Abstract
As most infectious organisms gain entry at mucosal surfaces, there is a great deal of interest in developing vaccines that elicit effective mucosal immune responses against pathogen challenge. Targeted vaccination is one of the most effective methods available to prevent and control infectious diseases. Mucosal vaccines can offer lower costs, better accessibility, needle free delivery, and a higher capacity for mass immunizations during pandemics. Both local mucosal immunity and robust systemic responses can be achieved through mucosal vaccination. Recent progress in understanding the molecular and cellular components of the mucosal immune system have allowed for the development of a novel mucosal vaccine platform utilizing specific dendritic cell-targeting peptides and orally administered lactobacilli to elicit efficient antigen specific immune responses against infections, including B. anthracis in experimental models of disease.
Introduction
As most infectious organisms gain entry at the mucosal surfaces of the hosts’ gastrointestinal, respiratory, and urogenital tracts, mucosal immunity and its manipulation in immunization are at the foreground of novel vaccine development. The ocular conjunctiva has also recently been appreciated as an additional mucosal route for human immunization [1]. In addition to interfacing with potential pathogens from the external environment, each of the aforementioned mucosae is also associated with a naturally occurring population of commensal microorganisms that can include bacteria, viruses, fungi, protozoa, and archea, known as its microbiota [2]. In particular, the gut harbors approximately 1014 bacteria, which is about ten times the number of cells in the entire human body [3]; thus, the mucosal immune system has evolved to maintain tolerance to its microbiota, while still responding to pathogenic invaders [4]. The relationship between this microbial community that colonizes the GI tract and the host is mutualistic, as these microbes influence the development of the host immune system [5]. In this review, we will summarize recent advances in mucosal vaccination strategies and explore dendritic cell-targeted platforms for the oral delivery of vaccine subunits for infectious disease prevention and control.
Mucosal vaccination
Vaccination prevents and controls infectious diseases. Mucosal infections, including influenza, tuberculosis, pneumonia, diarrheal diseases, and acquired immunodeficiency syndrome (AIDS), are major causes of morbidity and mortality, and act as socioeconomic burdens in both developing and developed countries [6]. Ironically, most vaccines have been based solely on empiric evidence, with little appreciation for the immune mechanisms by which they confer protective immunity [7]. The majority of vaccines used today are parenteral vaccines that are injected, but mucosal vaccines have considerable advantages compared to systemic injections including, ease of administration, improved practicality for mass vaccination in not requiring trained personnel or risking contaminated needle sticks, increased patient compliance, and ease of production due to a decreased need to purify bacterial by-products such as endotoxin, as the gut already harbors trillions of commensal bacteria [8]. Simplified production, storage, and administration methods of mucosal vaccines make them desirable for mass vaccination, especially during infectious disease outbreaks [9]. Thus, the challenge for mucosal vaccine design is to increase immunogenicity in order to induce both local and systemic immune responses and to provide an easier method of administration without compromising patient safety [10].
Mucosal Immunity
The capacity to induce local protective immunity within the mucosae, where pathogenic infection is initiated, is generally not possible with parenteral vaccination. Antigen presentation and lymphocyte priming in these tissues occur via specialized mucosal DCs in the local mucosal associated lymphoid tissue (MALT), which is located below the mucosal epithelium and is similar to peripheral lymph nodes with the exception of a much higher proportion of B cells relative to T cells; alternatively, epithelial microfold cells (M cells) can also capture luminal antigens and deliver them to underlying immune cells [10]. The hallmark of mucosal immunity and its distinction from systemic immunity is the local secretion of immunoglobulin A (IgA), which is relatively protease resistant and therefore, able to bind pathogens in gut [11]. To generate an efficacious immune response, mucosal vaccines must induce the production of antibodies at mucosal surfaces and systemically.
