Abstract
The chemokine Interferon gamma-induced protein 10 (IP-10) and human leukocyte antigen (HLA) are widely used indicators of glial activation and neuroinflammation and are up-regulated in many brain disorders. These inflammatory mediators have been widely studied in rodent models of brain disorders, but less work has been undertaken using human brain cells. In this study we investigate the regulation of HLA and IP-10, as well as other cytokines and chemokines, in microglia, astrocytes, pericytes, and meningeal fibroblasts derived from biopsy and autopsy adult human brain, using immunocytochemistry and a Cytometric Bead Array. Interferonγ (IFNγ) increased microglial HLA expression, but contrary to data in rodents, the anti-inflammatory cytokine transforming growth factor β1 (TGFβ1) did not inhibit this increase in HLA, nor did TGFβ1 affect basal microglial HLA expression or IFNγ-induced astrocytic HLA expression. In contrast, IFNγ-induced and basal microglial HLA expression, but not IFNγ-induced astrocytic HLA expression, were strongly inhibited by macrophage colony stimulating factor (M-CSF). IFNγ also strongly induced HLA expression in pericytes and meningeal fibroblasts, which do not basally express HLA, and this induction was completely blocked by TGFβ1, but not affected by M-CSF. In contrast, TGFβ1 did not block the IFNγ-induced increase in IP-10 in pericytes and meningeal fibroblasts. These results show that IFNγ, TGFβ1 and M-CSF have species- and cell type-specific effects on human brain cells that may have implications for their roles in adult human brain inflammation.
Introduction
Although the brain was long thought to have limited immunological activity, it is now appreciated that substantial immune activity occurs in the brain at a homeostatic level as well as during disease [1]. Markers of immune activation are ubiquitously used to track disease progress, correlate with symptomology, and have become a major target for disease therapies [2]. Brain-resident microglia are immune cells of myeloid origin. Microglia are the predominant antigen-presenting cell types of the brain and they perform a variety of functions including phagocytosis of debris, production of signalling molecules and monitoring extracellular ion levels [3]. Immune surveillance of the CNS is important for many homeostatic processes. However, neuroinflammation is thought to contribute to the pathogenesis of many neurological disorders [4]–[6]. A complete understanding of the phenotype of microglia in the adult human brain is still lacking as there is evidence that human adult microglia are different to fetal microglia and blood monocytes [7], [8]. Dystrophic microglia have been identified in the aged human brain and ‘microglial senescence’ is a possible contributor to neurological decline [9], [10]. Furthermore, immune responsiveness changes with age and over time microglia may become increasingly activated [11]. The “activated” microglial phenotype can be assessed in multiple ways, including expression of proteins involved in functional activities such as antigen presentation, morphological changes, and functional activation such as production of cytokines and chemokines.
Other cells apart from microglia have immune roles in the brain. Astrocytes perform many homeostatic functions which impact on immune activity in the CNS, for example maintaining BBB integrity, glutamate recycling, and potassium buffering [1]. Astrocytes also have many direct roles in the innate immunity of the CNS. They express innate immune receptors (e.g. TLR3 and CXCR3) and secrete soluble mediators which affect immune responses (e.g. TGFβ1, IL-6, and IL-10) [12], [13]. Astrocyte immune activity has been shown to play a specific role in several diseases including Alzheimer's disease (AD) [14] and epilepsy [15], partially through upregulated expression of pro-inflammatory cytokines.
Many other cells contribute to immune responses in the CNS, including cells at the blood-brain barrier such as pericytes [16]–[18], perivascular macrophages, perivascular mesenchymal stem cells [19] and other cells adjacent to the CNS parenchyma such as meningeal fibroblasts of the leptomeninges [1], [20]. We have previously identified and characterized a population of fibroblast-like cells in cultures of adult human brain tissue that express the fibroblast markers prolyl-4-hydroxylase and fibronectin [21], [22]. These cells do not express markers of microglia or astrocytes, and are likely to be of neurovascular origin as they also express markers of pericytes [19], [22]. Overall, this cell population expresses the fibroblast and pericyte markers prolyl-4-hydroxylase, vimentin, nestin, α-smooth muscle actin and platelet-derived growth factor receptor-β [22]. We refer to these cells as “pericytes”, in-line with the current literature [19], [22]. We show here that this cell population exhibits distinct immune characteristics. These cells are likely distributed throughout the CNS in ideal locations for immune interaction, both with cells of the periphery and of the CNS [19].
An essential aspect of neuroinflammation is cross-talk between different cells of the immune and central nervous systems via cell surface proteins and secreted molecules. Human leukocyte antigen (HLA) is a cell surface antigen presentation protein. HLA-DP, DQ and DR classes present extracellular antigens to T cells and are the human-specific versions of the class II Major Histo-Compatibility (MHC) complex in vertebrates. There are numerous reports of increased HLA and MHC class II expression with brain injury and disease processes in both rodent models and human post-mortem brain tissue [23]. For example, an increased number of HLA-DR positive microglia have been found in epileptic hippocampus compared to control human brain [24] and progressive accumulation and correlation to disease has been found for HLA-positive microglia in Huntington's and Alzheimer's disease brain tissue [25], [26]. MHC class II expression is increased in response to neuronal injury [27] and dense focal clusters of HLA-DR immunoreactivity are visible at senile plaques in AD gray matter [28]. While microglia are the predominant resident cell type in the brain to express HLA both in vitro and in situ, Styren et al. (1990) have shown that astrocytes in control and AD brains can also express HLA-DR [28]. Given its upregulation in so many diseases, the regulation of HLA in the adult human brain is of great interest. Substantial research has been conducted on the regulation of HLA expression [29], and it has become apparent that there can be species and cell type specific differences in its regulation.
A major cytokine known to influence HLA expression is the T-cell cytokine Interferon-y (IFNy). IFNy acts through the MHC Class II Transactivator (CIITA), the master regulator of MHC II gene expression [30]. Two other molecules which can affect HLA expression are Transforming Growth Factor β1 (TGFβ1) and Macrophage Colony-Stimulating Factor (M-CSF). The predominantly anti-inflammatory cytokine TGFβ1 has been shown to counteract the upregulation of HLA by IFNy via inhibition of the expression of IFNy-induced CIITA mRNA [30]–[33]. The effect of M-CSF on basal and IFNy-induced HLA-DR has previously been investigated in human fetal astrocytes and microglia [34], where it was found that M-CSF reduced HLA-DR in microglia but not astrocytes [34]. However, the relevance of these findings to the adult human brain is still to be determined.
The cytokine IFNy not only affects immune responses by inducing expression of cell surface proteins but also produces changes in glial cytokine and chemokine production. Interferon gamma-induced protein 10 (IP-10; CXCL10) is produced by a variety of cells in the brain in response to IFNy. This chemokine functions in selective trafficking of leukocytes, migration of glia and proliferation of various cell types [35]. IP-10 binds the G protein–coupled receptor CXCR3 [36]. CXCR3 expression has been reported in the developing human brain [37] and in human and rodent cultured microglia and astrocytes [38], [39].
IP-10 and CXCR3 have been shown to be increased in several neurological disease states [13]. IP-10 plays a particular role in viral infection as it is induced by the T-cell anti-viral cytokine IFNy. As such, IP-10 was found to be elevated in the CSF from patients with viral meningitis [40]. In a study of human brain tissue, IP-10 immunoreactivity was not detected in HIV-negative brains, but was present in HIV-positive brains and further found to be induced in human neurons by HIV infection in vitro [41]. Multiple sclerosis (MS) is an autoimmune demyelinating disease which involves a large recruitment of lymphocytes into the brain parenchyma. CXCR3-positive T cells are increased in blood [42] and brain tissue [43] of MS patients compared with healthy controls. Blocking IP-10 in the experimental autoimmune encephalomyelitis (EAE) mouse model of MS reduces the severity of the disease and the number of pathogenic T-cells in the inflamed CNS [40]. IP-10 is a common feature of other neurological conditions including AD [44] and glioma [45]. It is clear that IP-10 plays a profound role in neurological disease and the extracellular factors regulating IP-10 expression in the adult human brain require further investigation.
