expressed secretagogin (data not shown). Moreover, one might expect based on the density of Type 8 cells in the rat retina (see Fig. 6), that more SCGN-negative bipolar cell terminals would have been apparent in the ON sublamina if the Type 8 cell were devoid of SCGN labeling.
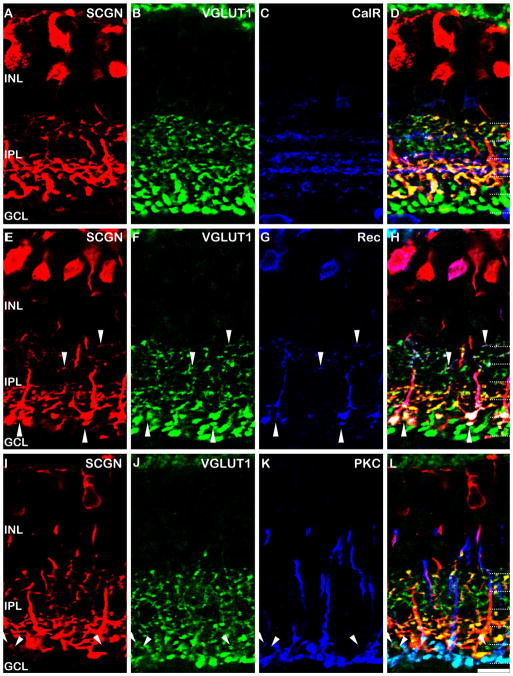
Figure 6.
Localization of secretagogin in the rat retina. A–D: Vertical sections of the rat retina immunolabeled for secretagogin (SCGN, A), VGLUT1 (B), and CalR (C). All of the secretagogin labeled axon terminals are also stained with VGLUT1 (D, yellow terminals); however, other axon terminals in S1, S2, and S5 and devoid of secretagogin staining (D, green terminals). E–H: Triple staining for secretagogin (E), VGLUT1 (F), and recoverin (Rec; G) with overlay in H. Recoverin labels the Type 2 OFF CBC (downward arrowheads) and the Type 8 ON CBC (upward arrowheads), both of which are also labeled for secretagogin. The Type 2 cell appears to be the only OFF bipolar cell to show secretagogin immunoreactivity. I–L: Triple staining for secretagogin (I), VGLUT1 (J), and PKC-α(K) with overlay in L. The vast majority of the ON CBC axon terminals are also labeled for secretagogin (L, yellow terminals); however a small number of axon terminals (arrowheads), located at the same level as the rod bipolar cell axon terminals (light blue) are devoid of secretagogin immunostaining. Dotted lines indicate the five IPL strata. Scale bar = 10 μm.
Developmental expression of secretagogin in the mouse retina
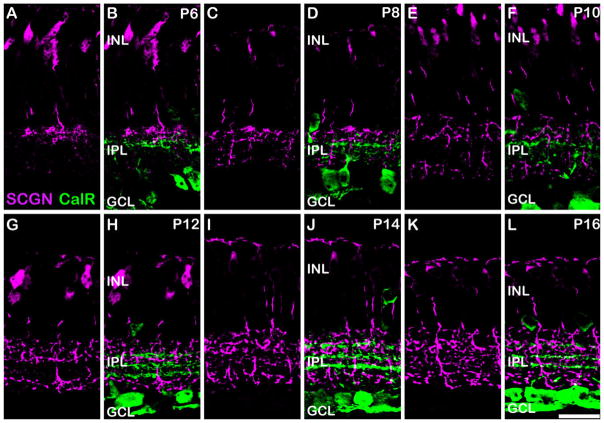
The developmental time-course of secretagogin expression is an important parameter that could be used in conjunction with functional changes to help elucidate its physiological role. No secretagogin immunoreactivity was observed in retinal sections from P2 or P4 (data not shown). Because of the known gradient in developmental maturation from central to peripheral retina, we acquired our images from central regions at each timepoint. The central band of calretinin staining, which divides the ON from OFF sublamina, is present at P6–P10, allowing us to determine whether the secretagogin-stained cells were ON or OFF cells. At P6, secretagogin staining was present in a subset of bipolar cells with axon terminals mostly restricted to the distal aspect of the IPL, suggesting that they were most likely OFF cells (Fig. 5A,B). Progressively more axon terminals were observed in the OFF sublamina, reaching an adult-like density by around P12 (Fig. 5G,H). An increase in the density of axon terminals was also seen in the S4 region of the ON sublamina between P8–12; however, few axon terminals were observed in the S3 region at this time. The first evidence of secretagogin immunoreactivity in the S3 region of the IPL was seen at P14–P16 (Fig. 5I–L); however, the intensity of staining was relatively weak compared to the other labeled axon terminals.
