Abstract
Background:
Hospital mortality has decreased over time for critically ill patients with various forms of brain injury. We hypothesized that the proportion of patients who progress to neurologic death may have also decreased.
Methods:
We performed a prospective cohort study involving consecutive adult patients with traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage or anoxic brain injury admitted to regional intensive care units in southern Alberta over a 10.5-year period. We used multivariable logistic regression to adjust for patient age and score on the Glasgow Coma Scale at admission, and to assess whether the proportion of patients who progress to neurologic death has changed over time.
Results:
The cohort consisted of 2788 patients. The proportion of patients who progressed to neurologic death was 8.1% at the start of the study period, and the adjusted odds of progressing to neurologic death decreased over the study period (odds ratio [OR] per yr 0.92, 95% confidence interval [CI] 0.87–0.98, p = 0.006). This change was most pronounced among patients with traumatic brain injury (OR per yr 0.87, 95% CI 0.78–0.96, p = 0.005); there was no change among patients with anoxic injury (OR per yr 0.96, 95% CI 0.85–1.09, p = 0.6). A review of the medical records suggests that missed cases of neurologic death were rare (≤ 0.5% of deaths).
Interpretation:
The proportion of patients with brain injury who progress to neurologic death has decreased over time, especially among those with head trauma. This finding may reflect positive developments in the prevention and care of brain injury. However, organ donation after neurologic death represents the major source of organs for transplantation. Thus, these findings may help explain the relatively stagnant rates of deceased organ donation in some regions of Canada, which in turn has important implications for the care of patients with end-stage organ failure.
Mortality has decreased among critically ill patients with various forms of brain injury in Canada and around the world.1–10 There have also been changes in the incidence of stroke and the rate of admission to hospital for traumatic brain injury, especially among younger people and those whose injuries are related to motor vehicle or bicycle crashes.5,6,10–13
Some countries have noted a possible decline in the total number of patients with neurologic death.14,15 Neurologic death (“brain death”) may occur when patients with brain injury experience progressive cerebral edema, complicated by transtentorial herniation. It is defined by the irreversible cessation of cerebral and brainstem functions, including respiration.16 Circulation and gas exchange persist only because of the use of mechanical ventilation. National guidelines exist for the diagnosis of neurologic death.17,18 We hypothesized that the proportion of patients with acute brain injury who progress to neurologic death may have decreased over time.
Methods
Cohort
The Alberta Health Services (Calgary Zone) Department of Critical Care Medicine administers critical care services to adults in Calgary. Four intensive care units (ICUs) serve local critically ill patients and are referral centres for southern Alberta and southeastern British Columbia. The University of Calgary’s Conjoint Health Research Ethics Board approved this study.
Prospective data are collected for all patients admitted to the regional ICUs. A point-of-care clinical information system is used for electronic charting. This system interfaces with bedside monitors to record clinical and laboratory data, which are then imported into a data warehouse. This database is continuously validated against the regional health records financial database.19
For every admission, the attending intensivist records the admission diagnosis using the Intensive Care National Audit and Research Centre coding method (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130271/-/DC1).20 The attending physician also documents the patient’s score on the Glasgow Coma Scale and the information required to calculate Acute Physiology and Chronic Health Evaluation II scores.21,22 Data analysts pursue missing data and send regular email reminders to the responsible physician until all information has been collected.
For patients who die in a regional intensive care unit, the responsible physician must document electronically whether the criteria for neurologic death were met.17,18 These data are verified by a quality-assurance process, in which all deaths are peer reviewed monthly by intensivists who were not directly involved in the deceased patient’s care. Failure to recognize neurologic death is surveyed, and feedback is provided to the clinicians involved.
The most common conditions to be complicated by neurologic death include traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage and anoxic brain injury.23 We identified all patients admitted with one of these conditions between Jan. 1, 2002, and June 30, 2012, in order to assess whether the proportion of patients who progress to neurologic death has changed over time.
To explore the possibility that intensivists may not be consistently diagnosing neurologic death when it occurs, we performed a chart review for patients who died in a regional intensive care unit between Jan. 1, 2011, and July 31, 2012. We reviewed the charts of patients whose admitting diagnoses included any of the following: traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage, anoxic brain injury, ischemic stroke, acute liver failure, brain tumour or central nervous system infection. Neurologic death was ruled out if the patient’s medical record revealed documentation of the presence of a motor response, preservation of brainstem reflexes or evidence of spontaneous respiration at the time of withdrawal of life-sustaining interventions.18 If there was insufficient documentation in the patient’s medical record to exclude neurologic death, the death was categorized as a possible missed case. Deaths were not classified as missed cases if the patient’s medical record indicated that neurologic death had been considered but not confirmed because of overt contraindications to organ donation. For patients with a score of 3 on the Glasgow Coma Scale and bilaterally fixed pupils, a second investigator independently reviewed the records. Disagreements were resolved by involvement of a third investigator.