Our study investigates the effects of cytokines IFNγ, TGFβ1 and M-CSF on adult human glial inflammatory mechanisms, namely the inducible expression of HLA-DP, DQ, DR and production of cytokines and chemokines by microglia, astrocytes, pericytes and meningeal fibroblasts. Parts of this work were presented to the XI International Congress of Neuroimmunology (ISNI) in Boston 2012 with an abstract published in the Journal of Neuroimmunology volume 253 page 115 (2012).
Methods
Tissue
Biopsy human temporal lobe tissue was from subjects receiving surgery for intractable epilepsy, and the research was approved by the Northern Regional Ethics Committee. All biopsy specimens were from temporal lobe epilepsy cases (n = 10) with varying degrees of mesial temporal sclerosis (neuropathological grade 3–4, where grade 4 is maximal severity). Autopsy adult human brain tissue (temporal lobe) from a range of neurologically diseased (Alzheimer's, n = 2; Huntington’s, n = 1; and Parkinson’s disease, n = 1) and normal individuals (n = 3) was obtained through the Neurological Foundation of New Zealand Human Brain Bank (University of Auckland Human Participants Ethics Committee). Informed written consent was obtained in all cases.
Human glial cell isolation and culture
Cells were obtained from adult human brain (middle temporal gyrus) tissue as previously described [21], [46], [47], and were cultured for ∼1 week prior to plating for experiments at 50,000 cells/ml in 96-well plates. This initial passaging of cells consisted of a mixed glial culture containing microglia, astrocytes and pericytes, as has been previously characterised [48]. All cultures were validated for cell phenotypes as per Gibbons et al. [21]. The percentage of different cell types varies between cultures, with an average of 13.2+/−1.3% PU.1-positive microglia, and 1.1+/−0.02% GFAP-positive astrocytes (mean +/− SEM, n = 3 cases) [48]. To obtain cultures of pericytes only, 3 or 4 subsequent passages were made (roughly 1 week apart, when cells had reached ∼90% confluence) and the negligibly dividing microglia and astrocytes were no longer present, as determined by immuno-labelling for microglia (PU.1) and astrocytes (GFAP) [21], [22].
Leptomeningeal explant cultures
To study the inflammatory role of meningeal fibroblasts, leptomeninges (from the same tissue as above) covering the middle temporal gyrus was carefully removed from underlying tissue using forceps. Small pieces of leptomeningeal tissue, ∼2×3 mm, were placed into wells of a 6-well plate with ∼850 µl (not so much that they were floating, but enough to surround them with nutrients) DMEM/F12 media supplemented with 10% fetal bovine serum (FBS), 1% Penicillin-Streptomycin-Glutamine (Gibco BRL) (final concentrations: penicillin (100 U/ml), streptomycin (100 µg/ml) and L-glutamine (0.29 mg/ml)). Half the volume of media was changed twice in the first week, and then a full media change was done every 3–4 days. Cells started to grow out of the tissue after ∼1 week. Leptomeningeal explants were passaged by moving to a new plate with forceps. For cytokine treatment, the explants were moved to a 24-well plate for 2 weeks to generate cells. The explants were then passaged into a new plate and the cells in the 24-well plate were left for 2 days before beginning cytokine treatment.
Cytokine treatment
Mixed primary human glial cell cultures were treated in 96-well plates. 1 µl cytokine was added to 100 µl media. Cells were treated with 1 ng/ml IFNy (in PBS with 0.1% BSA) at 0 and 48 h. Total time of IFNy treatment was 96 h. Cells were pre-treated with 10 ng/ml TGFβ1 (in 1 mM citric acid pH 3 with 0.1% BSA) or 25 ng/ml M-CSF (in H2O) at 0, 24 and 48 h. The last pre-treatment (at 48 h) was given at the same time as the first IFNy treatment.
Immunocytochemistry
Immunocytochemsitry was performed on cells as previously described using the following primary antibody and biotinylated secondary antibodies (see Table 1) [49].
Table 1. Antibodies used for immunocytochemistry.
| Antibody | Company | Catalogue # | Dilution |
| Mouse anti-HLA-DP, DQ, DR | Dako | M0775 | 1∶500 |
| Rabbit anti-PU.1 | Cell Signaling | 2258 | 1∶500 |
| Mouse anti-CD45 | Abcam | ab8216 | 1∶500 |
| Mouse anti-GFAP | Dako | Z0334 | 1∶5000 |
| Rabbit anti-IP-10 | Abcam | ab9807 | 1∶500 |
| Goat anti-rabbit IgG Alexa Fluor® 594 | Invitrogen | A11012 | 1∶500 |
| Goat anti-mouse IgG Alexa Fluor® 488 | Invitrogen | A11001 | 1∶500 |
| Goat anti-mouse IgG Alexa Fluor® 594 | Invitrogen | A11005 | 1∶500 |
| Goat anti-rabbit IgG Alexa Fluor® 488 | Invitrogen | A11008 | 1∶500 |
Quantitative image analysis of cell number, protein expression and microglial morphology
Immunocytochemical and morphological observations have been quantified using the Discovery-1 microscope (Molecular Devices) and Metamorph image analysis system as previously detailed and described [50], [51]. Microglial morphology was quantified as previously described [49].
Quantitative cytokine and chemokine measurement
Conditioned media from experiments was collected after 96 h IFNy treatment. The media was filtered using a 0.2 µm filter (Pall Life Sciences) and stored at −80°C until use. A Cytometric Bead Array (B.D Biosciences) was performed according to the manufacturer's instructions using a FACSAria II flow cytometer (B.D Biosciences) [52].
Statistical analysis
Data from representative experiments are displayed as mean ± standard error of the mean (SEM). Cells from at least 6 different individuals were used for experiments, except for quantitative cytokine/chemokine analysis for which 3 biopsy cases were used. The F-test and Bartlett's test were used to check for equal variances. Statistical analysis was carried out using t-tests and one-way ANOVA with Tukey's multiple comparison test where variances were equal, and the equivalent non-parametric test was used in cases of unequal variance (Mann Whitney test or Kruskal-Wallis test with Dunn's multiple comparison test). Statistically significant differences were set at P<0.05. Significant differences from vehicle (no cytokine treatment) are indicated.
Results
Meningeal fibroblasts were prepared from leptomeningeal explants, and dissociated cultures comprising pericytes, microglia and astrocytes were prepared as previously described [21], [46], [48].
Microglial expression of HLA-DP, DQ, DR is increased by IFNy and reduced by M-CSF but not by TGFβ1
Microglia are the predominant HLA-DP, DQ, DR-expressing cell type in human adult mixed glial cultures. Microglia from different cases express differing basal amounts of HLA-DP, DQ, DR. From 10 biopsy cases, 5 had high basal microglial HLA expression, 4 had moderate expression and 1 had low HLA expression (Table 2). Heterogeneity in HLA expression could not be explained by drug use or degree of sclerosis alone. We also did not observe any differences in HLA expression relating to differences in cellular composition of the cultures.
Table 2. Levels of HLA protein expression differ between biopsy cases.
| Case Number | Microglia | Astrocytes | Pericytes |
| 1 | High | High | None |
| 2 | High | High | None |
| 3 | High | Moderate | None |
| 4 | High | Moderate | None |
| 5 | Moderate | None | None |
| 6 | Low | Low | None |
| 7 | Moderate | Low | None |
| 8 | Moderate | Low | None |
| 9 | Moderate | None | None |
| 10 | High | Moderate | None |
In adult human mixed glial cultures, microglia and astrocytes express variable basal levels of HLA (qualitatively assessed by proportion of positive cells and intensity of staining). However, HLA expression is not observed on untreated brain-derived pericytes in culture.
