Abstract
Background
Neuroblastoma (NB) is the most common solid extracranial tumor in children. However, the molecular mechanism and progression of NB is largely unknown, and unfortunately, the prognosis is poor. Src-associated in mitosis with a molecular weight of 68 kDa (Sam68) is associated with carcinogenesis and neurogenesis. The present study aimed to investigate the clinical and prognostic significance of Sam68 in NB.
Methods
The expression of Sam68 in immortalized normal epithelial cells, NB cell lines, and in four cases of paired NB tissue and adjacent normal tissue from the same patient was examined using Western blotting, reverse transcription-polymerase chain reaction (PCR) and real-time reverse transcription-PCR. The proliferation of NB cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Furthermore, Sam68 protein expression was analyzed in 90 NB cases characterized as clinicopathological using immunohistochemistry. Statistical analyses were applied to evaluate the diagnostic value and associations of Sam68 with clinical parameters.
Results
Western blotting and reverse transcription-PCR showed that the expression level of Sam68 was markedly higher in NB cell lines than in the immortalized normal epithelial cells at both messenger RNA and protein levels. The MTT assay revealed that Sam68 expression supported proliferation of NB cells. Sam68 expression levels were significantly up-regulated in tumor tissues in comparison to the matched adjacent normal tissues from the same patient. Sam68 protein level was positively correlated with clinical stage (P<0.001), tumor histology (P<0.001), and distant metastasis (P=0.029). Patients with higher Sam68 expression had shorter overall survival time, whereas those with lower tumor Sam68 expression had longer survival time.
Conclusion
Our results suggest that Sam68 expression is associated with neuroblastoma progression and may represent a novel and valuable predictor for prognostic evaluation of neuroblastoma patients.
Keywords: Sam68, biomarker, prognosis, neuroblastoma
Background
Neuroblastoma (NB) is the most common solid extracranial tumor in children and also the most common childhood cancer diagnosed in children under 1 year old. Importantly, it has been suggested that the incidence of NB has increased in recent years.1,2 Due to their neural crest cell lineage, NBs may occur in the adrenal medulla (most common location) or anywhere along the sympathetic ganglia. NB is characterized by a wide range of clinical behavior. Generally, the therapy applied to NB patients depends on the risk category. Low-risk patients can be cured with surgery or just observed without treatment. Intermediate-risk NB is usually treated with surgery and chemotherapy. High-risk NB is treated with surgery, intensive chemotherapy, radiation therapy, bone marrow or hematopoietic stem cell transplantation, and targeted biologic therapies and immunotherapy.3 But still, recent therapeutic advance fails to significantly increase the 5-year survival rates of aggressive NB, despite of the expansion of knowledge about NB. Recent research has revealed that some molecules, such as v-myc myelocytomatosis viral related oncogene, neuroblastoma derived (MYCN), anaplastic lymphoma kinase (ALK), and protein kinase B (AKT/PKB), play crucial roles in the transformation of progenitor cells into NB and in the processes involved in NB proliferation, angiogenesis, invasion, migration, and metastasis.4–6 However, the underlying molecular mechanism remains poorly understood, and thus far, no ideal biomarker has been reported to specifically differentiate aggressive NB. Therefore, it is of great value to further understand the etiology of NB and to identify valuable diagnostic and prognostic markers as well as novel therapeutic targets of the disease.
Src-associated in mitosis with a molecular weight of 68 kDa (Sam68), originally identified as the first mitotic substrate of the tyrosine kinase Src in fibroblasts, is a member of the signal transduction and activation of RNA (STAR) family of RNA-binding proteins (RBPs).7,8 With a GRP33/Sam68/GLD1 (GSG or STAR) domain, the finding that Sam68 can interact with both RNA targets and other proteins suggests its possible regulation function in both RNA and protein metabolism.7,8 Subsequent studies have proved that Sam68 participated in messenger RNA (mRNA) processing, from transcription, to alternative splicing, to nuclear export.9–12 Meanwhile, Sam68 has been found to interact with numerous signaling proteins, such as Src and breast tumor kinase (BRK).13,14 Also, it has been reported that Sam68 acts as a regulator in tumor necrosis factor (TNF)-induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and apoptosis, involved in cell cycle regulation and apoptosis.15 All of this evidence suggests that Sam68 plays essential roles in cell proliferation, differentiation, and apoptosis, which affect carcinogenesis and tumor progression. In accordance with its oncogenic property, Sam68 has been found to be upregulated in renal cell carcinoma, breast cancer, cervical cancer, and prostate cancer.16–20 Previous findings highlight how aberrant regulation of Sam68 function corresponds with oncogenic transformation and cancer progression, thereby pointing to Sam68 as a potentially useful molecular biomarker for the identification of high-risk tumor phenotypes. Sam68 might be involved in supporting the proliferation and tumorigenicity of breast cancer cells and prostate cancer cells.18,20 In addition, recent evidence has linked Sam68 to neurogenesis.21,22 The Sam68 protein is upregulated upon neuronal differentiation of P19 cells, which fail to differentiate when Sam68 is knocked down by short hairpin RNAs, and the differentiation of primary neuronal progenitor cells from an embryonic mouse neocortex is suppressed by Sam68 depletion and promoted by Sam68 overexpression.21 Also, Sam68 has been reported to regulate neuronal activity-dependent alternative splicing of Neurexin-1.22 The above studies implicated that Sam68 might be a key regulator of carcinogenesis and neurogenesis.