To explore trends in referral patterns, we used data from the Alberta Trauma Registry to assess whether the proportion of patients with head trauma sent to Calgary from the 3 largest referral centres in southern Alberta (Red Deer, Medicine Hat and Lethbridge) has changed over time.
Statistical analysis
Continuous data are presented as medians with interquartile ranges. We performed between-group comparisons using the Kruskal–Wallis test, and we assessed categorical variables using χ2 analysis. Associations are presented as odds ratios (ORs) with 95% confidence intervals (CIs). We assessed changes over time in 3 ways. First, to compare patient characteristics, we divided the study period into sequential blocks of 3.5 years (Jan. 1, 2002, to June 30, 2005; July 1, 2005, to Dec. 31, 2008; and Jan. 1, 2009, to June 30, 2012). Second, to assess temporal changes in the annual proportion of patients who progress to neurologic death, we used the Cochran–Armitage test for trend. Third, we modelled the effect of time (in units of 365 d) using multivariable logistic regression, adjusted for age and Glasgow Coma Scale score, both of which are associated with prognosis in patients with brain injury.
Using multivariable logistic regression, we compared the proportion of patients who progressed to neurologic death before and after various developments in our region: mutual patient care rounds involving critical care and neurosurgical services began on July 1, 2005 (only at the regional neurosurgical centre); a comprehensive management protocol for traumatic brain injury was implemented on Aug. 18, 2008 (only for trauma patients); and patients with neurologic diagnoses were clustered in a neurocritical care “pod” within a larger multidisciplinary unit starting on June 1, 2010 (only at the neurosurgical centre). In each case, we compared data from 3 years before and after the practice modification. If fewer than 3 years of data were available, we compared the maximum equal number of days before and after the change.
Results
Patient characteristics
The number of people eligible for Alberta health insurance coverage in the Calgary Zone increased from 1 067 058 in 2001 to 1 408 647 in 2011. Between 2002 and mid-2012, we identified 2788 patients with traumatic brain injury, subarachnoid hemorrhage, intracerebral hemorrhage or anoxic brain injury (8% of admissions to the regional ICUs).
The median age and score on the Glasgow Coma Scale among patients did not change over time (Table 1). The most common diagnosis was traumatic brain injury, but the proportion decreased slightly over time, while the proportion of patients with anoxic brain injury increased (p = 0.05). This change was greater among comatose patients (Glasgow Coma Scale ≤ 8): the proportion of patients with traumatic brain injury decreased from 49% to 42% over the study period, while the proportion of patients with anoxic injury increased from 22% to 32% (p = 0.004).
Table 1:
Characteristics of critically ill patients with traumatic brain injury, anoxic brain injury, subarachnoid hemorrhage or intracerebral hemorrhage admitted to an intensive care unit in Calgary, Alberta, from Jan. 1, 2002, to June 30, 2012
| Characteristic | Median (IQR) or % (no.) | p value* | ||
|---|---|---|---|---|
| Jan. 1, 2002, to June 30, 2005, n = 924 | July 1, 2005, to Dec. 31, 2008, n = 1019 | Jan. 1, 2009, to June 30, 2012, n = 845 | ||
| Age, yr | 49 (29–66) | 50 (31–64) | 51 (34–65) | 0.2 |
| Female | 34.5 (319) | 34.2 (348) | 37.0 (313) | 0.4 |
| Diagnosis | 0.05 | |||
| Traumatic brain injury | 55.1 (509) | 55.4 (565) | 50.5 (427) | |
| Anoxic brain injury | 16.1 (149) | 17.9 (182) | 21.9 (185) | |
| Subarachnoid hemorrhage | 15.3 (141) | 14.6 (149) | 14.0 (118) | |
| Intracerebral hemorrhage | 13.5 (125) | 12.1 (123) | 13.6 (115) | |
| Glasgow Coma Scale score | 7 (4–10) | 7 (4–10) | 7 (3–11) | 0.9 |
| APACHE II score | ||||
| Full score | 20 (14–26) | 19 (13–26) | 19 (13–26) | 0.3 |
| Modified score† | 10 (6–15) | 9 (6–15) | 9 (5–14) | 0.2 |
Note: APACHE = Acute Physiology and Chronic Health Evaluation, IQR = interquartile range.