HLA expression in adult human microglia was increased by exposure to IFNy (1 ng/ml), regardless of basal levels of expression (Fig. 1A and B). Adult human glial cultures were immunostained for the microglial transcription factor PU.1 and the percentage of HLA-immunopositive microglia was found to significantly increase with IFNy (Fig. 1G). The number of HLA-immunopositive microglia and the intensity of HLA expression were both increased by IFNy.
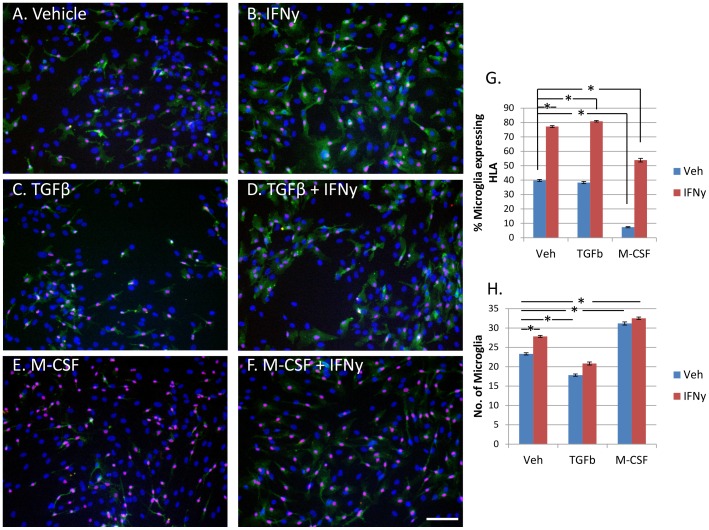
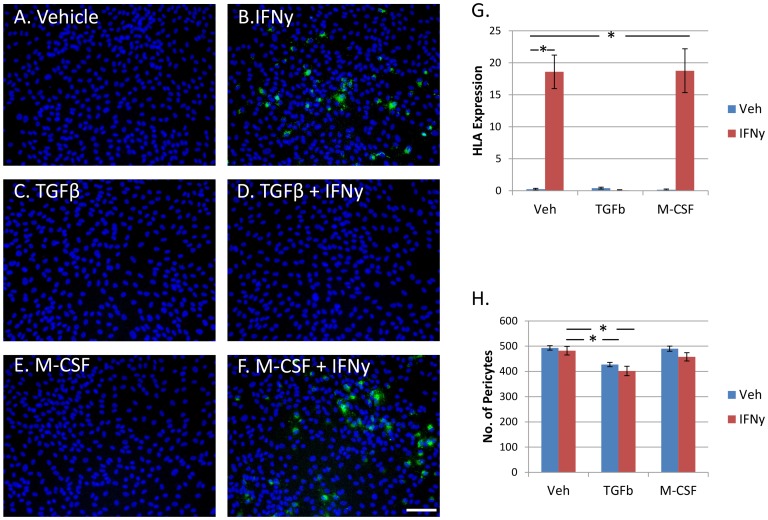
Figure 1. Microglial expression of HLA-DP, DQ, DR is increased by IFNy, not changed by TGFβ1, and reduced by M-CSF.
A) Adult human PU.1+ve microglia (pink) express variable levels of HLA-DP, DQ, DR (green) in basal conditions without any treatment. All nuclei are labelled with Hoechst (blue). B) IFNy (1 ng/ml, 96 h) increased microglial expression of HLA-DP, DQ, DR, as well as HLA-DP, DQ, DR expression by astrocytes and pericytes in the mixed glial culture. C) TGFβ1 (10 ng/ml) did not affect microglial HLA-DP, DQ, DR expression alone, or when enhanced by IFNy treatment (D). E) M-CSF (25 ng/ml) reduced basal HLA-DP, DQ, DR expression in microglia and also decreased IFNy-enhanced HLA-DP, DQ, DR expression in microglia (F). Scale bar = 100 µm. G) A significant increase in percentage of HLA-positive microglia is found with IFNy treatment. No change in microglial HLA-DP, DQ, DR expression is seen for TGFβ1 treatment, but M-CSF significantly reduces microglial HLA-DP, DQ, DR protein expression (N = 12). H) The number of microglia per image, as measured by PU.1-immunopositive cells, is significantly increased by IFNy and M-CSF. However, TGFβ1 significantly reduces microglial cell number (N = 12).
Contrary to the literature on rodent studies, TGFβ1 (10 ng/ml) treatment of human adult microglia did not reduce (or enhance) IFNy-induced HLA expression. Furthermore, no effect of TGFβ1 was observed for basal (vehicle-treated) microglial HLA expression (Fig. 1C, D and G).
We have previously reported that M-CSF-treated adult human microglia have reduced expression of HLA compared to vehicle-treated microglia [49]. Here we further report that M-CSF (25 ng/ml) in combination with IFNy significantly reduced the IFNy-mediated increase in microglial HLA expression (Fig. 1E, F and G).
Microglial cell number is increased by IFNy and M-CSF, but reduced by TGFβ1
We have previously reported an increase in microglia number following M-CSF treatment [49]. Despite increased numbers of microglia we found simultaneously reduced HLA expression (Fig. 1G and H). IFNy was also found to slightly increase microglia number compared to vehicle. However, the increase in microglial cell number produced by IFNy was not as great as for M-CSF (Fig. 1H). Although TGFβ1 did not influence microglial expression of HLA, it did significantly reduce microglial cell number (Fig. 1H).
IFNy treatment results in microglia with a more rounded morphology
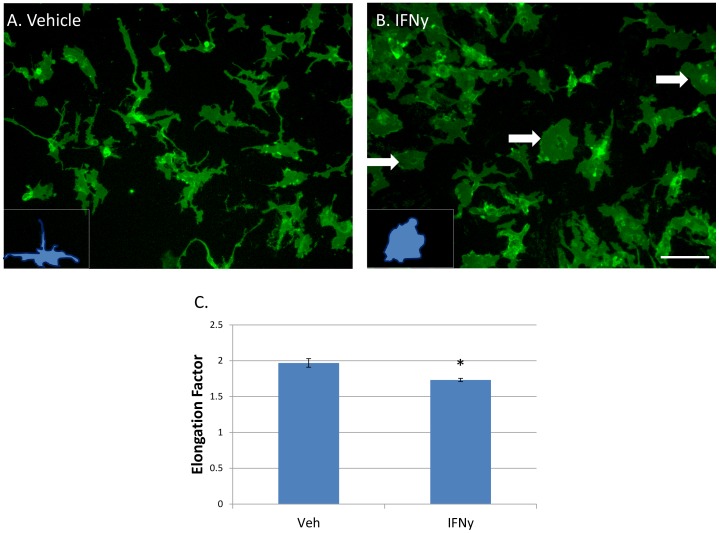
The morphology of untreated adult human microglia in vitro is heterogeneous, with cells having variable protrusions and extensions. Microglial morphology is presumed to relate to their function, although exactly how is currently unclear. Round ‘amoeboid’ microglia are traditionally viewed as activated, inflammatory microglia [53]. We observed a quantifiable change in microglial morphology following 96 h IFNy treatment toward a rounded, less ramified shape (Fig. 2A and B). The ‘elongation’ of microglia was quantifiably reduced by IFNy as shown using the Elliptical Form Factor image analysis tool in MetaMorph software (Fig. 2C).
Figure 2. IFNy produces a change in microglia morphology to a more rounded, less elongated form.
A) Adult human microglia immunolabelled with the cell surface marker CD45 have a heterogeneous morphology in basal conditions without any treatment. B) IFNy (1 ng/ml, 96 h) resulted in microglia with a rounder morphology (arrows). Insets in A) and B) show representative morphology of cells. Scale bar = 100 µm. C) Quantification of the ‘rounding’ effect using Metamorph Elliptical Form Factor (a measure of elongation) image analysis demonstrates a significant shift in microglia shape following IFNy treatment to a more rounded and less elongated form (N = 12).