Figure 5.
Developmental expression of secretagogin in the mouse retina. Vertical sections from mouse retina aged postnatal day (P) 6–16, immunolabeled for secretagogin (magenta) and calretinin (CalR; green). A,B: P6. The central band of calretinin immunostaining is present and divides the ON and OFF sublaminae of the IPL. Most of the secretagogin stained bipolar cell axon terminals are located distal to the central band of calretinin staining suggesting their likely identity as OFF CBCs. C,D: P8. More secretagogin stained bipolar cell terminals are seen in the distal and proximal IPL. The density of bipolar cell terminals continues to increase at P10 (E–F) and P12 (G–H); however, the S3 region of the IPL remains relatively spared of secretagogin labeling. At P14 (I–J) and P16 (K–L), a higher number of axon terminals are seen in the S3 region of the IPL; however, the intensity of staining of these terminals appears to be comparatively weaker than the axon terminals in other strata. Scale bar = 20 μm.
Expression of secretagogin in the rat retina
While the staining pattern appears to be well preserved across species (mouse, rat, and rabbit; Fig. 2), we wanted to determine whether the subtype-specific labeling observed in mouse is conserved in a closely related species, the rat (Fig. 6). The diversity of rat bipolar cells has been previously described (see fig. 1D of Euler et al., 1996) and we used this classification and nomenclature for this study. We focused first on the OFF sublamina of the IPL. Triple labeling of secretagogin with CalR and VGLUT1 revealed that a subset of VGLUT1-positive axon terminals in S1, as well as all of the VGLUT1-positive axon terminals in S2, did not show secretagogin immunoreactivity (Fig. 6A–D). Immunostaining for recoverin, a marker of the Type 2 OFF CBCs in the rat retina (Euler et al., 1995; Chun et al., 1999), revealed that all of the secretagogin-positive axon terminals in the OFF sublamina also contained recoverin (Fig. 6E–H, downward arrowheads). These data indicate that some species variability exists in the type of OFF-bipolar cells stained by secretagogin. We also analyzed secretagogin expression in the ON-sublamina of the rat retina. In addition to labeling the Type 2 OFF CBC, recoverin labels the Type 8 ON CBC in the rat (Euler et al., 1995; Chun et al., 1999). All of the Type 8 cell axon terminals were also labeled with secretagogin (Fig. 6E–F, upward arrowheads). Finally, we triple-labeled for VGLUT1, secretagogin, and PKC to establish whether all of the ON CBCs in the rat retina were secretagogin-positive. Figure 6I–L shows that almost all ON CBC axon terminals were secretagogin-positive. However, we observed a sparse number of axon terminals, located at the S4/S5 margin, that lacked secretagogin labeling. Given that only Type 8 and Type 9 cells stratify in this region of the IPL, and that we have shown secretagogin staining in the Type 8 cell, it seems likely that, as in the mouse retina, these spared axon terminals are Type 9 (blue cone selective bipolar) cells.
DISCUSSION
This study demonstrates, for the first time, the protein expression of secretagogin in a subset of cone bipolar cells in the mammalian retina. We have provided a detailed analysis of the specific subtypes of bipolar cells that express secretagogin in the mouse and rat retina and have detailed the developmental time-course of secretagogin expression in the mouse. In the following section we discuss the implications of these findings with regard to the potential physiological roles of this protein.
Comparison of secretagogin expression with other calcium-binding proteins
The data presented here accord with a previous study describing the gene expression of secretagogin in the mouse retina (Kim et al., 2008). The Scgn gene was identified as a novel marker of ON and OFF bipolar cells using serial analysis of gene expression and in situ hybridization, although the specific subtypes of bipolar cell were not identified. Our findings extend these genetic studies at the protein level, and demonstrate broad expression of the secretagogin protein within ON and OFF cone bipolar cells. It is noteworthy that there is at least one subtype of ON-cone bipolar cell, and one subtype of OFF-cone bipolar in both rat and mouse, that does not express secretagogin, and it is tempting to speculate that the omission of the protein may convey specialized functional properties that are essential to each pathway.