However, whether Sam68 regulation occurs in NB progression remains unknown. In this study, we report for the first time the overexpression of Sam68 in both NB cell lines and patient samples of NB. Furthermore, Sam68 overexpression correlates with clinicopathologic characteristics of NB patients. Our results suggest that Sam68 might be a valuable diagnostic and prognostic marker as well as a novel therapeutic target of NB.
Materials and methods
Cell lines
The primary-cultured immortalized normal epithelial cells as control cells were maintained in bronchial epithelial growth medium (Lonza Group Ltd, Basel, Switzerland), supplemented with 5 ng/mL epithelial growth factor, 70 ng/mL phosphoethanolamine, and 10% fetal bovine serum. Neuroblastoma cell lines (obtained from American Type Culture Collection, ATCC), including IMR-32, M17, LAN-5, LAN-6, SK-N-SH, and SH-SY5Y (a subclone of SK-N-SH), were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin. Cells were maintained in a humidified atmosphere at 37°C with 5% CO2.
Vectors and retroviral infection
Two Sam68 RNA interference (RNAi) oligonucleotides were cloned into the pSuper-retro-puro vector to generate pSuper-retro-Sam68-RNAi#1 and pSuper-retro-Sam68-RNAi#2. Both the RNAi vector and phosphatidylinositol kinase (PIK) plasmids were generous gifts from Professor Libing Song, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China. Both pSuper-retro-Sam68(s) combined with PIK vector and pSuper-retro-Sam68(s) combined with blank vector as control were packed into virus using the calcium phosphate method. Retroviral production and infection were performed as described previously.23 Stable cell lines expressing Sam68 RNAi(s) were selected with 0.5 ug/L of puromycin 48 hours after infection. After 10-day selections, the SH-SY5Y cell lysates prepared from the pooled population of cells in the sample buffer were fractionated on sodium-dodecyl-sulfate-polyacrylamide-gel-electrophoresis (SDS-PAGE) for the detection of the Sam68 protein level.
Patient information and tissue specimens
This study was conducted on a total of 90 paraffin-embedded NB specimens from female patients, which were histopathologically and clinically diagnosed as NB at the First Affiliated Hospital of Sun Yat-sen University from 1995 to 2005. For the use of these clinical materials for research purposes, prior patients’ consent and approval were obtained from the First Affiliated Hospital of Sun Yat-sen University and the Institutional Research Ethics Committee. All samples were collected and analyzed with prior written informed consent from the children’s parents.
Clinical and pathological classification and staging were determined according to the classification criteria proposed by the International Neuroblastoma Staging System (INSS). Clinical information on the samples is summarized in Table 1. The percentages of tumor purity in sections adjacent to the regions used for RNA extraction were estimated during routine histopathologic analysis.
Table 1.
Correlations between Sam68 expression and clinicopathologic characteristics of neuroblastoma
| Characteristics | n | Sam68 expression
|
Chi-square test, P-value | |
|---|---|---|---|---|
| Low or none, number of cases (%) | High, number of cases (%) | |||
| Gender | ||||
| Male | 57 | 10 (11.11) | 23 (25.56) | 0.822 |
| Female | 33 | 16 (17.78) | 41 (45.56) | |
| Age (years) | ||||
| ≤1 | 19 | 9 (10.00) | 10 (11.11) | 0.045 |
| >1 | 71 | 17 (18.89) | 54 (60.00) | |
| Clinical stage | ||||
| 1 | 4 | 4 (4.44) | 0 (0.00) | <0.001 |
| 2A/2B | 19 | 11 (12.22) | 8 (8.89) | |
| 3 | 23 | 6 (6.67) | 17 (18.89) | |
| 4 | 40 | 4 (4.44) | 36 (40.00) | |
| 4S | 4 | 1 (1.11) | 3 (3.33) | |
| Tumor histology | ||||
| Undifferentiated | 48 | 7 (7.78) | 41 (45.56) | <0.001 |
| Differentiated | 29 | 16 (17.78) | 13 (14.44) | |
| GNB | 13 | 3 (3.33) | 10 (11.11) | |
| Primary tumor site | ||||
| Adrenal | 58 | 15 (16.67) | 43 (47.78) | 0.394 |
| Extra-adrenal | 32 | 11 (12.22) | 21 (23.33) | |
| Metastases | ||||
| Yes | 54 | 11 (12.22) | 43 (47.78) | 0.029 |
| No | 36 | 15 (16.67) | 21 (23.33) | |
Note: Clinical and clinicopathologic classification and staging were determined according to International Neuroblastoma Staging System (INSS) criteria.