Astroglial expression of HLA-DP, DQ, DR is increased by IFNy but not affected by TGFβ1 or M-CSF
Astrocytes from different cases express differing basal amounts of HLA-DP, DQ, DR. From 10 biopsy cases, 2 had high basal astrocytic HLA expression, 3 had moderate expression and 5 had low or no HLA expression (Table 2). Basal astrocytic expression of HLA was generally higher when microglial HLA expression was high (Table 2), but the percentage of astrocytes expressing HLA (<10%) was lower than for microglia (40%, Fig. 1G and 3G).
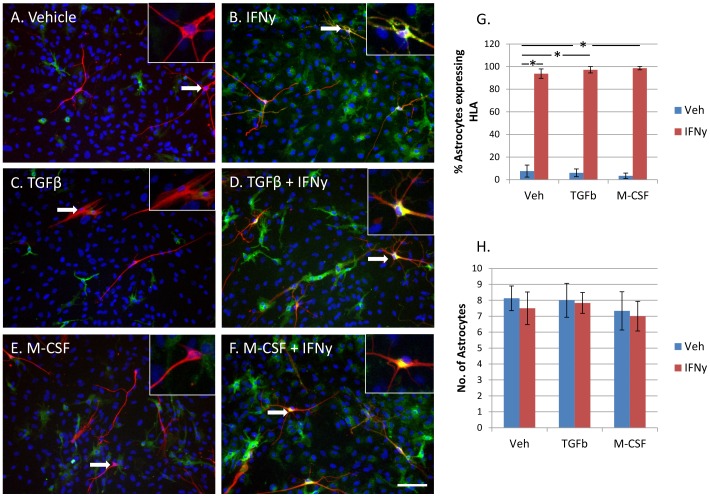
Figure 3. Astrocytic expression of HLA-DP, DQ, DR is increased by IFNy, and not changed by TGFβ1 or M-CSF.
A) Adult human GFAP +ve astrocytes (red) express variable levels of HLA-DP, DQ, DR (green) in basal conditions without any treatment. B) IFNy (1 ng/ml, 96 h) increased astroglial expression of HLA-DP, DQ, DR. C) TGFβ1 (10 ng/ml) did not affect astrocyte HLA-DP, DQ, DR expression alone, or when enhanced by IFNy treatment (D). E) M-CSF (25 ng/ml) also did not affect basal HLA-DP, DQ, DR expression in astrocytes or IFNy-enhanced HLA-DP, DQ, DR expression in astrocytes (F). Insets show close-up examples of astrocytes indicated by arrows. Scale bar = 100 µm. G) A significant increase in percentage of HLA-DP, DQ, DR-immunopositive astrocytes is found with IFNy treatment. Neither TGFβ1 nor M-CSF significantly affect astrocyte HLA-DP, DQ, DR protein expression (N = 12). H) Quantification of GFAP-immunopositive astrocyte cell number (per well) following treatment with IFNy, TGFβ1 or M-CSF does not result in any significant differences compared to vehicle-treated cells (N = 12).
Astrocytes were identified in human adult mixed glial cultures by expression of glial fibrillary acidic protein (GFAP). The number of astrocytes expressing HLA, and the amount of HLA expressed, was greatly increased in all cases by exposure to IFNy (Fig. 3A, B and G). TGFβ1 and M-CSF had no effect on IFNy-induced astrocytic HLA expression (Fig. 3D, F and G). TGFβ1 and M-CSF also did not influence basal HLA expression by astrocytes (Fig. 3C, E and G).
While IFNy increased HLA expression in astrocytes, it did not influence the number of GFAP-immunopositive astrocytes. TGFβ1 and M-CSF did not affect GFAP-immunopositive astrocyte cell number either (Fig. 3H).
IFNy induces HLA-DP, DQ, DR expression in brain-derived pericytes
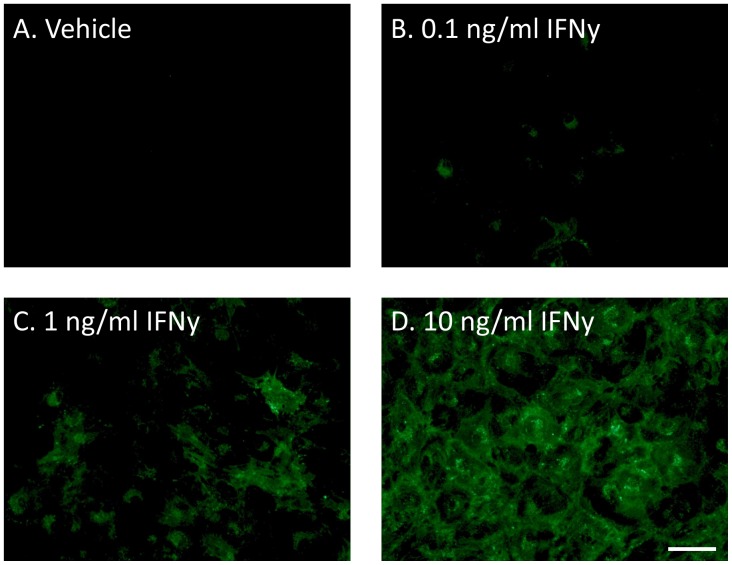
We next investigated HLA induction in the third population of cells in our mixed human glial cultures: pericyte cells [21], [22]. Pure cultures of brain pericytes were obtained after 3–4 passages of mixed glial cultures as they are the predominant cell type to divide basally in culture [21]. These cells do not express HLA basally without stimulation (Table 2 and Fig. 4A). However, upon exposure to IFNy they elicit a robust response by increasing HLA expression in a concentration-dependent fashion (Fig. 4). This response was seen for cultures of pericytes from both biopsy and post-mortem tissue from a range of neurologically diseased (Epilepsy, Alzheimer's, Huntington's and Parkinson's disease) and normal individuals. Pericytes had the same response whether in mixed cultures with microglia and astrocytes, or in cultures of pericytes alone.
Figure 4. Brain-derived pericytes do not express HLA-DP, DQ, DR protein under basal conditions but it is induced by IFNy in a concentration-dependent manner.
A) In normal culture conditions of DMEM/F12 +10% FBS +1% PSG brain pericytes do not express HLA-DP, DQ, DR protein. However, IFNy (0.1–10 ng/ml, 96 h) induced a concentration-dependent increase in HLA-DP, DQ, DR expression (B-D). Microglia and astrocytes are not present in cultures after 3–4 passages, producing a culture of pericytes only. Scale bar = 50 µm.
IFNy-induced pericyte HLA-DP, DQ, DR expression is inhibited by TGFβ1 but not by M-CSF
Whereas no effect of TGFβ1 on HLA induction was seen for adult human microglia, there was a major inhibition effect of TGFβ1 on brain pericytes (Fig. 5D and G). This response was seen for pericytes alone and within mixed glial cultures with microglia and astrocytes present. Conversely, whereas microglial HLA induction was reduced by M-CSF, pericytes were unaffected (Fig. 5F and G). This is expected from previous findings of the M-CSF receptor (c-fms) being expressed only on microglia in primary human mixed glial cultures [49]. IFNy or M-CSF treatment had no effect on total number of pericytes as measured by Hoechst staining of nuclei in pericyte-only cultures (Fig. 5H). However, TGFβ1 was found to reduce pericyte cell number (Fig. 5H).
Figure 5. IFNy-induced expression of HLA-DP, DQ, DR in brain-derived pericytes is inhibited by TGFβ1 but not by M-CSF.