The cellular expression of secretagogin displayed some variability in the rodent retina (Table 3). We did not attempt to identify the cell types expressing secretagogin in the rabbit retina due to the lack of sufficient subtype-specific bipolar cell markers. In the mouse retina, secretagogin appeared to be expressed by all OFF bipolar cells except the Type 1 bipolar cell. In contrast, in the rat retina the Type 2 bipolar was the only type of OFF CBC to express secretagogin. Another notable difference was the absence of secretagogin in the Type 7 ON CBC of the mouse retina, while the rat homolog of this cell appeared to express secretagogin. In both the rat and mouse retina, a sparse subset of axon terminals stratifying in S5 of the IPL lacked secretagogin expression. It is likely that these unlabeled terminals represent Type 9 (blue cone bipolar cells) since these cells are sparse and known to stratify in this region of the IPL (Haverkamp et al., 2005).
TABLE 3.
Summary of Secretagogin Expression in Rodent Bipolar Cells
| OFF Bipolar Cells
|
ON Bipolar Cells
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | Type 6 | Type 7 | Type 8 | Type 9 | RBC | |
| Mouse | × | ✓ | ✓ | ✓ | ✓ | ✓ | × | ✓? | ×? | × |
| Rat | × | ✓ | × | × | ✓ | ✓ | ✓ | ✓ | ×? | × |
Cell type classification from Ghosh et al. (2004) for mouse and from Euler et al. (1996) for rat.
RBC, rod bipolar cell.
A number of calcium-binding proteins have been previously identified in specific subtypes of bipolar cells. CaBP1 and CaBP5 proteins are close relatives of calmodulin and have been localized to different populations of bipolar cells. CaBP1 is expressed in Type 1 and 2 OFF CBCs (Haeseleer et al., 2000; Haverkamp et al., 2003), while CaBP5 is expressed in Type 3 OFF CBCs, Type 5 ON CBCs, and RBCs (Haeseleer et al., 2000; Haverkamp et al., 2003). Recoverin is expressed only in Type 2 OFF CBCs of the retina (Haverkamp et al., 2003), while the closely related protein calsenilin was recently shown to label Type 4 OFF CBCs (Haverkamp et al., 2008). Of these calcium-binding proteins, CaBP5 is the only one to have a functional role ascribed to it. In the CaBP5 knockout mouse, rod-mediated responses of retinal ganglion cells showed reduced light sensitivity compared with wildtype ganglion cells (Rieke et al., 2008) and the authors suggest that this may be due to a direct interaction of CaBP5 with the voltage-gated calcium channel Cav1.2 in rod bipolar cells. Despite this putative functional role, the intracellular distribution of CaBP5 is diffuse within the bipolar cells, and therefore diffuse intracellular distribution, as is seen with secretagogin and all of the aforementioned calcium-binding proteins, does not preclude a localized functional role. Indeed, the overlap of multiple calcium-binding proteins within subclasses of bipolar cells is consistent with diverse functional roles.
Developmental expression of secretagogin
During development, the first evidence for secretagogin immunostaining was observed at P6, a finding consistent with gene expression studies that showed a sharp rise in Scgn expression between P4 and P6 (Kim et al., 2008). The complete staining of the bipolar cells from their dendrites to axon terminals makes secretagogin a useful marker to track changes in bipolar cell stratification patterns during development, and to compare the development of rod and cone bipolar cells. At P6, secretagogin staining was mainly confined to cells projecting axon terminals to the OFF sub-lamina of the IPL, but from P8 onwards secretagogin-positive cells were visible in the ON sublamina. This suggests that OFF bipolar cells develop and mature earlier than the ON bipolar cells. This temporal pattern has been suggested from the development of VGLUT1 expression, which arises in OFF BC terminals prior to ON BC terminals (Sherry et al., 2003); however, robust expression of VGLUT1 does not occur until P8, 2 days after the first signs of secretagogin expression. The S3 region of the ON sub-lamina was the last to develop secretagogin immunoreactivity, and given that the Type 5 bipolar cells stratify exclusively at this level (Haverkamp et al., 2009), this finding may indicate that Type 5 cells develop relatively later than other cone bipolar cell types.