Abbreviations: GNB, ganglioneuroblastoma; Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
RNA extraction, reverse transcription, and real-time polymerase chain reaction (PCR)
Total RNA from cultured cells and fresh tissues was extracted using the Trizol reagent (Life Technologies) according to the manufacturer’s instruction. The extracted RNA was pretreated with ribonuclease-free deoxyribonuclease, and about 2 ug of RNA from each sample was used for complementary DNA (cDNA) synthesis with an IScriptcDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). For PCR-mediated amplification of Sam68 cDNA, an initial amplification using Sam68 specific primers was done with denaturation at 95°C for 10 minutes, followed by 30 denaturation cycles at 95°C for 60 seconds, primers annealing at 55°C for 30 seconds, and primers extension at 72°C for 30 seconds. Upon completion of the cycling steps, a final extension at 72°C for 5 minutes was carried out before the reaction mixture was stored at 4°C. Real-time PCR was employed to quantify the fold of increase of Sam68 mRNA in each of the primary NB tissues relative to the paired adjacent noncancerous tissues from the same patient, by a Bio-Rad CFX96 sequence detection system with SsoFast EvaGreen Supermix (Bio-Rad Laboratories). Reverse transcription-PCR and real-time PCR primes were designed using the Primer Express Software Version 2.0 (Life Technologies) and the primers sequences were: Sam68 forward primers: 5′-ATGAAGCTTATGGCCAGGAC-3′; Sam68 reverse primers: 5′-CAGAAGCCAGAATGCAGAGT-3′; Sam68 probe: 5′-CAGAATATTATGACTATGGACATGG-3′; glyceraldehyde-3 phosphate dehydrogenase (GAPDH) forward prime: 5′-CCAATGTGTCCGTCGTGGAT-3′; GAPDH reverse primers: 5′-TGCTGTTGAAGTCGCAGGAG-3′; and GAPDH probe: 5′-CATCACTGCCACCCAGAAGACTGTG-3′. Expression data were normalized to the geometric mean of the housekeeping gene GAPDH to control the variability in expression levels and calculated as 2–[(Ct of Sam68) – (Ct of GAPDH)], where Ct represents the threshold cycle for each transcript.
Western blotting
Western blotting analysis was performed according to standard methods as described previously,24 using anti-Sam68 rabbit polyclonal antibody (1:1,000; Santa Cruz Biotechnology, Inc, Dallas, TX, USA). Blotting membranes were stripped and reprobed with anti-GAPDH antibody (1:1,000; Sigma-Aldrich, St Louis, MO, USA) as a loading control.
3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay
Cells, seeded on 96-well plates, were stained at the indicated time point (0, 24h, 48h, 72h, 96h) with 100 mL of sterile MTT dye (0.5 mg/mL, Sigma-Aldrich) for 4 hours at 37°C, followed by removal of the culture medium and the addition of 150 mL of dimethyl sulphoxide (DMSO) (Sigma-Aldrich). The absorbance was measured at 570 nm, with 655 nm as the reference wavelength. All experiments were performed in triplicate.
Immunohistochemistry
Immunohistochemistry staining was carried out using Histostain-Plus Kits (Life Technologies) following the manufacturer’s protocols. Two independent pathologists blinded to the clinical parameters conducted the immunoreactivity score (IRS) for Sam68 expression. The staining results were scored based on the following criteria: i) percentage of positive tumor cells in the tumor tissue: 0 (0%), 1 (1%–10%), 2 (11%–50%), and 3 (51%–100%); ii) staining intensity: 0 (no staining), 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). IRS was calculated as staining intensity score × proportion of positive tumor cells, which scores as 0, 1, 2, 3, 4, 6, and 9. Cutoff values for Sam68 were chosen on the basis of a measure of heterogeneity with a log-rank test statistical analysis with respect to overall survival. An optimal cutoff value was identified: IRS ≥4 was used to define tumors as high Sam68 expression and IRS ≤3 as low expression of Sam68.
Statistical analysis
All statistical analyses were carried out using the SPSS version 13.0 statistical software packages (IBM Corporation, Armonk, NY, USA). The correlation between Sam68 expression and the clinicopathological characteristics was analyzed by a chi-square test. Survival curves were plotted by the Kaplan–Meier method and compared with the log-rank test. The significance of various variables for survival was evaluated by univariate and multivariate Cox regression analyses. P<0.05 in all cases was considered statistically significant.