A) Vehicle-treated pericytes (Hoechst-labelled nuclei) do not express HLA-DP, DQ, DR protein. B) IFNy induces a major up-regulation of HLA-DP, DQ, DR protein (green) in brain pericytes. C) TGFβ1 treatment alone does not induce expression of HLA-DP, DQ, DR in these cells. However, TGFβ1 completely inhibits the IFNy-stimulated increase in HLA-DP, DQ, DR (D). M-CSF affects neither basal (E) nor IFNy-induced (F) HLA-DP, DQ, DR expression in brain pericytes. Scale bar = 100 µm. G) HLA-DP, DQ, DR is induced by treatment with IFNy, and inhibited by simultaneous exposure to TGFβ1 but not M-CSF (N = 12). H) Pericyte cell number (per well) is not influenced by IFNy or M-CSF but is significantly decreased by TGFβ1 (N = 12).
IFNy also induces meningeal fibroblasts to express HLA-DP, DQ, DR
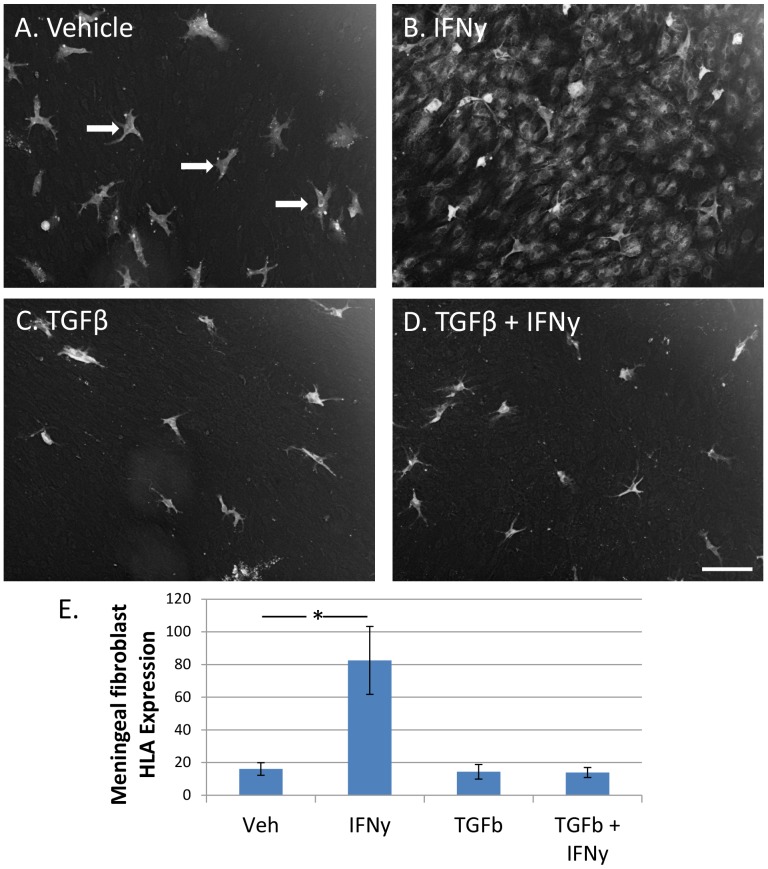
To study the induction of HLA-DP, DQ, DR in meningeal fibroblasts we undertook explant culture studies. Explant cultures were generated from leptomeninges overlying the middle temporal gyrus from both biopsy epilepsy specimens and autopsy specimens. The explant cultures generated cells over 1–2 weeks in 24-well plates. Once confluent, the explants were removed (and placed in a new 24-well plate) and the remaining adherent cells were characterised using antibodies to prolyl-4-hydroxylase and fibronectin for meningeal fibroblasts, and CD45 and PU.1 for leptomeningeal/perivascular macrophages. The majority of cells (>95%) were prolyl-4-hydroxylase and fibronectin-immunopositive meningeal fibroblast cells, with scattered CD45 and PU.1-immunopositive leptomeningeal/perivascular macrophages. HLA-DP, DQ, DR was absent in untreated meningeal fibroblasts, but present in leptomeningeal/perivascular macrophages (Fig. 6A). This pattern of staining matches closely that found in the pericyte cells and microglia derived from dissociated mixed glial cultures used in this study. IFNγ induced strong expression of HLA in meningeal fibroblasts (Fig. 6B and E). This expression was again completely blocked in meningeal fibroblasts by TGFβ1 (Fig. 6D). Interestingly, consistent with the failure of TGFβ1 to reduce microglial HLA expression, it also failed to reduce the expression of HLA in leptomeningeal/perivascular macrophages (Fig. 6).
Figure 6. IFNy-induced expression of HLA-DP, DQ, DR in meningeal fibroblasts is completely blocked by TGFβ1.
A) In vehicle-treated leptomeningeal explant cultures only leptomeningeal/perivascular macrophages express HLA-DP, DQ, DR (indicated by arrows). B) IFNy increases intensity of HLA expression on macrophage cells and greatly induces HLA expression in meningeal fibroblasts. C) TGFβ1 has no effect on basal leptomeningeal/perivascular macrophage or meningeal fibroblast HLA expression. D) However, TGFβ1 completely inhibits IFNy-induced meningeal fibroblast HLA expression, without affecting leptomeningeal/perivascular macrophage HLA expression. DAB brightfield images have been inverted for image analysis. Scale bar = 100 µm. E) Quantification of HLA expression shows a massive increase in HLA expression in leptomeningeal explant cultures with IFNy but not with TGFβ1 + IFNy (N = 12).
IFNy treatment of primary adult human mixed glia results in increased pro-inflammatory cytokine and chemokine release
Another important function of microglia is production and secretion of cytokines. To assess the effect of IFNy on the production of these immune signalling molecules, we measured an array of cytokines and chemokines in the conditioned media of vehicle control and IFNy-treated mixed glial cultures (containing microglia, astrocytes and brain pericytes) using a Cytometric Bead Array (B.D Biosciences). A total of 16 cytokines was assessed, of which 10 (GM-CSF, IFNy, TNF, interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-7, IL-12p70 and IL-13) were not detected in conditioned media from IFNy-treated, nor vehicle-treated, adult human mixed glia cultures. IL-10 and MIP-1α were detected at very low levels (<5 pg/ml) in both IFNy-treated and non-treated cells' conditioned media. IL-6, IL-8, IP-10 and MCP-1 were expressed at moderate levels in vehicle-treated cells' conditioned media (0.5–10 ng/ml). With IFNy treatment, there was no change in IL-8 concentration. IL-6 concentration was increased with IFNy (418±42 [mean ± SEM] pg/ml for vehicle treatment vs 576±39 pg/ml for IFNy treatment, n = 3; P = 0.0519), though not to statistical significance (Fig. 7A). MCP-1 concentration was significantly increased with IFNy (8104±608 pg/ml for vehicle treatment vs 10190±437 pg/ml for IFNy treatment, n = 3; P = 0.0493) (Fig. 7B). However, the biggest IFNy-induced change was an increase in IP-10 concentration from 2021±782 pg/ml for vehicle treatment to 36860±10140 pg/ml for IFNy treatment (n = 3; P = 0.0267) (Fig. 7C).
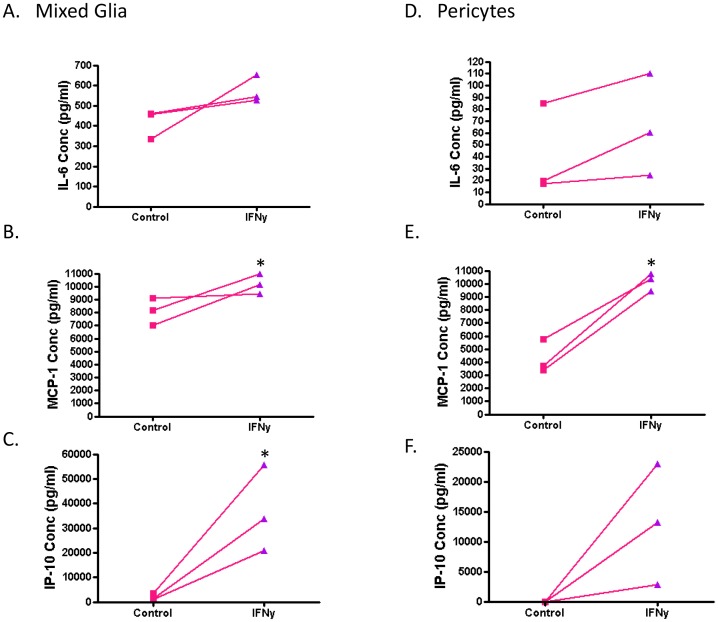
Figure 7. IFNy increases pro-inflammatory cytokine and chemokine release from adult human mixed glial cultures and brain-derived pericytes.