Functional role of secretagogin in the retina
The members of the EF-hand family of calcium-binding proteins are generally assigned to one of two groups: those that are actively involved in calcium sensing and signaling and those that act as passive calcium buffers. The calcium sensors have a lower affinity for calcium and undergo significant conformational changes in response to calcium binding, while the buffers have high affinity but show little conformational change. Recent evidence suggests that this dichotomy may not be so distinct, since both calretinin and calbindin D28-K, which were previously considered to be pure calcium buffers, may also show some calcium sensor function (reviewed in Schwaller, 2009). It has been demonstrated that secretagogin, which shows high sequence homology to calretinin and calbindin D-28K (Wagner et al., 2000), undergoes a significant conformational change upon calcium binding, suggesting a role in calcium sensing/signaling (Rogstam et al., 2007). However, these authors also suggested that since only 0.5%–14% of secretagogin has calcium bound at resting calcium concentrations, it could provide some buffering capacity.
One possible site of action of secretagogin is in the dendrites or soma, where it could modulate the characteristics of the postsynaptic currents. Although both RBCs and ON CBCs respond to glutamate through activation of the metabotropic glutamate receptor, mGluR6, these two cell types show dissimilar functional responses. ON CBCs respond to a sustained light flash with a sustained response, while rod bipolar cells display an initial transient peak followed by a smaller sustained component (Berntson and Taylor, 2000; Puthussery et al., 2009). The transient-sustained conductance seen in RBCs is known to be calcium-dependent, since inclusion of BAPTA in the recording pipette produces a sustained response, comparable to that seen in ON CBCs (Berntson et al., 2004). The molecular basis for this functional difference is unknown. However, recent evidence suggests that the cation channel coupled to the mGluR6 receptor is a TRP channel, possibly TRPM1 (Bellone et al., 2008; Morgans et al., 2009; Shen et al., 2009). Immunohistochemical evidence suggests that this channel is localized to the dendrites and soma of ON bipolar cells (Morgans et al., 2009). Since the conductance of TRP channels can be modulated by calcium and calcium-binding proteins (reviewed in Gordon-Shaag et al., 2008) it is possible that secretagogin could modulate TRP channel function in subtypes of bipolar cells. Future physiological recordings from identified subtypes of ON cone bipolar cells will be required to test this hypothesis.
Another possible site of secretagogin action is within the axon terminals where it could modulate transmitter release. Rogstam et al. (2007) identified the synaptosomal associated protein SNAP-25 as a highly specific binding protein for secretagogin. These proteins have been shown to interact with high affinity in the presence of calcium and to a lesser extent in the absence of calcium. SNAP-25 is a component of the SNARE complex and is involved in exocytosis. Given the evidence for expression of SNAP-25 in bipolar cell axon terminals (Brandstätter et al., 1996) it is tempting to speculate that secretagogin could be involved in the exocytotic release of glutamate from bipolar cell axon terminals. Again, we would expect to see that release of glutamate from Types 7 and 9 bipolar cells would be distinct. The development of a secretagogin knockout mouse will be an important tool for identifying the functional contribution of secretagogin to bipolar cell function.
Acknowledgments
Grant sponsor: NHMRC (Australia) Overseas Biomedical Fellowship; Grant number: 520033 (to T.P.); Grant sponsor: American Australian Association Fellowship (to T.P.); Grant sponsor: National Eye Institute (NEI); Grant number: EY017095 (to W.R.T.).
The authors thank Dr. Ludwig Wagner for generously providing the secretagogin antibody and Dr. Silke Haverkamp for providing retinas from the Gustducin-8.4GFP mouse, which was originally generated by Dr. Robert Margolskee.