Results
Upregulation of Sam68 in NB cells
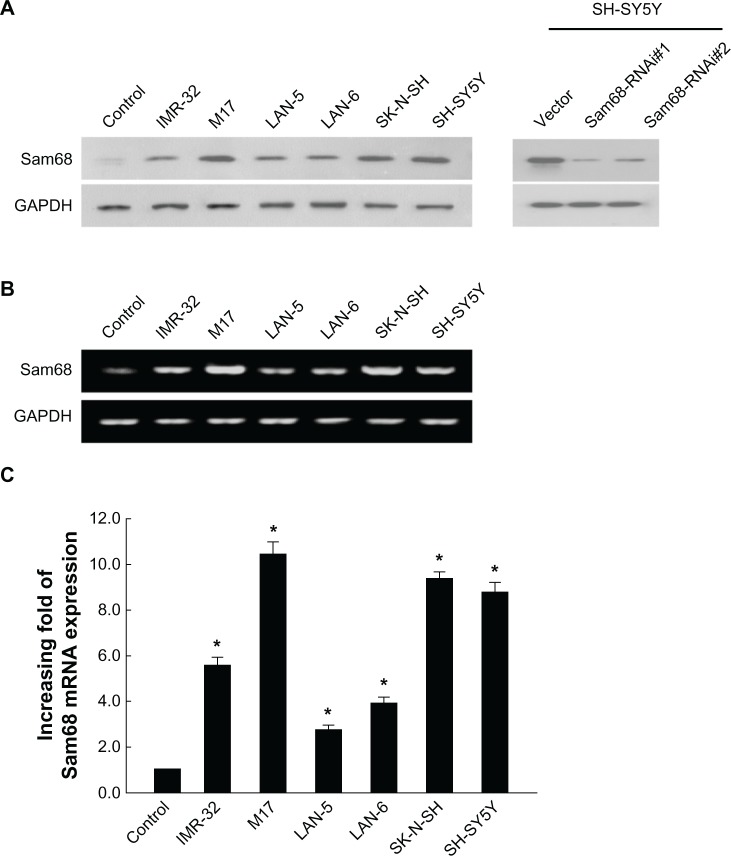
Western blotting analysis showed that Sam68 protein was highly expressed in all NB cell lines, whereas it was weakly detected in the immortalized normal epithelial cells (Figure 1A). Reverse transcription-PCR and real-time reverse transcription-PCR were carried out to detect the mRNA level. As shown in Figure 1B and C, in parallel with upregulation of the Sam68 protein, the six NB cell lines consistently showed significantly higher levels of Sam68 mRNA in comparison with the control cell lines.
Figure 1.
Upregulation of Sam68 protein and mRNA in neuroblastoma cell lines.
Notes: (A) Western blotting showed the expression level of Sam68 protein in the normal control cells, cultured neuroblastoma cell lines (IMR-32, M17, LAN-5, LAN-6, SK-N-SH, and SH-SY5Y), and the SH-SY5Y neuroblastoma cell line stably transduced with retroviral vectors expressing Sam68 RNAi(s) (Sam68-RNAi#1 and Sam68-RNAi#2) or with control vector virus (vector). (B) Expression of Sam68 mRNA in the normal control cells and neuroblastoma cell lines by reverse transcription-PCR. (C) The average ratios of Sam68 expression quantified by real-time reverse transcription-PCR in the normal control cells and neuroblastoma cell lines. Expression levels were normalized for GAPDH. Columns, mean from three parallel experiments; bars, standard deviation; *P<0.05 (Student’s t-test).
Abbreviations: GAPDH, glyceraldehyde-3 phosphate dehydrogenase; mRNA, messenger RNA; PCR, polymerase chain reaction; RNAi, RNA interference; Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
To validate the specificity of the Sam68 antibody used in the Western blotting and the following immunohistochemical staining experiments, we tested the antibody on SH-SY5Y cell lines stably transduced with retroviral vectors expressing Sam68 RNAi(s). As shown in supplemental Figure 2, there are no significant differences in growth rate, cell morphology and gene expression between the mock (SH-SY5Y cells) and the Vector. Western blotting analysis showed that the antibody detected decreased expression of Sam68 protein in the cells (Figure 1A, right), which indicates that the Sam68 antibody used in our study specifically recognizes the Sam68 protein.
Figure 2.
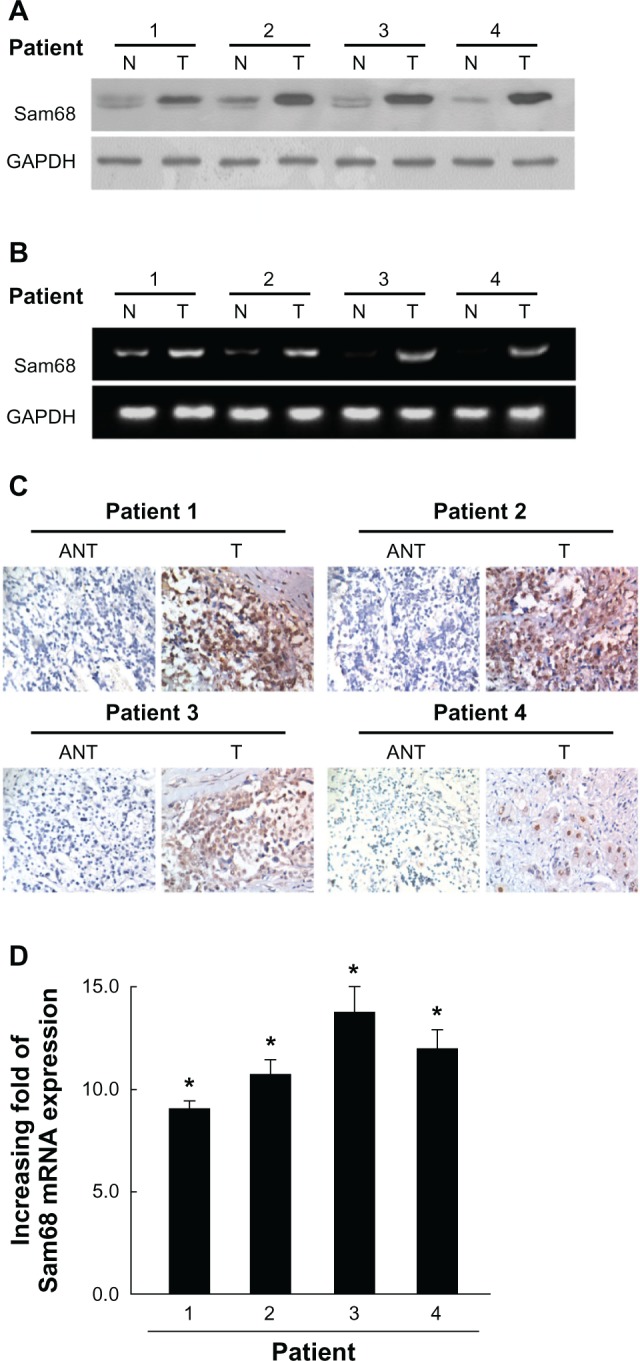
Overexpression of Sam68 in primary neuroblastoma tissues.