Nonparametric testing (Kruskal–Wallis test) was used for continuous variables and χ2 testing was used for discrete variables.
Age and Glasgow Coma Scale score are components of the APACHE II score. The modified score was calculated by subtracting the impact of these variables.
Temporal trends
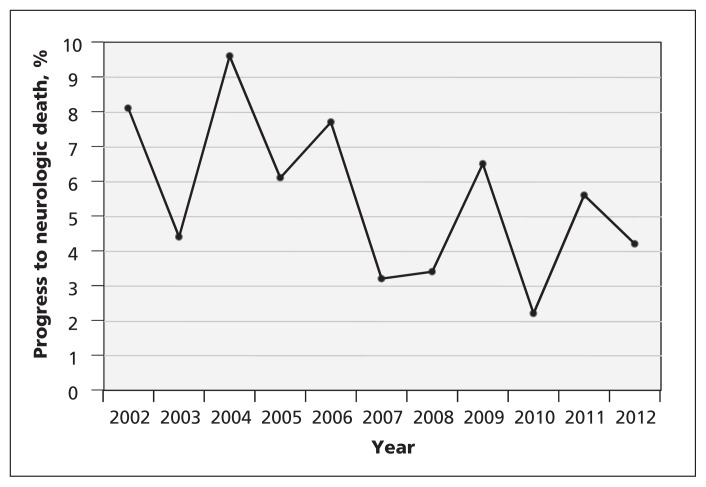
The percentage of patients who progressed to neurologic death was 8.1% in 2002 and decreased through the study period (Cochrane–Armitage test for trend, p = 0.01; Figure 1). The decrease was most pronounced among patients with traumatic brain injury (p = 0.003). This was also observed when the data were assessed by 3.5-year period (Jan. 1, 2002, to June 30, 2005: 6.1%; July 1, 2005, to Dec. 31, 2008: 3.4%; Jan. 1, 2009, to June 30, 2012: 2.8%; p = 0.02; Table 2). Neurologic death among patients with subarachnoid hemorrhage and intracerebral hemorrhage decreased over time; however, these changes were not statistically significant (p = 0.3 for both). The increased proportion of patients with anoxic injury who progressed to neurologic death was not significant (p = 0.8). The results did not vary by season, either for the whole cohort or for the subgroup with traumatic brain injury (3.9% January–March; 4.2% April–June; 4.3% July–September; 4.1% October–December; p = 1.0). Hospital mortality decreased over time for patients with traumatic brain injury, subarachnoid hemorrhage and intracerebral hemorrhage (p = 0.005), but not for those with anoxic brain injury (p = 0.7). The characteristics of patients who progressed to neurologic death are presented in Table 3.
Figure 1:
Proportion of neurocritical care patients in Calgary intensive care units who progressed to neurologic death from Jan. 1, 2002, to June 30, 2012. Cochrane–Armitage trend test: p = 0.01.
Table 2:
Outcomes of critically ill patients with traumatic brain injury, anoxic brain injury, subarachnoid hemorrhage or intracerebral hemorrhage admitted to an intensive care unit in Calgary, Alberta, from Jan. 1, 2002, to June 30, 2012
| Characteristic | % (no. of patients)* | p value† | ||
|---|---|---|---|---|
| Jan. 1, 2002, to June 30, 2005 | July 1, 2005, to Dec. 31, 2008 | Jan. 1, 2009, to June 30, 2012 | ||
| Neurologic death | ||||
| Total, n = 2788 | 6.9 (64/924) | 5.2 (53/1019) | 4.7 (40/845) | 0.1 |
| Traumatic brain injury, n = 1501 | 6.1 (31/509) | 3.4 (19/565) | 2.8 (12/427) | 0.02 |
| Anoxic brain injury, n = 516 | 4.0 (6/149) | 8.2 (15/182) | 5.9 (11/185) | 0.3 |
| Subarachnoid hemorrhage, n = 408 | 10.6 (15/141) | 10.7 (16/149) | 8.5 (10/118) | 0.8 |
| Intracerebral hemorrhage, n = 363 | 9.6 (12/125) | 2.4 (3/123) | 6.1 (7/115) | 0.06 |
| Hospital mortality | ||||
| Total, n = 2788 | 42.0 (388/924) | 39.0 (397/1019) | 38.8 (328/845) | 0.3 |
| Traumatic brain injury, n = 1501 | 27.5 (140/509) | 26.9 (152/565) | 23.4 (100/427) | 0.3 |
| Anoxic brain injury, n = 516 | 73.8 (110/149) | 67.6 (123/182) | 73.0 (135/185) | 0.4 |
| Subarachnoid hemorrhage, n = 408 | 49.6 (70/141) | 40.9 (61/149) | 34.7 (41/118) | 0.05 |
| Intracerebral hemorrhage, n = 363 | 54.4 (68/125) | 49.6 (61/123) | 45.2 (52/115) | 0.4 |
| Length of stay in intensive care unit, median, d (IQR) | ||||
| All patients | 3.0 (1.4–7.8) | 4.1 (1.7–10.6) | 3.8 (1.7–8.6) | < 0.001 |
| Patients who died in an intensive care unit | 2.1 (0.7–4.6) | 2.0 (0.9–5.5) | 2.1 (0.9–4.8) | 0.2 |
Note: IQR = interquartile range.