Mucosal dendritic cells
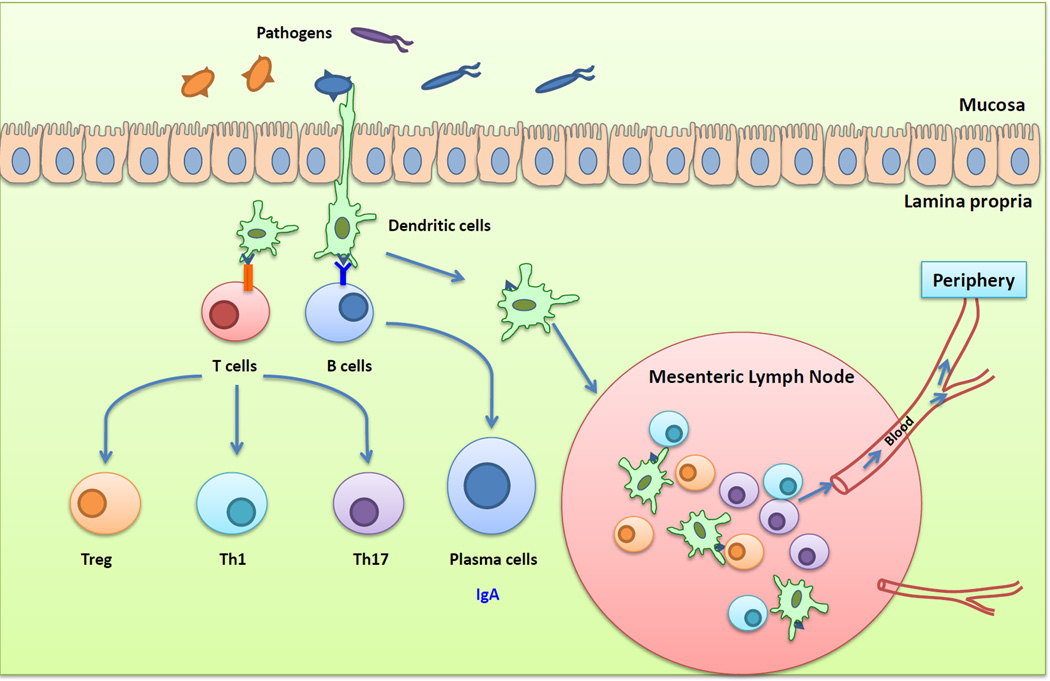
Dendritic cells (DCs) play central roles in the initiation and polarization of antigen-specific immune responses (Figure 1). DCs are found throughout the MALT of the mucosae, including the isolated lymphoid follicles and the draining lymph nodes, as well as in the subepithelial lamina propria [12]. DCs are typically divided into conventional DCs and plasmacytoid DCs (pDCs). pDCs are a minor population found in the blood, lymphoid tissues, and lamina propria that produce large amounts of type I interferon [13]. Lamina propria DCs are further classified via expression of the α-integrin, CD103. CX3CR1−CD103+ DCs differentiate from conventional myeloid DC precursors, while CX3CR1+CD103− DCs express the fractalkine receptor, CX3CR1, and are derived from monocytes [14, 15]. CX3CR1+ DCs have long stellate protrusions that interdigitate between the epithelial cells to sample antigens in the lumen of the gut and typically do not migrate. However, the initiation of mucosal T cell immunity is thought to be confined to organized lymphoid tissue and not the lamina propria. Thus, luminal antigens must be transported to MALT by CD103+ DCs to prime T lymphocytes and to imprint on them the expression of the gut homing receptors, CCR9 and α4β7 integrin [16]. The production of cytokines such as interleukin (IL)-6, IL-10, IL-12, IL-23, transforming growth factor (TGF)-β, and metabolites by macrophages and DCs, promote the induction and polarization of the T helper cell response, resulting in Th1, Th2, Th17, or regulatory T cells (Tregs) [17].
Figure 1. The intestinal immune system.
Pathogens or bacterial gene products can be captured by DC subsets situated within the epithelial layer or residing beneath the epithelial dome. These cells can either activate T and B cells directly or migrate into the mesenteric lymph nodes to present processed immunogens to T and B cells. Activated T and B cells can migrate into the periphery, poised to induce specific immunity against pathogen challenge.