Notes: (A) Expression of Sam68 protein in each of the primary neuroblastoma tissues (T) and adjacent noncancerous tissues (N) paired from the same patient by Western blotting. (B) Reverse transcription-PCR analysis of Sam68 mRNA expression in each of the primary neuroblastoma tissues (T) and adjacent noncancerous tissues (N) paired from the same patient. GAPDH was probed as a loading control. (C) Sam68 expression levels were upregulated in the primary neuroblastoma tissues (T) in comparison to the paired adjacent noncancerous tissues (ANT) from the same patient, as examined by immunohistochemistry. (D) The average tumor/noncancerous (T/ANT) ratios of Sam68 expression quantified by real-time reverse transcription-PCR. Expression levels were normalized for GAPDH. Columns, mean from three parallel experiments; bars, standard deviation; *P<0.05 (Student’s t-test).
Abbreviations: ANT, adjacent noncancerous tissues; GAPDH, glyceraldehyde-3 phosphate dehydrogenase; mRNA, messenger RNA; N, noncancerous tissues; PCR, polymerase chain reaction; Sam68, Src-associated in mitosis with a molecular weight of 68 Kd; T, neuroblastoma tissues.
High Sam68 level correlates with active proliferation
Cell proliferation was observed in six NB cell lines (Figure S1), showing that cell growth is parallel to Sam68 protein and mRNA expression. This proved that Sam68 might enhance proliferation ability in NB.
Overexpression of Sam68 in NB
To determine whether the upregulation of Sam68 in NB cell lines is clinically correlated with NB progression, we carried out Western blotting analysis on four pairs of matched adjacent normal adrenal medulla tissue and adrenal-original NB samples. As shown in Figure 2A, Sam68 was found to be differentially overexpressed in all four examined human tumor samples paired with adjacent normal tissues from the same patients. This finding is consistent with the results obtained in our immunohistochemical analysis (Figure 2C). Reverse transcription-PCR (Figure 2B) and real-time reverse transcription-PCR (Figure 2D) revealed the degrees of Sam68 mRNA upregulation in tumor samples. These results, obtained from clinical samples, confirm that higher levels of Sam68 are expressed in cancer lesions than in surrounding tumor-adjacent regions.
Increased Sam68 expression correlates with clinicopathologic characteristics of NB patients
We further examined the possible correlations between expression levels of Sam68 and clinicopathologic characteristics of NB. As summarized in Table 1, analysis of 90 NB samples indicated that Sam68 expression was correlated with age (P=0.045), clinical stage (P<0.001), tumor histology (P<0.001), and distant metastasis (P=0.029). However, our analyses did not show significant associations between Sam68 expression and other clinical features including gender and primary tumor site.
High Sam68 expression is associated with poor prognosis of patients with NB
Spearman correlation analysis revealed that higher Sam68 expression level was associated with shorter survival time (P<0.001), with a correlation coefficient of −0.484. As shown in Table 2, Spearman correlations of Sam68 expression level to clinical stage, tumor histology, and metastases were 0.439 (P<0.001), 0.271 (P=0.010), and 0.230 (P=0.029), respectively. Kaplan–Meier analysis showed that survival time was significantly different between the low and high Sam68 expression groups (P<0.001). As shown in Figure 3, the cumulative 5-year survival rate was 54.70% in the low Sam68 expression group, but it was only 30.16% in the high expression group. In addition, we did multivariate survival analysis to determine whether Sam68 expression level was an independent prognostic factor (Table 3). In this analysis, clinical stage and Sam68 expression were each recognized as independent prognostic factors. Thus, our findings clearly indicate that Sam68 expression level has a significant correlation with the prognosis of NB.
Table 2.