Unless otherwise stated.
Nonparametric testing (Kruskal–Wallis test) was used for continuous variables and χ2 testing was used for discrete variables.
Table 3:
Characteristics of critically ill patients with traumatic brain injury, anoxic brain injury, subarachnoid hemorrhage or intracerebral hemorrhage who progressed to neurologic death in Calgary, Alberta, from Jan. 1, 2002 to June 30, 2012
| Characteristic | Median (IQR) or % (no. of patients) | p value† | ||
|---|---|---|---|---|
| Jan. 1, 2002, to June 30, 2005, n = 64 | July 1, 2005, to Dec. 31, 2008, n = 53 | Jan. 1, 2009, to June 30, 2012, n = 40 | ||
| Age, yr | 42 (24–56) | 45 (26–58) | 44 (35–56) | 0.6 |
| Female | 50.0 (32) | 45.3 (24) | 40.0 (16) | 0.6 |
| Diagnosis | ||||
| Traumatic brain injury | 48.4 (31) | 35.8 (19) | 30.0 (12) | 0.04 |
| Anoxic brain injury | 9.4 (6) | 28.3 (15) | 27.5 (11) | |
| Subarachnoid hemorrhage | 23.4 (15) | 30.2 (16) | 25.0 (10) | |
| Intracerebral hemorrhage | 18.8 (12) | 5.7 (3) | 17.5 (7) | |
| Glasgow Coma Scale at admission | 3 (3–3.5) | 3 (3–3) | 3 (3–3) | 0.5 |
| APACHE II score* | ||||
| Full score | 29 (25–34) | 30 (26–34) | 29 (24–34) | 0.7 |
| Modified score | 16 (13–20) | 17 (14–21) | 15 (13–20) | 0.6 |
| Length of stay in intensive care unit, d | 0.9 (0.7–1.6) | 1.1 (0.7–1.7) | 1.3 (1.0–2.0) | 0.06 |
| Family approached regarding organ donation‡ | 96.9 (62) | 98.1 (52) | 95.0 (38) | 0.7 |
| Family consented to organ donation | 81.0 (50) | 81.0 (42) | 76 (29) | 0.7 |
Note: APACHE = Acute Physiology and Chronic Health Evaluation, IQR = interquartile range.
Age and Glasgow Coma Scale score are components of the APACHE II score. The modified score was calculated by subtracting the impact of these variables.
Nonparametric testing (Kruskal–Wallis test) was used for continuous variables and χ2 testing was used for discrete variables.
Four patients’ families were not approached about organ donation because of an overt contraindication to donation; 1 patient sustained cardiac arrest before the family could be approached.
Multivariable analysis
When we used logistic regression to adjust for age and score on the Glasgow Coma Scale, there was a reduction in the proportion of patients who progressed to neurologic death over time (OR per yr 0.92, 95% CI 0.87–0.98, p = 0.006; Table 4). Consistent with the findings of our unadjusted analysis, these findings were largely because of a reduction among patients with traumatic brain injury (Table 4; OR per yr 0.87, 95% CI 0.78–0.96, p = 0.005). None of the specific practice modifications introduced in our region were individually associated with a reduction in neurologic death, although a possible trend was observed with the implementation of a regional protocol for care of patients with traumatic brain injury (OR 0.60, 95% CI 0.35–1.05, p = 0.07).
Table 4:
Multivariable analysis assessing the odds of progression to neurologic death per year, from Jan.1, 2002 to June 30, 2012
| Variable | Adjusted OR per yr (95% CI)* | p value |
|---|---|---|
| All patients | 0.92 (0.87–0.98) | 0.006 |
| Diagnosis | ||
| Traumatic brain injury, n = 1501 | 0.87 (0.78–0.96) | 0.005 |
| Anoxic brain injury, n = 516 | 0.96 (0.85–1.09) | 0.6 |
| Subarachnoid hemorrhage, n = 408 | 0.98 (0.87–1.11) | 0.8 |
| Intracerebral hemorrhage, n = 363 | 0.94 (0.82–1.09) | 0.4 |
Note: CI = confidence interval, OR = odds ratio.