Vaccine delivery systems
As it is located in close proximity to a large number of commensal microbes, the mucosal immune system uses a multifaceted regulatory system to maintain a balance between pathogen surveillance and tolerance to commensal microbes and dietary antigens. This propensity for tolerance to mucosal antigens means that mucosally-delivered antigens are typically less immunogenic than antigens delivered by another route, and require potent adjuvants, vectors, or delivery platforms for effective mucosal vaccination [6, 18]. Because of their functional specialties and the differential expression of pattern recognition receptors on mucosal DC subsets, microbial products do not bias these leukocytes to evoke common types of immune responses (Th1 or Th2). To stimulate appropriate adaptive immune responses within the mucosa, antigens likely need to be targeted to the correct DC subset with distinctive adjuvants [19]. Recent strategies for developing preventative and therapeutic vaccines have focused on the ability to deliver immunogenic microbial subunits to DCs in a targeted and prolonged manner [20]. Targeted vaccines would decrease the need for adjuvants to enhance the immune response. Despite decades of research, there are still only several adjuvants licensed for use, including alum (an aluminum salt), oil-in-water emulsions, and AS04 (the TLR4 ligand, monophosphoryl lipid A, adsorbed to alum) [7]. Accordingly, there is an urgent need to identify novel adjuvants in order to mobilize efficacious immunity with vaccination against bacterial challenge.
Lactic acid bacteria as mucosal vaccine vectors
Targeting DCs with an antigen delivery system offers tremendous potential for the development of new vaccines. Lactic acid bacteria (LAB) including, Lactobacillus acidophilus, Lactococcus lactis, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus gasseri, represent an alternative platform for mucosal delivery of therapeutic molecules, as opposed to “classical delivery systems” such as attenuated pathogens, microparticles, or liposomes [21]. These bacteria are non-pathogenic, have been shown to be very effective in delivering antigens to the mucosae, and are “generally regarded as safe” (GRAS) due to their long history of consumption in fermented foods in the human diet [22]. In addition to imunomodulatory properties, the ability to be genetically modified, and to survive gastric transit and assume a close physical association with the intestinal epithelium, make LAB good candidates for the development of live vaccine vectors targeting immunogens to the intestinal mucosa [22].
The Gram-positive lactobacilli have conserved pathogen-associated molecular patterns (PAMPs) such as peptidoglycan, cell wall polysaccharides, lipoproteins, and lipoteichoic acid (LTA) anchored in the cytoplasmic membrane; however, the chemical structure of these conserved molecules appear to vary between different strains in terms of polymer composition and length [23]. This variability in surface molecules would explain the differential capabilities of various LAB species and strains to activate innate cells (e.g., DCs), induce regulated inflammatory responses against infection, control the balance between T cell subset responses (e.g., Th1 and Th2), and enhance IgA, showing a range of outcomes that appear to promote tolerance on one end of the spectrum and immune activation on the other; qualities which promote the usefulness of these bacteria as live vaccine vectors [24–26].
Targeted dendritic cell vaccines
As DCs represent the interface of the innate and adaptive immunity, DC-targeting strategies are pivotal in the induction of antigen-specific immunity. DCs shape the adaptive immune response by internalizing and processing antigens through MHC class I and class II pathways and presenting these peptides to CD4+ and CD8+ T lymphocytes. The ability to present peptides derived from exogenous antigens on MHC I or “cross-presentation” is critical for CD8+ immunity against pathogens like influenza A virus that do not primarily infect DCs [27]. Therefore, targeting DCs with such an antigen-delivery system provides tremendous potential in developing new vaccines.