Spearman correlation analysis between Sam68 and clinical pathologic factors
| Variables | Sam68 expression level
|
|
|---|---|---|
| Correlation coefficient | P-value | |
| Survival time | −0.484 | <0.001 |
| Clinical staging | 0.439 | <0.001 |
| Tumor histology | 0.271 | 0.010 |
| Metastases | 0.230 | 0.029 |
Abbreviation: Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
Figure 3.
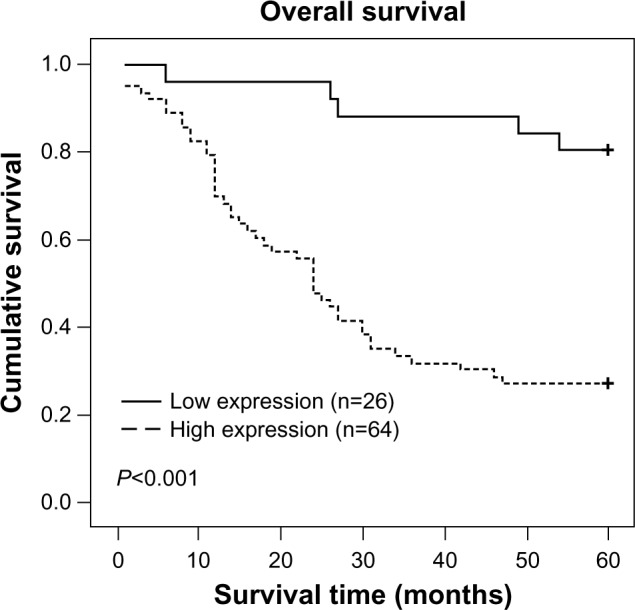
Patients with high Sam68 expression had shorter survival time.
Notes: Kaplan–Meier curves with univariate analyses (log-rank) for patients with low Sam68 expression versus high Sam68 expression tumors showed that the cumulative 5-year survival rate was 54.70% in the low Sam68 expression group (n=26, thick line), but it was only 30.16% in the high-expression group (n=64, dotted line).
Abbreviation: Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
Table 3.
Univariate and multivariate analyses of various prognostic variables in patients with neuroblastoma
| Univariate analysis
|
Multivariate analysis
|
|||||
|---|---|---|---|---|---|---|
| Number of patients | P-value | Regression coefficient (standard error) | P-value | Relative risk | 95% confidence interval | |
| Clinical stage | ||||||
| Advanced (3 and 4) | 73 | <0.001 | 1.638 (0.437) | 0.007 | 3.359 | 1.394–8.094 |
| Early (1, 2, and 4S) | 17 | |||||
| Sam68 | ||||||
| Low | 26 | <0.001 | 1.817 (0.473) | 0.003 | 4.189 | 1.613–10.875 |
| High | 64 | |||||
Abbreviation: Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
Discussion
In this study, we presented the first evidence of Sam68 upregulation in NB. Also, we aimed at evaluating the possibility of using Sam68 as a potential clinical indicator to identify aggressive NB, as well as a prognostic marker for patient survival in cancer. We found that expression of Sam68 was upregulated at both the mRNA and protein levels, in NB cell lines as compared with the control cells. Here we refer to the results of our study. Also, we showed that paired neuroblastoma lesions and adjacent normal tissues had been found to express Sam68 differently, with the tumor lesions displaying higher expression of Sam68. Furthermore, our immunostaining studies showed that the expression level of Sam68 protein significantly correlated with NB clinical characteristics and reduced survival time of patients with high Sam68 expression. Taken together, our study suggests that Sam68 might represent a novel indicator for differentiating aggressive NB, thus guiding the individual therapeutic strategies.
NB is such a heterogeneous disease that it can spontaneously regress, mature, or display an aggressive, therapy-resistant phenotype. Increasing evidence suggests that the biological and molecular features of NB significantly influence its phenotype and are highly predictive of its clinical behaviors.4–6,25,26 The previous classification system takes into consideration the histologic features of NB such as the degree of cellular differentiation, Schwannian stroma, and the mitosis-karyorrhexis index, in addition to the age of the patient.27 Recently, several clinically relevant and statistically significant molecular markers such as MYCN gene amplification status, chromosome 11q aberration, and DNA ploidy were incorporated into the new classification system.28 Even so, the risk classification system of NB is far from satisfactory. For this reason, seeking diagnostic and prognostic markers as well as novel therapeutic targets of NB is definitely essential.