LITERATURE CITED
- Adamus G, Amundson D. Epitope recognition of recoverin in cancer associated retinopathy: evidence for calcium-dependent conformational epitopes. J Neurosci Res. 1996;45:863–872. doi: 10.1002/(SICI)1097-4547(19960915)45:6<863::AID-JNR23>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004;21:913–924. doi: 10.1017/S095252380421611X. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Wässle H, Betz H, Morgans CW. The plasma membrane protein SNAP-25, but not syntaxin, is present at photoreceptor and bipolar cell synapses in the rat retina. Eur J Neurosci. 1996;8:823–828. doi: 10.1111/j.1460-9568.1996.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Chun MH, Kim IB, Oh SJ, Chung JW. Synaptic connectivity of two types of recoverin-labeled cone bipolar cells and glutamic acid decarboxylase immunoreactive amacrine cells in the inner plexiform layer of the rat retina. Vis Neurosci. 1999;16:791–800. doi: 10.1017/s0952523899164174. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Euler T, Schneider H, Wässle H. Glutamate responses of bipolar cells in a slice preparation of the rat retina. J Neurosci. 1996;16:2934–2944. doi: 10.1523/JNEUROSCI.16-09-02934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EVJ, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Witkovsky P. Cholinergic, but not the rod pathway-related glycinergic (All), amacrine cells contain calretinin in the rat retina. Neurosci Lett. 1998;247:179–182. doi: 10.1016/s0304-3940(98)00323-1. [DOI] [PubMed] [Google Scholar]
- Gartner W, Lang W, Leutmetzer F, Domanovits H, Waldhausl W, Wagner L. Cerebral expression and serum detectability of secretagogin, a recently cloned EF-hand Ca(2+)-binding protein. Cereb Cortex. 2001;11:1161–1169. doi: 10.1093/cercor/11.12.1161. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Gordon-Shaag A, Zagotta WN, Gordon SE. Mechanism of Ca(2+)-dependent desensitization in TRP channels. Channels (Austin) 2008:2. doi: 10.4161/chan.2.2.6026. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CL, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. Five members of a novel Ca(2+)-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley TL, Pochet R, Baizer L, Burton MD, Crabb JW, Parmentier M, Polans AS. Calbindin D-28K immunoreactivity of human cone cells varies with retinal position. Vis Neurosci. 1995;12:301–307. doi: 10.1017/s0952523800007987. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Functional organization of cone bipolar cells in the rat retina. J Neurophysiol. 1997;77:1716–1730. doi: 10.1152/jn.1997.77.4.1716. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Ghosh KK, Hirano AA, Wässle H. Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol. 2003;455:463–476. doi: 10.1002/cne.10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Specht D, Majumdar S, Zaidi NF, Brandstätter JH, Wasco W, Wässle H, Tom Dieck S. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101. doi: 10.1002/cne.21612. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Inta D, Monyer H, Wässle H. Expression analysis of green fluorescent protein in retinal neurons of four transgenic mouse lines. Neuroscience. 2009;160:126–139. doi: 10.1016/j.neuroscience.2009.01.081. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23:518–529. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillem GS, Dacheux RF. Rabbit cone bipolar cells: correlation of their morphologies with whole-cell recordings. Vis Neurosci. 2001;18:675–685. doi: 10.1017/s0952523801185019. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Duvoisin RM, Zheng SX, Nelson SM, Zhang J, Jeffrey BG. The TRPM1 channel: a potential role in the ON-bipolar cell light response. IOVS. 2009;2009:43. ARVO E-Abstract 1413. [Google Scholar]
- Pochet R, Pasteels B, Seto-Ohshima A, Bastianelli E, Kitajima S, Van Eldik LJ. Calmodulin and calbindin localization in retina from six vertebrate species. J Comp Neurol. 1991;314:750–762. doi: 10.1002/cne.903140408. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur J Neurosci. 2009;29:1533–1542. doi: 10.1111/j.1460-9568.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Lee A, Haeseleer F. Characterization of Ca2+-binding protein 5 knockout mouse retina. Invest Ophthalmol Vis Sci. 2008;49:5126–5135. doi: 10.1167/iovs.08-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogstam A, Linse S, Lindqvist A, James P, Wagner L, Berggard T. Binding of calcium ions and SNAP-25 to the hexa EF-hand protein secretagogin. Biochem J. 2007;401:353–363. doi: 10.1042/BJ20060918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell Mol Life Sci. 2009;66:275–300. doi: 10.1007/s00018-008-8564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- Wagner L, Oliyarnyk O, Gartner W, Nowotny P, Groeger M, Kaserer K, Waldhausl W, Pasternack MS. Cloning and expression of secretagogin, a novel neuroendocrine-and pancreatic islet of Langerhans-specific Ca2+-binding protein. J Biol Chem. 2000;275:24740–24751. doi: 10.1074/jbc.M001974200. [DOI] [PubMed] [Google Scholar]
- Wässle H, Dacey DM, Haun T, Haverkamp S, Grünert U, Boycott BB. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Vis Neurosci. 2000;17:591–608. doi: 10.1017/s0952523800174097. [DOI] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky L, Isaacs KR, Jacobowitz DM. Calretinin mRNA and immunoreactivity in the medullary reticular formation of the rat: colocalization with glutamate receptors. Brain Res. 1996;741:123–133. doi: 10.1016/s0006-8993(96)00908-0. [DOI] [PubMed] [Google Scholar]
- Wong GT, Ruiz-Avila L, Margolskee RF. Directing gene expression to gustducin-positive taste receptor cells. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]