Here each data point indicates an individual case (n = 3). Control and IFNy-treated samples from the same case are indicated by connecting lines. A) IL-6 secretion is slightly, but not significantly, increased by IFNy treatment of mixed glia (microglia, astrocytes and brain-derived pericytes). B) MCP-1 production is significantly increased by IFNy in mixed glia cultures. C) Adult human mixed glia produce a low basal level of IP-10 which is markedly increased by IFNy. D) Relatively low concentrations of IL-6 production by pericytes are not changed by IFNy. E) Pericyte cell cultures produce comparable levels of MCP-1 to mixed glial cultures when stimulated with IFNy. F) Pericytes release IP-10 upon IFNy stimulation only.
Cultures of pericytes alone (passage 5 – no microglia or astrocytes present as determined by immunolabelling) did not secrete IP-10 under vehicle conditions but did with IFNy (13003±5798 pg/ml, n = 3), albeit to a lesser extent than the mixed glial cultures (Fig. 7F). Pericyte-only cultures also had lower basal secretion of IL-6 and MCP-1 but whereas IL-6 concentration was not changed by IFNy treatment (Fig. 7D), MCP-1 concentration was increased as for mixed glial cultures (4293±735 pg/ml for vehicle treatment vs 10190±387 pg/ml for IFNy treatment, n = 3; P = 0.0021) (Fig. 7E).
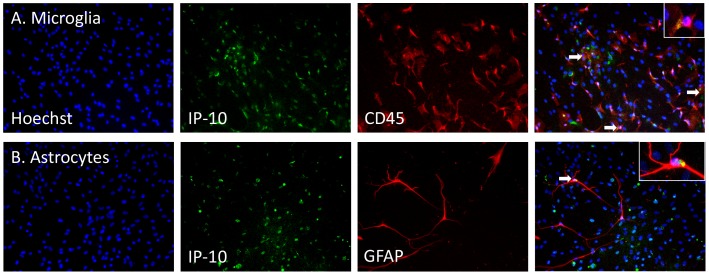
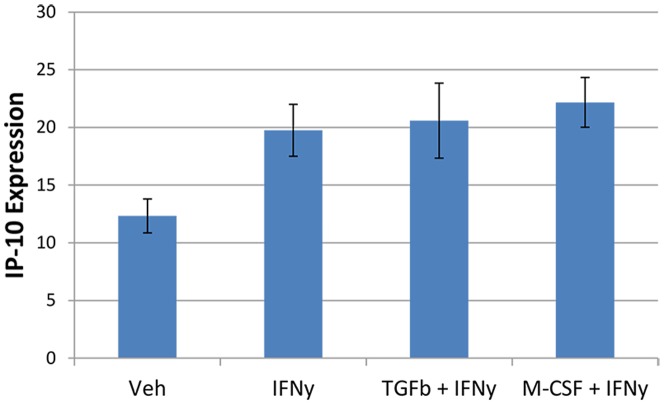
The increase in IP-10 production with IFNy treatment can also be visualised by immunocytochemistry. This revealed that IP-10 is produced by both microglia and astrocytes in our mixed glial cultures and thus is likely to be secreted into the extracellular environment by multiple cell types (Fig. 8). In pericyte-only cultures, IP-10 staining is increased by IFNy. Unlike the inhibition of HLA expression by TGFβ1, the IFNy-induction of IP-10 expression was not blocked by TGFβ1 (Fig. 9). We did not observe an effect of M-CSF on IP-10 levels in pericytes either (Fig. 9).
Figure 8. IP-10 is expressed by microglia and astrocytes in primary adult human mixed glial cultures.
A) Following IFNy treatment (1 ng/ml, 96 h) IP-10 expression (green) is co-localised with CD45-immunopositive microglia (red). All nuclei are labelled with Hoechst (blue). Hoechst, IP-10 and CD45 and overlaid in the far right image. B) GFAP-immunopositive astrocytes (red) express IP-10 following IFNy treatment. Hoechst, IP-10 and GFAP are overlaid in the far right image. Scale bar = 100 µm. Arrows indicate high levels of IP-10 expression and insets show close-up examples of cells expressing IP-10.
Figure 9. IFNy induces IP-10 expression in pericytes and is not affected by TGFβ1 or M-CSF.
Following IFNy treatment, IP-10 expression is significantly increased in pericytes from basal levels. Simultaneous treatment with either TGFβ1 or M-CSF does not affect the levels of IP-10 expression induced by IFNy (N = 12).
Discussion
Our findings demonstrate that HLA-DP, DQ, DR is an inducible protein which is not expressed constitutively by all adult human microglia, and that levels of HLA expression vary between individuals (Table 2). This study investigates glia from a number of neurologically diseased and normal human brains. However, no correlations were observed between particular disease states and neuroinflammatory protein expression. With the use of larger numbers of brains and a broader range of disease grades this information may be obtainable. Despite variable basal HLA expression, microglia from all cases consistently showed increased HLA with IFNy treatment (Fig. 1). In our studies we have used an antibody which targets HLA classes DP, DQ and DR. It will be interesting to see if the changes in expression we observed are due to one or more particular classes.
The results of the present study, together with previous work, suggest that the effects of TGFβ1 on IFNy-induced HLA expression are species specific as well as cell type specific. We found that TGFβ1 did not affect HLA expression in adult human microglia, either at basal levels or with IFNy-induction (Fig. 1). Conversely, TGFβ1 blocked IFN-y-induced enhancement of CIITA in murine macrophages and microglia [8], [30], [54], and human macrophage U937 cells [55]. This differential finding between rodent and human microglia is of major importance for understanding human neuroinflammation, especially given the emphasis in the literature of the anti-inflammatory properties of TGFβ1 [56].
We show that although TGFβ1 did not affect microglial HLA, M-CSF significantly reduced HLA expression by microglia (Fig. 1 and 10). The effect of M-CSF on basal and IFNy-induced HLA-DR has previously been investigated in human fetal astrocytes and microglia [34]. Similar to our results, they found reduced HLA-DR with M-CSF for microglia but not astrocytes [34]. It has been found that IFNy-mediated MHC-II induction in rodents was significantly muted in tumor microglia/macrophages compared with normal brain [57]. As M-CSF has been demonstrated to be upregulated in brain tumors [58], [59], it could be a possible mediator of decreased HLA expression within tumors.
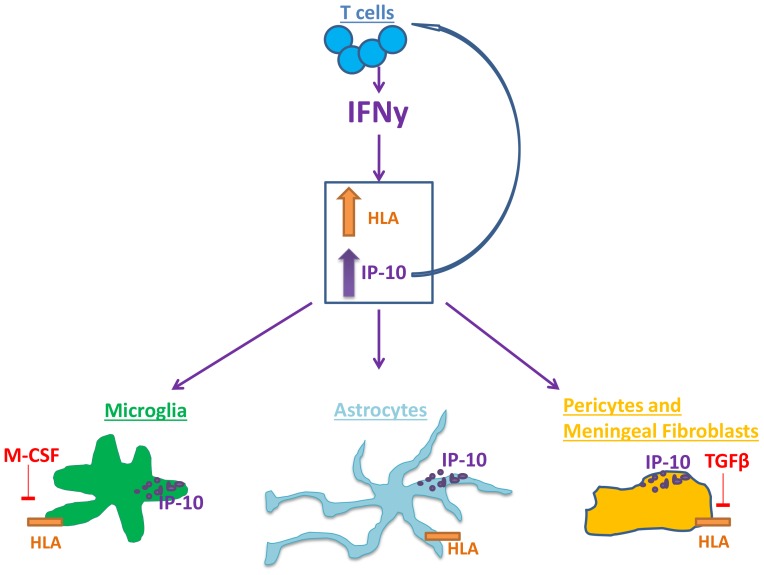
Figure 10. Differential regulation of HLA and IP-10 in adult human microglia, astrocytes, brain pericytes and meningeal fibroblasts by IFNy, TGFβ1 and M-CSF.