Adjusted for age and Glasgow Coma Scale score at admission. Each of these variables was strongly associated with the odds of progressing to neurological death (p < 0.001).
Medical records review
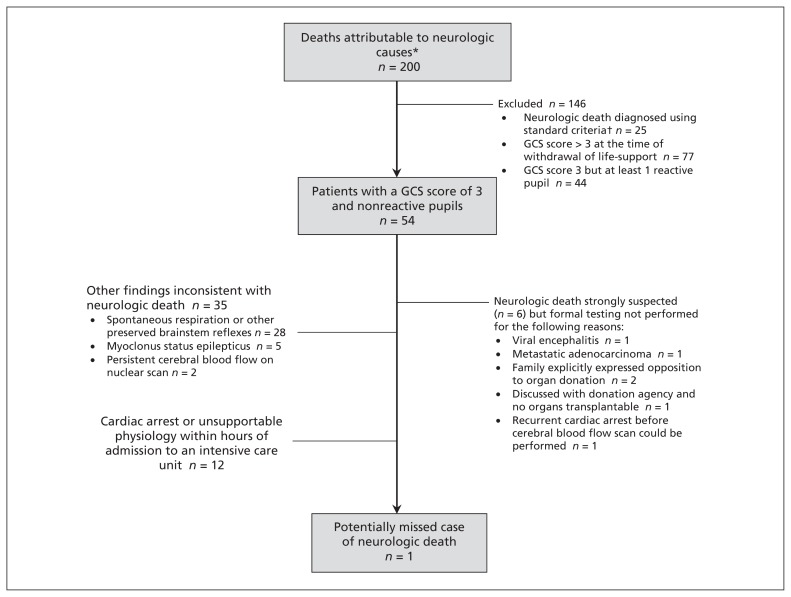
To assess whether our findings might be explained by inconsistent identification of neurologic death, we performed a review of medical records. Between Jan. 1, 2011, and July 31, 2012, we identified 200 consecutive patients with a primary neurologic diagnosis who died in a regional intensive care unit. Of these, neurologic death was diagnosed in 25 cases. Of the remaining 175 cases, over 95% of patients underwent withdrawal of life-sustaining interventions. The possibility of neurologic death could not be excluded in 7 cases. In 6 of these cases, neurologic death was considered before withdrawal of mechanical ventilation, but confirmatory testing was not performed for reasons that would have precluded organ donation (Figure 2). We identified 1 patient for whom there was the possibility that neurologic death was missed.
Figure 2:
Results of a retrospective chart review of all deaths attributable to neurologic causes in Calgary intensive care units from Jan. 1, 2011, to July 31, 2012. Clinical declaration of neurologic death was performed by 2 experts (intensivist, neurologist or neurosurgeon). *Consecutive deaths due to traumatic brain injury (37 patients), subarachnoid hemorrhage (14), intracerebral hemorrhage (27), ischemic stroke (16), anoxic brain injury (92), acute liver failure (4), brain tumour (1), fat embolism (1) or central nervous system infection (8). †As per Canadian consensus guidelines.18 Note: GCS = Glasgow Coma Scale
The annual proportion of admissions with traumatic brain injury from our 3 major referral centres who had an Abbreviated Injury Score of at least 3 (serious injury) ranged from 12.7% to 15.9%; there was no temporal trend.
Interpretation
Main findings
In this population-based study, we found that the proportion of patients with brain injury who progressed to neurologic death decreased during the study period, particularly among those with traumatic brain injury.
The reasons for our findings cannot be determined with certainty from these data, but the change may reflect positive societal and health care system developments in injury prevention and care. Consistent with our observations, Alberta Transportation reported that annual traffic-related fatalities decreased between 2006 and 2010 (from 404 to 307), as did nonfatal injury collisions (from 18 831 to 13 552), despite consistent population growth. Similar trends have been observed elsewhere in Canada and reported nationally by Transport Canada.11,12 The availability of airbags in automobiles and the use of helmets during cycling or other recreational activities are examples of preventative measures that are increasing in use across Canada and have been associated with reductions in the incidence and severity of traumatic brain injury.1,11–13,24–27
The care of patients with brain injury has advanced over the past decade. Numerous changes have been instituted in our region, which have been associated with improvements in mortality and neurologic recovery.9 Examples include the development of a regional protocol for the care of patients with traumatic brain injury, the presence of fellowship-trained neurocritical care specialists, and the selective use of decompressive craniectomy.28–30 Comparable resources to those in our region exist at other Canadian academic medical centres. However, we were unable to show that any one of these interventions was significantly associated with the observed reduction in neurologic death.