It has been clearly documented in previous studies that Sam68 was associated with various biological processes, such as signal transduction, RNA metabolism, cell cycle regulation, and apoptosis, suggesting divergent roles for Sam68 in cancer.15–20 At first, it was reported that Sam68 knockout fibroblast cells exhibited anchorage-independent growth, defective contacted inhibition, and formed metastatic tumors in nude mice.29 So Sam68 was originally defined as a tumor suppressor protein. However, other studies have shown that Sam68 contributes to cancer progression, by which Sam68 could positively affect cancer cell proliferation and cell cycle progression.30, 31 Modem et al reported that heat shock protein 22 (Hsp22) (HspB8/H11) knockdown induced Sam68 expression and stimulated proliferation of glioblastoma cells in U87 glioblastoma cells, which contained higher levels of Hsp22 than the other cell lines tested; Hsp22 knockdown dramatically increased both Sam68 mRNA and protein, altered cellular morphology, and enhanced cell proliferation, which was associated with a sharp decrease in G0/G1 and a corresponding increase in S and G2/M phases in exponentially growing cultures.30 Chen et al suggested a mechanism by which the Src family kinase-Fyn heterogenous nuclear ribonucleoprotein A2B1 (HnRNPA2B1) and Sam68 to coordinate and regulate apoptosis, thus promoting the proliferation and metastasis of pancreatic cancer.31 Researchers have identified a role of Sam68 in TNF-induced NF-κB activation and apoptosis.15 One study suggested that Sam68 acted as a convergence point for ERK signaling pathway and potentially in the process of metastasis.32 Nevertheless, the biological significance of Sam68 in cancer development and progression is still poorly understood, and needs to be further studied.
In our study, upregulation of Sam68 in NB was confirmed from several aspects, including examination of protein expression and Sam68 mRNA in NB cell lines in comparison with those in immortalized normal epithelial cells, observation of Sam68 level and cancer proliferation in different NB cell lines, comparative analysis of Sam68 expressions in paired tumor tissues and adjacent normal tissues, and a clear showing of a high level of expression of Sam68 in a relatively large number of NB specimens. The oncogenic potential of Sam68 has been implicated as it promoted NB cell proliferation. Moreover, we found a significant correlation between Sam68 expression and clinicopathologic characteristics of NB. NB patients with higher Sam68 expression had shorter overall survival time than patients with lower Sam68 expression (P<0.001). Apparently, further studies are needed to investigate the correlations of Sam68 with the above mentioned molecular markers used in the current classification system. These results not only suggest a potentially promising usefulness of Sam68 as a novel indicator for differentiating aggressive NB and its prognosis, but also warrant further studies on a possible link between the biological function of Sam68 and the pathogenesis of NB, which might eventually lead to the development of a novel therapeutic target for the disease.
In conclusion, our study evaluated the possibility of using Sam68 as a clinically relevant indicator for NB progression and as a prognostic marker for patient survival in NB. In combination with other biomarkers of NB, Sam68 might be valuable for evaluating the diagnosis and prognosis, as well as providing potential targets for new therapeutic approaches for children with NB. Hence, further study on the mechanism by which Sam68 is involved in the development and progression of NB and prospective research on the prognostic significance of Sam68 are required.
Supplementary figures
The neuroblastoma cell growth as determined by MTT assay.
Abbreviation: MTT, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide.
Cell growth and Sam68 expression in SH-SY5Y cells (mock) and vector-infected cells (vector).
Notes: (A) The cell growth of mock and vector as determined by MTT assay. (B) Cultured cells under microscope. (C) Western blotting shows the expression level of Sam68 protein.
Abbreviations: MTT, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide; Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.
Acknowledgments
This study was supported by the Science and Technology Plans of Guangdong Province, China (No. 2010B060900026) and the Guangdong Provincial Natural Science Foundation (No. 9151008901000045).
Authors’ contributions
Zhe Xu conceived and designed the experiments. Xiaohong Zhao, Zuoqing Li and Benfu He performed the experiments. Zuoqing Li, Juncheng Liu, Suisheng Li, Li Zhou, Cuiling Pan and Zhe Yu analyzed the data. Xiaohong Zhao wrote and edited the manuscript. All authors read, critically revised and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Heck JE, Ritz B, Hung RJ, Hashibe M, Boffetta P. The epidemiology of neuroblastoma: a review. Paediatr Perinat Epidemiol. 2009;23(2):125–143. doi: 10.1111/j.1365-3016.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 3.Haase GM, Perez C, Atkinson JB. Current aspects of biology, risk assessment, and treatment of neuroblastoma. Semin Surg Oncol. 1999;16(2):91–104. doi: 10.1002/(sici)1098-2388(199903)16:2<91::aid-ssu3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Verissimo CS, Molenaar JJ, Fitzsimons CP, Vreugdenhil E. Neuroblastoma therapy: what is in the pipeline? Endocr Relat Cancer. 2011;18(6):R213–R231. doi: 10.1530/ERC-11-0251. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Lee JS, Guo F, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21(3):362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartelet H, Oligny LL, Vassal G. AKT pathway in neuroblastoma and its therapeutic implication. Expert Rev Anticancer Ther. 2008;8(5):757–769. doi: 10.1586/14737140.8.5.757. [DOI] [PubMed] [Google Scholar]