The T cell pro-inflammatory cytokine IFNy upregulates HLA and IP-10 protein expression in adult human brain glial cells, pericytes and meningeal fibroblasts. Microglial HLA was increased by IFNγ (1 ng/ml for 96 h). M-CSF (25 ng/ml), but not TGFβ1 (10 ng/ml), was found to decrease microglial HLA expression. Astrocytic expression of HLA was also increased by IFNγ, and not modulated by TGFβ1 or M-CSF. Brain pericytes and meningeal fibroblasts do not basally express HLA but have a marked induction on exposure to IFNγ, which was blocked by TGFβ1. IFNγ increased adult human microglia, astrocyte and pericyte expression and release of pro-inflammatory cytokines and chemokines, particularly IP-10. IP-10 may be involved in leukocyte trafficking into the CNS.
Both M-CSF and IFNy increased the number of microglia in culture, although M-CSF had a greater effect (Fig. 1H). We and others have previously shown an increase in microglial cell number with M-CSF treatment [34], [49]. Given that M-CSF reduced microglial HLA expression whereas IFNy increases HLA, it was surprising to find a similar effect of M-CSF and IFNy on microglial cell number. M-CSF increases proliferation of adult human microglia [49] but this was not observed for IFNy (data not shown).
We found that TGFβ1 reduced microglia cell number and this could in fact be a mechanism by which TGFβ1 exerts anti-inflammatory effects. Previous reports in rodents have shown that TGFβ1 inhibits microglial proliferation [60], [61]. Our results show a similar effect of TGFβ1 on human and rodent microglial cell number, but a differential effect of TGFβ1 on microglial HLA expression.
Our immunocytochemistry and morphological analysis show increased rounding of IFNy-treated adult human microglia, with increased HLA-DP, DQ, DR staining (Fig. 2). Immunohistochemistry of brains of adult humans with MS has shown HLA-DR+ cells with oval morphology within MS lesions, whereas cells just outside the lesion and in the normal appearing parenchyma had a more ramified morphology [62]. Furthermore, expression of HLA class II molecules was noted to be less intensive on rod-shaped microglia compared to neighbouring ramified microglia in neurologically diseased human brain tissue [63]. To complement this finding we report here that IFNy treatment produces the opposite effect of rounded microglial morphology with increased HLA expression. ‘Activated microglia’ cannot be solely defined by morphology or expression of a single cell surface marker [11]. However, together with increased HLA-DP, DQ, DR and IP-10 expression, this change in morphology is suggestive of a pro-inflammatory microglial phenotype.
We also reiterate previous findings that HLA can be expressed by other brain cell types apart from microglia. We demonstrate that HLA is expressed by a small percentage of astrocytes under basal culture conditions and that they readily increase HLA expression upon IFNy stimulation (Fig. 3). Early studies of HLA-DR expression on cultured human adult astrocytes similarly found that a small proportion expressed HLA-DR and that there was a concentration-dependent increase in HLA-positive astrocytes with IFNy stimulation [62], [64]. While microglia are the predominant cell type to express HLA both in vitro and in situ, Styren et al. (1990) have shown that astrocytes in control and AD brains can express HLA-DR, although they are reported to be rare compared to HLA-DR-positive microglia [28].
Astrocytes were not responsive to either TGFβ1 or M-CSF when analysed for HLA expression (Fig. 3). We have previously reported that GFAP-positive astrocytes in mixed human adult glial cultures are negative for M-CSF receptor protein [49]. Astrocytes have however been shown to produce TGFβ1 and M-CSF which then act on other brain cells [34], [65]. These differential cell type responses to TGFβ1 and M-CSF show that astrocytes have a distinct immune phenotype and have an important role in brain immune responses.
A study investigating the expression of the IFNy receptor on human cells and tissue found astrocytes to be the predominant cell type with IFNy receptor expression [66]. Astrocytes, but not microglia or oligodendrocytes, expressed IFNy receptor in diseased and normal human brain tissue [66]. On the other hand, cultured human microglia, astrocytes and oligodendrocytes showed constitutive expression of IFNy receptor protein. While confirming IFNy receptor expression on microglia in vitro, this finding calls into question the physiological in vivo relevance of the effect of IFNy on microglia. However it will be important to confirm these immunohistochemical double-label results with in situ hybridization and a range of antisera to the IFNy receptor. If this result is validated by other studies it suggests that astrocytes are the main cells contributing to IFNy-mediated neuroinflammation in the brain.
The pericyte cell population did not express HLA in basal culture conditions, either in mixed glial cultures or in later passage (passage 3–5) pericyte-only cultures (Table 2). Exposure to IFNy resulted in a concentration-dependent increase in HLA expression by these brain pericyte cells (Fig. 4). These results show that brain pericyte cells have the capacity to be directed towards an immune role and may be an important target for treating neuroinflammation. Indeed, previous studies in rodents have shown that pericytes are involved in brain inflammation [18].
Despite TGFβ1 not affecting the microglial HLA response, TGFβ1 had a dramatic effect on HLA induction in brain pericytes (Fig. 10). TGFβ1 completely blocked IFNy-induced HLA expression in these cells (Fig. 5). Similar reports of TGFβ1 modulation of HLA expression have been made for human cells with fibroblast characteristics from other regions of the body [67], [68]. The finding that M-CSF does not influence brain pericyte expression of HLA is consistent with our previous observation that these cells don't express the receptor for M-CSF [49].
To further study the cellular basis of the induction of HLA in other non-glial cells, we undertook explant culture studies of leptomeninges tissue. These explants gave rise to cell cultures consisting predominantly of meningeal fibroblasts, with scattered leptomeningeal/perivascular macrophages. Leptomeningeal-explant derived meningeal fibroblasts responded to IFNy by expressing HLA in a similar fashion to dissociated brain pericyte cells (Fig. 6B). Furthermore, TGFβ1 abolished this induction (Fig. 6D and E). These results suggest that meningeal fibroblasts derived from leptomeninges and brain-derived pericytes respond in a common way to IFNy and TGFβ1, and indeed it is likely that our cultures from both dissociated brain tissue and leptomeninges contain mixtures of both cell types. As leptomeningeal/perivascular macrophages were also present in these cultures, it is possible that they can influence the response of meningeal fibroblasts to these cytokines, as might be expected in vivo.
The upregulation of HLA in individuals with neurological disease identifies HLA as an important molecule in the adult human brain, and one that may be important for communication with peripheral T cells. Increased intercellular adhesion molecule-1 (ICAM-1) expression has been found in epileptic and AD brains, and increased infiltration of CD8- and CD4- positive T lymphocytes was found in the hippocampus of patients with hippocampal sclerosis [69], [70]. ICAM-1 may aid T cell infiltration into the brain parenchyma where they could interact with antigen-presenting cells. However it is still unknown to what extent T cell activation occurs in the brain, and what factors govern this immune activation. The leptomeninges has been demonstrated to be a location of T cell contact with phagocytic antigen-presenting cells and a point of entry of encephalogenic T cells into the CNS [71], [72]. Live cell two-photon imaging of rats has revealed T cells moving out of leptomeningeal blood vessels and into the subarachnoid space where they interact with antigen-presenting cells and subsequently invade the CNS parenchyma [72]. In addition, the T cells became reactivated and upregulated pro-inflammatory cytokines and receptors including IFNy and CXCR3. Our data showing that both brain pericytes and meningeal fibroblasts can be induced to express HLA (as well as the chemokine IP-10) support this previous work and indicate that cells in the vasculature and meninges play major roles in brain inflammation.