An alternative explanation for our findings could be that intensivists are inconsistently recognizing neurologic death. However, in our detailed review of 200 consecutive deaths over a 19-month period, we found only a single case in which the diagnosis may have been missed.
It is possible that some patients in our study would have progressed to neurologic death if the withdrawal of life-sustaining interventions had been delayed for longer. However, we found that the length of stay among deceased patients has not decreased, suggesting that life-sustaining interventions are not being withdrawn earlier than in the past. Another possibility is that referral patterns could have changed, whereby patients destined for neurologic death are transferred less often from peripheral centres.31 We found no evidence to support such a trend.
Patients who have been declared neurologically dead constitute the majority of deceased organ donors in Canada. Contemporary data indicate that donation after neurologic death accounts for about one-half of kidney transplants, more than three-quarters of liver transplants, over 90% of pancreas and lung transplants, and all heart and small bowel transplants.32 Our results likely help explain the relatively stagnant or even declining rates of deceased organ donation in some Canadian jurisdictions.33 Thus, our findings highlight the limitations of using the common metric of the number of donors per million population as a measure of the “performance” of an organ donation and transplantation system. Although this index facilitates comparisons of donation activity, improvements in the prevention and care of brain injury may contribute to a reduction in the number of organ donors.34
Some patients who do not progress to neurologic death still have a poor prognosis, such that life-sustaining interventions are withdrawn with the expectation of imminent death. Donation after cardiocirculatory death is offered in such cases at some Canadian hospitals.35,36 Our data suggest that this practice has greater potential to increase donation rates in Canada than efforts aimed at improving the recognition of neurologic death. Increased transplantation rates attributable to donation after cardiocirculatory death have been observed in other countries.15,37 Outcomes using organs recovered after cardiocirculatory death have been favourable in Canada. However, the number of organs that can be transplanted is lower, liver and lung graft survival is somewhat worse, and cardiac transplantation is not performed in this context.36 Some clinicians continue to have concerns about donation after cardiocirculatory death, especially related to physicians’ imperfect ability to predict prognosis and marked variability in practices related to the withdrawal of life-sustaining interventions.38–40
Comparison with other studies
It remains somewhat uncertain to what extent these findings can be generalized to other jurisdictions. Although neurologic death appears to have decreased in some other countries, investigators have been unable to determine specific reasons.14,15,37,41 The Canadian Institute for Health Information regularly collects data regarding the number of “potential donors” who meet the criteria for neurologic death that have been referred to provincial organ donation agencies.32,33 Definitions and reporting practices vary considerably across provinces, limiting the reliability and comparability of these data. Nevertheless, the number of “neurologically dead potential donors” in this database has decreased modestly, from a maximum of 18.7 per million population in 2005 to as low as 15.7 per million in 2010 (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.130271/-/DC1), despite national efforts to promote awareness of neurologic death and organ donation.18 This decline has been more pronounced in some Canadian provinces (e.g., Quebec and Alberta) than in others.
Limitations
Canadian guidelines for the neurologic determination of death were modified in 2006.18 Differences compared with previous guidelines are subtle and unlikely to have influenced our results.17,18
About 40–45 patients per year with cardiac arrest are cared for in a separate coronary care unit in our region, which was not included in this analysis. The average mortality rate for these patients is about 40%.42 During the 10.5-year study period, there were only 3 referrals of neurologically deceased patients for organ donation from this unit, such that its exclusion is unlikely to have influenced our results.
Conclusion
Our finding that a reduced proportion of patients with brain injury progresses to neurologic death suggests that initiatives aimed at improving road safety, preventing injuries during recreational activities, and improving prehospital and in-hospital care have had an effect and should continue to be promoted.
However, the rates of donation after neurologic death in Canada are unlikely to rise in the future. Thus, if organ transplantation rates are to increase, it will need to occur through alternative approaches, such as living donation, donation after cardiocirculatory death and innovations aimed at improving the use of donated organs.
Supplementary Material
Footnotes
Competing interests: Andreas Kramer is medical director of the Southern Alberta Organ and Tissue Donation Program; Danny Zuege is the former medical director of this program. Christopher Doig is the former head of the Canadian Council of Donation and Transplantation. He is a coauthor of the Canadian Guidelines for the Neurological Determination of Death. No competing interests declared by David Zygun.