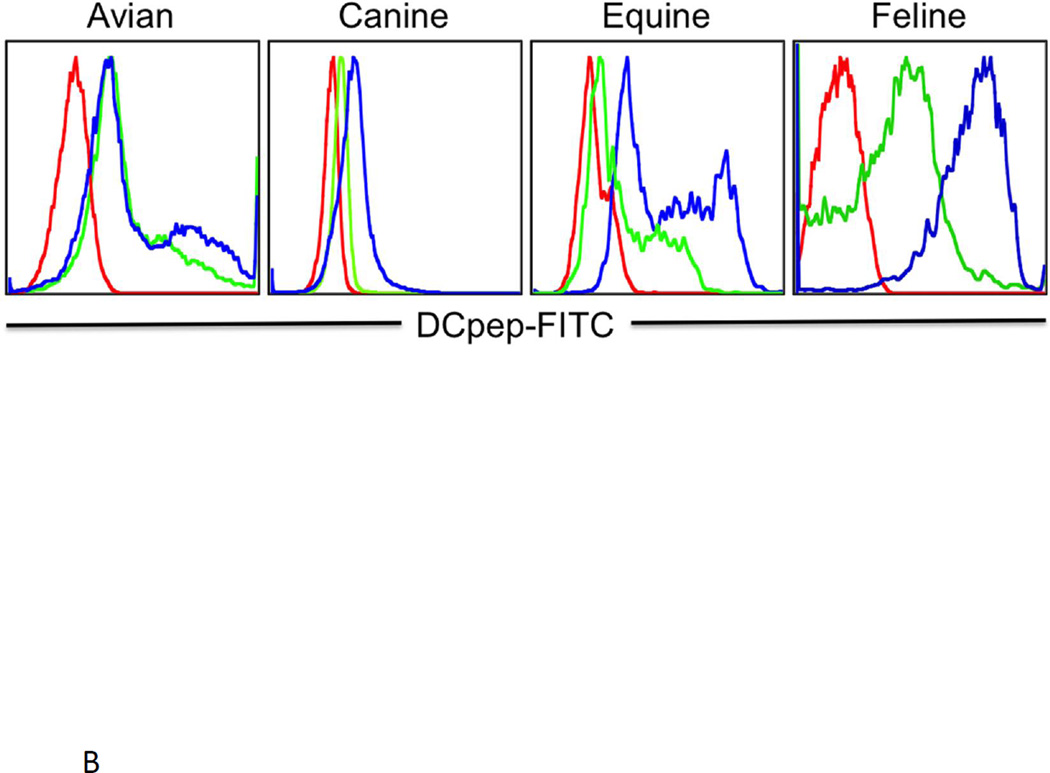
With the goal of ascertaining specific DC-binding peptides that would increase antigen immunogenicity and enhance naïve T cell priming, our laboratory identified several DC-targeting peptides, including a peptide deemed “DC-pep” that specifically bound to human CD1a+DRbrightCD11cbright DCs after screening a 12-mer peptide phage display library (Figure 2a) [28]. Interestingly, this peptide also recognizes the conserved region of its ligand on avian, canine, equine, and feline DCs; thus, this ligand is currently under intensive scrutiny (Figure 2b). Using hepatitis C virus (HCV) nonstructural protein 3 (NS3) as the immunogen, we showed that the genetic fusion of this “DC-pep” to the C terminus of NS3 enhanced DC activation, resulting in increased interferon (IFN)-γ and tumor necrosis factor (TNF)-α by CD4+ and CD8+ T cells from HCV-infected patients. Notably, the NS3-DC pep fusions did not modify the phenotype or function of the DCs, emphasizing the proficiency of these peptide fusions in directing antigens to DC subsets without inducing anergy [28]. We also recently showed that CD4+ and CD8+ T cell epitopes of the folate receptor can be targeted to this “DCpep” to elicit high avidity CD4+ T cells that mobilize immunity against breast cancer in vivo [29] suggesting the feasibility of targeted vaccines using DC binding peptides.
Figure 2.
a. Identifying 12-mer peptides that specifically bind to DCs. The Ph.D.™-12 Phage Display Peptide Library with a complexity of 1011 independent 12-mer peptide sequences fused to pIII was first incubated with non-dendritic cells including, B cells, T cells, monocytes, and macrophages. The non-bound phages were then panned with human DCs. These phage peptide selections were repeated at least four times. The enriched DC-specific phage peptide library was then amplified, and the DNA isolated to identify peptide sequences.
b. The ability to bind targeting peptides by DCs across different species. Identified 12-mer peptides were biotinylated to be screened for their binding to DCs derived from peripheral blood samples from various species, including avian, canine, equine, and feline DCs, and analyzed by a Fortessa cytometer. Experiments were repeated at least three times.
The currently licensed vaccine against Bacillus anthracis in the U.S. consists of partially purified preparations of protective antigen (PA) and aluminum hydroxide that is subcutaneously or intramuscularly administered at weeks 0, 2, 4 and at 6, 12, and 18 months with annual boosters [30]. To improve upon this vaccine approach, our laboratory exploited the DC-targeting strategy to specifically direct the immunogenic PA, to DCs by using LAB as vectors to induce optimal intestinal and systemic immune responses against B. anthracis infection (Figure 3). This platform provides numerous advantages, as L. acidophilus and L. gasseri survive well but only transiently colonize the gastrointestinal tract, can favorably stimulate DCs, and are potent adjuvants themselves. Furthermore, a mucosal, DC-targeted approach increases the bioavailability of a vaccine subunit when delivered orally by LAB [31]. To accomplish this, L. acidophilus or L. gasseri were engineered to express the targeted vaccine, such that when given orally, they protected the mice against B. anthracis infection. These lactobacilli expressing PA-DCpep vaccines induced local IgA secretion, T cell immunity, and high titers of anti-PA antibodies [32]. This is important, as protective immunization against anthrax correlates with the PA-neutralizing humoral response elicited by vaccination [30].