- 7.Fumagalli S, Totty NF, Hsuan JJ, Courtneidge SA. A target for Src in mitosis. Nature. 1994;368(6474):871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SJ, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368(6474):867–871. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 9.Paronetto MP, Cappellari M, Busa R, et al. Alternative splicing of the cyclin D1 proto-oncogene is regulated by the RNA-binding protein Sam68. Cancer Res. 2010;70(1):229–239. doi: 10.1158/0008-5472.CAN-09-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan P, Gaughan L, Dalgliesh C, et al. The RNA-binding and adaptor protein Sam68 modulates signal-dependent splicing and transcriptional activity of the androgen receptor. J Pathol. 2008;215(1):67–77. doi: 10.1002/path.2324. [DOI] [PubMed] [Google Scholar]
- 11.Batsche E, Yaniv M, Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol. 2006;13(1):22–29. doi: 10.1038/nsmb1030. [DOI] [PubMed] [Google Scholar]
- 12.Paronetto MP, Achsel T, Massiello A, Chalfant CE, Sette C. The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol. 2007;176(7):929–939. doi: 10.1083/jcb.200701005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espejo A, Côté J, Bednarek A, Richard S, Bedford MT. A protein- domain microarray identifies novel protein-protein interactions. Biochem J. 2002;367(Pt 3):697–702. doi: 10.1042/BJ20020860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derry JJ, Richard S, Valderrama Carvajal H, et al. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol. 2000;20(16):6114–6126. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakrishnan P, Baltimore D. Sam68 is required for both NF-κB activation and apoptosis signaling by the TNF receptor. Mol Cell. 2011;43(2):167–179. doi: 10.1016/j.molcel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Li J, Zheng H, et al. Expression and cytoplasmic localization of SAM68 is a significant and independent prognostic marker for renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2685–2693. doi: 10.1158/1055-9965.EPI-09-0097. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Yu CP, Zhong Y, et al. Sam68 expression and cytoplasmic localization is correlated with lymph node metastasis as well as prognosis in patients with early-stage cervical cancer. Ann Oncol. 2012;23(3):638–646. doi: 10.1093/annonc/mdr290. [DOI] [PubMed] [Google Scholar]
- 18.Song L, Wang L, Li Y, et al. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010;222(3):227–237. doi: 10.1002/path.2751. [DOI] [PubMed] [Google Scholar]
- 19.Bielli P, Busà R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18(4):R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 20.Busa R, Paronetto MP, Farini D, et al. The RNA-binding protein Sam68 contributes to proliferation and survival of human prostate cancer cells. Oncogene. 2007;26(30):4372–4382. doi: 10.1038/sj.onc.1210224. [DOI] [PubMed] [Google Scholar]
- 21.Chawla G, Lin CH, Han A, Shiue L, Ares M, Black DL. Sam68 regulates a set of alternatively spliced exons during neurogenesis. Mol Cell Biol. 2009;29(1):201–213. doi: 10.1128/MCB.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iijima T, Wu K, Witte H, et al. SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell. 2011;147(7):1601–1614. doi: 10.1016/j.cell.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol. 2000;20(1):273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zhang N, Song LB, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14(11):3319–3326. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Jaboin J, Dennis PA, Thiele CJ. Genetic and pharmacologic identification of Akt as a mediator of brain-derived neurotrophic factor/TrkB rescue of neuroblastoma cells from chemotherapy-induced cell death. Cancer Res. 2005;65(6):2070–2075. doi: 10.1158/0008-5472.CAN-04-3606. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb I, Goldstein D, Irish J, et al. Expression patterns of Trk-A, Trk-B, GRP78, and p75 NRT in olfactory neuroblastoma. Hum Pathol. 2009;40(9):1330–1335. doi: 10.1016/j.humpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Shimada H. Tumors of the neuroblastoma group. Pathology (Phila) 1993;2(1):43–59. [PubMed] [Google Scholar]
- 28.Zage PE, Louis CU, Cohn SL. New aspects of neuroblastoma treatment: ASPHO 2011symposium review. Pediatr Blood Cancer. 2012;58(7):1099–1105. doi: 10.1002/pbc.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Li L, Nisson PE, et al. Neoplastic transformation and tumorigenesis associated with sam68 protein deficiency in cultured murine fibroblasts. The Journal of Biological Chemistry. 2000;275(51):40195–40201. doi: 10.1074/jbc.M006194200. [DOI] [PubMed] [Google Scholar]
- 30.Modem S, Chinnakannu K, Bai U, Reddy GP, Reddy TR. Hsp22 (HspB8/H11) knockdown induces Sam68 expression and stimulates proliferation of glioblastoma cells. J Cell Physiol. 2011;226(11):2747–2751. doi: 10.1002/jcp.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZY, Cai L, Zhu J, et al. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis. 2011;32(10):1419–1426. doi: 10.1093/carcin/bgr088. [DOI] [PubMed] [Google Scholar]
- 32.Locatelli A, Lofgren KA, Daniel AR, Castro NE, Lange CA. Mechanisms of HGF/Met signaling to Brk and Sam68 in breast cancer progression. Horm Cancer. 2012;3(1–2):14–25. doi: 10.1007/s12672-011-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The neuroblastoma cell growth as determined by MTT assay.
Abbreviation: MTT, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide.
Cell growth and Sam68 expression in SH-SY5Y cells (mock) and vector-infected cells (vector).
Notes: (A) The cell growth of mock and vector as determined by MTT assay. (B) Cultured cells under microscope. (C) Western blotting shows the expression level of Sam68 protein.
Abbreviations: MTT, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide; Sam68, Src-associated in mitosis with a molecular weight of 68 Kd.