Disclaimer: Christopher Doig is a member of the CMA Board and was not involved in the editorial decision-making process for this article.
This article has been peer reviewed.
Contributors: Andreas Kramer and David Zygun conceived the study. Andreas Kramer collected the data, performed the analysis, wrote the first draft and revised the manuscript. Christopher Doig and Danny Zuege reviewed the medical records. David Zygun, Christopher Doig and Danny Zuege assisted with interpretation of the data and participated in the writing and revising of the manuscript. All of the authors approved the final version submitted for publication.
References
- 1.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. MMWR Surveill Summ 2011;60:1–32 [PubMed] [Google Scholar]
- 2.Mayo NE, Nadeau L, Daskalopoulou SS, et al. The evolution of stroke in Quebec: a 15-year perspective. Neurology 2007;68: 1122–7 [DOI] [PubMed] [Google Scholar]
- 3.Nieuwkamp DJ, Setz LE, Algra A, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009;8:635–42 [DOI] [PubMed] [Google Scholar]
- 4.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology 2010;74:1494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsay MP, Hill M, Kapral MK, et al. Stroke. In: Tracking heart disease and stroke in Canada. Ottawa (ON): Public Health Agency of Canada; 2009. Available: www.phac-aspc.gc.ca/publicat/2009/cvd-avc/pdf/cvd-avs-2009-eng.pdf (accessed 2013 July 18). [Google Scholar]
- 6.Feigin VL, Lawes CMM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69 [DOI] [PubMed] [Google Scholar]
- 7.Rea TD, Crouthamel M, Eisenberg MS, et al. Temporal patterns in long-term survival after resuscitation from out-of-hospital cardiac arrest. Circulation 2003;108:1196–201 [DOI] [PubMed] [Google Scholar]
- 8.Kitamura T, Iwami T, Kawamura T, et al. Nationwide improvements in survival from out-of-hospital cardiac arrest in Japan. Circulation 2012;126:2834–43 [DOI] [PubMed] [Google Scholar]
- 9.Kramer AH, Zygun DA. Declining mortality in neurocritical care patients: a cohort study in Southern Alberta. Can J Anaesth. 2013;60:966–75 [DOI] [PubMed] [Google Scholar]
- 10.Head injuries in Canada: a decade of change (1994–1995 to 2003–2004). Ottawa (ON): Canadian Institute for Health Information; 2006. Available: https://secure.cihi.ca/free_products/ntr_head_injuries_2006_e.pdf (accessed 2013 July 18). [Google Scholar]
- 11.Canadian motor vehicle traffic collision statistics. Ottawa (ON): Transport Canada; 2010. Available: www.tc.gc.ca/eng/roadsafety/tp-1317.htm#2 (accessed 2013 July 18). [Google Scholar]
- 12.Alberta traffic collision statistics. Edmonton (AB): Alberta Transportation Office of Traffic Safety; 2010. Available: www.transportation.alberta.ca/Content/docType47/Production/AR2010.pdf (accessed 2013 July 18). [Google Scholar]
- 13.Dennis J, Ramsay T, Turgeon AF, et al. Helmet legislation and admissions to hospital for cycling related head injuries in Canadian provinces and territories: interrupted time series analysis. BMJ 2013;346:f2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jochmans I, Darius T, Kuypers D, et al. Kidney donation after circulatory death in a country with a high number of brain dead donors: 10-year experience in Belgium. Transpl Int 2012;25: 857–66 [DOI] [PubMed] [Google Scholar]
- 15.Summers DM, Counter C, Johnson RJ, et al. Is the increase in DCD organ donors in the United Kingdom contributing to a decline in DBD donors? Transplantation 2010;90:1506–10 [DOI] [PubMed] [Google Scholar]
- 16.Wijdicks EF. The diagnosis of brain death. N Engl J Med 2001; 344:1215–21 [DOI] [PubMed] [Google Scholar]
- 17.Canadian Neurocritical Care Group Guidelines for the diagnosis of brain death. Can J Neurol Sci 1999;26:64–6 [PubMed] [Google Scholar]
- 18.Shemie SD, Doig C, Dickens B, et al. Severe brain injury to neurological determination of death: Canadian forum recommendations. CMAJ 2006;174:S1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahpori R, De Los Angeles A, Laupland K. Information management framework: a model for clinical departments. Stud Health Technol Inform 2009;143:81–6 [PubMed] [Google Scholar]
- 20.de Keizer NF, Bonsel GJ, Goldfad C, et al. The added value that increasing levels of diagnostic information provide in prognostic models to estimate hospital mortality for adult intensive care patients. Intensive Care Med 2000;26:577–84 [DOI] [PubMed] [Google Scholar]