Figure 3. Induction of intestinal and systemic immune responses against B. anthracis infection by L. gasseri expressing the targeted PA-DCpep vaccine against B. anthracis.
The vaccine subunit PA of B. anthracis can be targeted by a 12-mer DC binding peptide (DCpep) expressed by L. gasseri. Mice orally treated with L. gasseri expressing the fusion protein targeted vaccine demonstrated the induction of local mucosal, as well as systemic immunity against B. anthracis infection.
Subsequent studies have shown that treatment of mice with different doses of L. gasseri expressing PA-DCpep induces local colonic immune responses, resulting in the activation of innate cells (e.g., DCs), which induce Th1, Th17, and Treg cell immune responses and also trigger phenotypic maturation and the release of critical pro-inflammatory cytokines by DCs and macrophages [33]. A systemic immune response is also elicited, as mice orally treated with L. gasseri expressing PA-DCpep showed higher serum levels of IgG-subclasses. Additionally, data obtained from these studies clearly showed that consumption of high numbers of L. gasseri expressing the vaccine of interest did not induce any adverse effects in vaccinated mice. Studies are ongoing to pinpoint the role of such an oral vaccine in the activation of colonic B cells and in the generation of germinal center B cells, all of which play a critical role in the elimination of infections. L. gasseri expressing PA-DCpep, which innately contains immunostimulatory lipoteichoic acid (LTA) and unmethylated DNA (CpG), also increased the gene expression of Toll-like receptors, C-type lectin receptors and NOD-like receptors in the colon [33], indicating that such a potent vaccine recruits several pattern recognition receptors to mobilize protection against invading microbes. Thus, lactobacilli-based delivery systems offer tremendous practical advantages. Recombinant antigens such as PA do not require chemical coupling agents, and the recombinant bacteria can be administered orally, whereupon both mucosal and systemic immune responses are elicited [31].
Nonetheless, plasmid-based vaccines inherently pose several disadvantages including, potential horizontal transfer of antibiotic resistance genes and diminished expression of the desired gene in the absence of selection pressure. To overcome these issues, the vaccine subunit-gene can potentially be integrated into chromosomal DNA of bacteria by insertional sequence (IS) elements, attP/intergrase systems, or homologous recombination using suicide or temperature-sensitive replicons. Recently, this last method has been improved upon by the Klaenhammer laboratory, in which genes were integrated into multiple chromosomal locations with little to no deletion of native DNA, enabling selection of one or more integration sites that can provide enhanced gene expression [34].
Concluding remarks
To further improve the induction of immunity against infectious diseases, vaccine DC-targeting peptides encoded in the genomes of beneficial engineered bacteria may be used to direct vaccine subunits in future clinical trials, whereupon oral delivery systems such as the aforementioned Gram-positive bacteria can be employed that can be easily cultured and prepared for the consumption as a vaccine.
Highlights.
A brief overview of mucosal immunity, with emphasis on intestinal immunity is given
Advantages of mucosal vaccines are discussed
We describe the identification of a unique dendritic cell-binding peptide
We report a novel mucosal vaccine platform utilizing genetically engineered lactobacilli
Acknowledgements
This work was supported in part by the National Institutes of Health Grant 1RO1AI098833-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflict of interest to disclose.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
□ of special interest
□□ of outstanding interest
- 1.Barisani-Asenbauer T, Inic-Kanada A, Belij S, et al. The ocular conjunctiva as a mucosal immunization route: a profile of the immune response to the model antigen tetanus toxoid. PLoS One. 2013;8:e60682. doi: 10.1371/journal.pone.0060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. □□ This review provides an excellent overview of the gut microbiota and host interactions. In addition, there is a helpful text box highlighting the different T helper lymphocyte subsets and their particular functions in mucosal immunity.
- 5.Dongarra ML, Rizzello V, Muccio L, et al. Mucosal immunology and probiotics. Curr Allergy Asthma Rep. 2013;13:19–26. doi: 10.1007/s11882-012-0313-0. [DOI] [PubMed] [Google Scholar]
- 6.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012;1:50–63. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nature immunology. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 9.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland DB, Fagarasan S. IgA synthesis: a form of functional immune adaptation extending beyond gut. Curr Opin Immunol. 2012;24:261–268. doi: 10.1016/j.coi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Rescigno M. Dendritic cells in oral tolerance in the gut. Cell Microbiol. 2011;13:1312–1318. doi: 10.1111/j.1462-5822.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 13. Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. □□ This is a very thorough and comprehensive review discussing the roles of the different subsets of dendritic cells, including tables and figures that list the differential expression of phenotypic markers, the functions, and anatomic locations of the dendritic cell subsets. There is also a helpful table describing how to characterize mouse tissue dendritic cells in vivo.
- 14.Varol C, Vallon-Eberhard A, Elinav E, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Rescigno M. Dendritic cells in bacteria handling in the gut. J Leukoc Biol. 2011;90:669–672. doi: 10.1189/jlb.0311141. [DOI] [PubMed] [Google Scholar]
- 16.Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. 2013;91:232–239. doi: 10.1038/icb.2012.79. [DOI] [PubMed] [Google Scholar]
- 17.Chinen T, Rudensky AY. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev. 2012;245:45–55. doi: 10.1111/j.1600-065X.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujkuyama Y, Tokuhara D, Kataoka K, et al. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012;11:367–379. doi: 10.1586/erv.11.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duluc D, Gannevat J, Anguiano E, et al. Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 2013;6:626–638. doi: 10.1038/mi.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nature reviews. Immunology. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leblanc JG, Aubry C, Cortes-Perez NG, et al. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett. 2013 doi: 10.1111/1574-6968.12159. [DOI] [PubMed] [Google Scholar]
- 22.Wells J. Mucosal vaccination and therapy with genetically modified lactic acid bacteria. Annu Rev Food Sci Technol. 2011;2:423–445. doi: 10.1146/annurev-food-022510-133640. [DOI] [PubMed] [Google Scholar]
- 23. van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013;34:208–215. doi: 10.1016/j.it.2013.01.005. □ This paper concisely reviews the differential immune responses elicited by lactobacilli and discusses some of the molecular mechanisms for species and strain specificity in the resulting immunity.
- 24.Mohamadzadeh M, Duong T, Hoover T, Klaenhammer TR. Targeting mucosal dendritic cells with microbial antigens from probiotic lactic acid bacteria. Expert Rev Vaccines. 2008;7:163–174. doi: 10.1586/14760584.7.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Mohamadzadeh M, Klaenhammer TR. Specific Lactobacillus species differentially activate Toll-like receptors and downstream signals in dendritic cells. Expert Rev Vaccines. 2008;7:1155–1164. doi: 10.1586/14760584.7.8.1155. [DOI] [PubMed] [Google Scholar]
- 26.Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A. 2005;102:2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waithman J, Mintern JD. Dendritic cells and influenza A virus infection. Virulence. 2012:3. doi: 10.4161/viru.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel TJ, Morris C, Brumlik M, et al. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J Immunol. 2004;172:7425–7431. doi: 10.4049/jimmunol.172.12.7425. [DOI] [PubMed] [Google Scholar]
- 29.Erskine CL, Krco CJ, Hedin KE, et al. MHC class II epitope nesting modulates dendritic cell function and improves generation of antigen-specific CD4 helper T cells. J Immunol. 2011;187:316–324. doi: 10.4049/jimmunol.1100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitlaru T, Altboum Z, Reuveny S, Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev. 2011;239:221–236. doi: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Mohamadzadeh M, Durmaz E, Zadeh M, et al. Targeted expression of anthrax protective antigen by Lactobacillus gasseri as an anthrax vaccine. Future Microbiol. 2010;5:1289–1296. doi: 10.2217/fmb.10.78. [DOI] [PubMed] [Google Scholar]
- 32.Mohamadzadeh M, Duong T, Sandwick SJ, et al. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kathania M, Zadeh M, Lightfoot YL, et al. Colonic immune stimulation by targeted oral vaccine. PloS one. 2013;8:e55143. doi: 10.1371/journal.pone.0055143. □ Original work indicating the efficacy of an oral targeted vaccine that elicits local and systemic immune responses with no adverse effects.
- 34. Douglas GL, Klaenhammer TR. Directed chromosomal integration and expression of the reporter gene gusA3 in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2011;77:7365–7371. doi: 10.1128/AEM.06028-11. □ Continuation of work from an active research laboratory that constantly improves the chromosomal insertion of genes of interest.