- 21.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–4 [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29 [PubMed] [Google Scholar]
- 23.Cloutier R, Baran D, Morin JE, et al. Brain death diagnoses and evaluation of the number of potential organ donors in Quebec hospitals. Can J Anaesth 2006;53:716–21 [DOI] [PubMed] [Google Scholar]
- 24.Stewart TC, Girotti MJ, Nikore V, et al. Effect of airbag deployment on head injuries in severe passenger motor vehicle crashes in Ontario, Canada. J Trauma 2003;54:266–72 [DOI] [PubMed] [Google Scholar]
- 25.Karkhaneh M, Rowe BH, Saunders LD, et al. Bicycle helmet use four years after the introduction of helmet legislation in Alberta, Canada. Accid Anal Prev 2011;43:788–96 [DOI] [PubMed] [Google Scholar]
- 26.Russell K, Christie J, Hagel BE. The effect of helmets on the risk of head and neck injuries among skiers and snowboarders: a meta-analysis. CMAJ 2010;182:333–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haider AH, Saleem T, Bianiuk JW, et al. An evidence-based review: efficacy of safety helmets in the reduction of head injuries in recreational skiers and snowboarders. J Trauma Acute Care Surg 2012;73:1340–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel HC, Menon DK, Tebbs S, et al. Specialist neurocritical care and outcome from head injury. Intensive Care Med 2002; 28:547–53 [DOI] [PubMed] [Google Scholar]
- 29.Kramer AH, Zygun DA. Do neurocritical care units save lives? Measuring the impact of specialized ICUs. Neurocrit Care 2011; 14:329–33 [DOI] [PubMed] [Google Scholar]
- 30.Albanese J, Leone M, Alliez JR, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med 2003;31:2535–8 [DOI] [PubMed] [Google Scholar]
- 31.Tenn-Lyn NA, Doig CJ, Shemie SD, et al. Potential organ donors referred to Ontario neurosurgical centres. Can J Anaesth 2006; 53:732–6 [DOI] [PubMed] [Google Scholar]
- 32.E-statistics report on transplant waiting list and donor statistics. Ottawa (ON): Canadian Institute for Health Information; 2013. Available: www.cihi.ca/CIHI-ext-portal/internet/en/document/types+of+care/specialized+services/organ+replacements/report_stats2012 (accessed 2013 July 18). [Google Scholar]
- 33.Canadian organ replacement register annual report: treatment of end-stage organ failure in Canada, 2001 to 2010. Ottawa (ON): Canadian Institute for Health Information; Available: https://secure.cihi.ca/free_products/2011_CORR_Annua_Report_EN.pdf (accessed 2013 July 18). [Google Scholar]
- 34.Barnieh L, Baxter D, Boiteau P, et al. Benchmarking performance in organ donation programs: dependence on demographics and mortality rates. Can J Anaesth 2006;53:727–31 [DOI] [PubMed] [Google Scholar]
- 35.Shemie SD, Baker AJ, Knoll G, et al. Donation after cardiocirculatory death in Canada. CMAJ 2006;175:S1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez-Alejandro R, Wall W, Jevnikar A, et al. Organ donation after cardiac death: donor and recipient outcomes after the first three years of the Ontario experience. Can J Anaesth 2011. 58: 599–605 [DOI] [PubMed] [Google Scholar]
- 37.Kumar R, Shekar K, Widdicombe N, et al. Donation after cardiac death in Queensland: review of the pilot project. Anaesth Intensive Care 2012;40:517–22 [DOI] [PubMed] [Google Scholar]
- 38.Turgeon AF, Lauzier F, Simard JF, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011;183:1581–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Downie J, Kutcher M, Rajotte C, et al. Eligibility for organ donation: a medico-legal perspective on defining and determining death. Can J Anaesth 2009;56:851–63 [DOI] [PubMed] [Google Scholar]
- 40.Wijdicks EF, Rabinstein AA. Absolutely no hope? Some ambiguity of futility of care in devastating acute stroke. Crit Care Med 2004;32:2332–42 [PubMed] [Google Scholar]
- 41.Saidi RF, Markmann JF, Jabbour N, et al. The faltering solid organ donor pool in the United States (2001–2010). World J Surg 2012;36:2909–13 [DOI] [PubMed] [Google Scholar]
- 42.Champagne P, Tilley S, Taylor R, et al. Correlates of outcomes for 131 consecutive cardiac arrest patients having undergone therapeutic hypothermia at a single Canadian tertiary referral medical center. Can J Cardiol 2012;28:S161 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.