Cytokines/chemokines are a major system of brain communication as there is mounting evidence that endogenous cytokines/chemokines in the brain act together with neurotransmitter and neuropeptide systems to control brain function [73]. We report extensive release of pro-inflammatory chemokines IP-10 and MCP-1 following IFNy treatment of adult human mixed glial cultures (Fig. 7B and C). IL-6 was present at lower levels under control conditions and not significantly increased by IFNy (Fig. 7A). Pure cultures of brain pericytes had lower basal cytokine/chemokine expression but also demonstrated a massive increase in MCP-1 and IP-10 release with IFNy treatment (Fig. 7E and F). Within mixed glial cultures the increase in MCP-1 may be largely from the pericytes as the relative increase in MCP-1 is much greater for pericyte-only cultures than for mixed glial cultures. Alternatively, the presence of microglia and astrocytes in mixed glial cultures may also be limiting MCP-1 release from pericytes. IL-6 levels were higher in the mixed glial cultures than in the pure pericyte cultures, suggesting that astrocytes or microglia are the main source of this cytokine. The increase in IP-10 release from mixed glial cultures is likely produced by all cell types present as we demonstrate immunocytochemical labelling of IP-10 production in microglia and astrocytes (Fig. 8), and pericyte-only cultures secrete IP-10 after IFNy treatment (Fig. 7F). Meningeal fibroblasts grown from explant cultures also expressed IP-10 in response to IFNy (data not shown), suggesting that meningeal fibroblasts are also a potential source of this chemokine in the inflamed brain.
IP-10 and MCP-1 can also be released by human fetal and simian adult astrocytes in response to IFNy [74]. Astrocytes and microglia have increased expression of IP-10 in several infectious and neurotoxic contexts including AD, ischemia and LPS-challenge [44], [75]–[77]. There is also evidence to suggest that not only glial cells but neuronal cells too can release chemokines to attract T cells into the brain parenchyma [78]. Adult human brain microvascular endothelial cells have been shown to upregulate IP-10 in response to IFNy [79]. The brain-derived pericytes and meningeal fibroblasts are also likely to be in ideal locations (i.e. blood vessels and leptomeninges) to convey systemic inflammatory signals to brain glia and neurons, acting as a gate-way between peripheral physiology and the CNS [80]. In cases of viral infection, Dionne et al. (2011) have demonstrated, using a brain slice culture model, that at least some of the IP-10 production and functional effects induced by viral infection are brain specific [81]. Interestingly, Durafourt et al. (2012) found IP-10 to be upregulated following activation in human microglia, but not in human macrophages, suggesting that IP-10 may be expressed by brain microglia more than peripheral macrophages in adult humans [82].
IP-10 expression is generally associated with loss of neuronal viability, however a direct mechanism has not always been established [78], [83]–[85]. As the IP-10 receptor CXCR3 is expressed by numerous cell types, IP-10 could act on a variety of cell types to eventuate in neuronal cell death. However, astrocytes and microglia have been found to respond differently to IP-10, and cellular background has been shown to determine CXCR3 signaling, highlighting cell type specificity in response to chemokines [39], [86].
IP-10 protein is expressed by macrophages in MS lesions and IP-10 and MCP-1 are expressed by astrocytes at the rim of MS lesions, while both microglia and astrocytes express the IP-10 and MCP-1 receptors CXCR3 and CCR2 respectively [43], [87]. CXCR3-positive astrocytes were also found to be increased in the CNS of HIV-positive patients, in ischaemic infarcts and in astrocytic neoplasms [13]. It has been suggested that IP-10-positive cells may represent a novel population of cells to target pharmacologically in a broad range of neurodegenerative conditions [88]. The effect of IP-10 on neuronal viability in the adult human brain remains unknown and pharmacologic reduction of IP-10 expression requires further exploration in the context of the adult human brain.
In conclusion, HLA and IP-10 are major players in neuroinflammation. Numerous studies have investigated their regulation, however few studies have been performed with human cells. This study used primary human adult glia to demonstrate species and cell type specificity in response to IFNy, TGFβ1 and M-CSF. While IFNy induced inflammatory responses in all human brain cell types studied, TGFβ1 and M-CSF have anti-inflammatory effects on specific cell populations (Fig. 10). In particular our studies have demonstrated that not only do the “classical” brain immune cells (microglia and astrocytes) show immune activation, but human brain pericytes and meningeal fibroblasts also show dramatic immune activation. This data is likely to have relevance for neuroinflammation in the adult human brain and more studies are warranted to determine the regulators of this neuroinflammation.
Acknowledgments
The authors would like to thank the tissue donors for their generous donation of brain tissue for research; Lynair Roberts, specialist epilepsy nurse at Auckland City Hospital, for co-ordination of tissue collection; Claire Lill and Inna Semenyajenko, from the Centre for Brain Research Biobank, for help with tissue processing; and Kristina Burkert for flow cytometry assistance.
Funding Statement
This study was performed with the help of funding from Gravida - National Centre for Growth and Development, Neurological Foundation of New Zealand, Tertiary Education Commission, Coker Charitable Trust, Hugh Green Foundation and the Health Research Council of New Zealand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. The Journal of Clinical Investigation 122: 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Politis M, Piccini P (2012) Positron emission tomography imaging in neurological disorders. Journal of Neurology 259: 1769–1780. [DOI] [PubMed] [Google Scholar]
- 3. Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394. [DOI] [PubMed] [Google Scholar]
- 4. Klegeris A, McGeer EG, McGeer PL (2007) Therapeutic approaches to inflammation in neurodegenerative disease. Current Opinion in Neurology 20: 351–357. [DOI] [PubMed] [Google Scholar]
- 5. Khandelwal PJ, Herman AM, Moussa CEH (2011) Inflammation in the early stages of neurodegenerative pathology. Journal of Neuroimmunology 238: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker L, Sills GJ (2012) Inflammation and Epilepsy: The Foundations for a New Therapeutic Approach in Epilepsy? Epilepsy Curr 12: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambert C, Desbarats J, Arbour N, Hall JA, Olivier A, et al. (2008) Dendritic Cell Differentiation Signals Induce Anti-Inflammatory Properties in Human Adult Microglia. The Journal of Immunology 181: 8288–8297. [DOI] [PubMed] [Google Scholar]
- 8. Abutbul S, Shapiro J, Szaingurten-Solodkin I, Levy N, Carmy Y, et al. (2012) TGF-β signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60: 1160–1171. [DOI] [PubMed] [Google Scholar]
- 9. Streit WJ (2006) Microglial senescence: does the brain's immune system have an expiration date? Trends in Neurosciences 29: 506–510. [DOI] [PubMed] [Google Scholar]
- 10. Lopes KO, Sparks DL, Streit WJ (2008) Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia 56: 1048–1060. [DOI] [PubMed] [Google Scholar]
- 11. Perry VH (2010) Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathologica 120: 277–286. [DOI] [PubMed] [Google Scholar]
- 12. Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends in Immunology 28: 138–145. [DOI] [PubMed] [Google Scholar]
- 13. Goldberg SH, Van Der Meer P, Hesselgesser J, Jaffer S, Kolson DL, et al. (2001) CXCR3 expression in human central nervous system diseases. Neuropathology and Applied Neurobiology 27: 127–138. [DOI] [PubMed] [Google Scholar]
- 14. Li C, Zhao R, Gao K, Wei Z, Yin MY, et al. (2011) Astrocytes: Implications for Neuroinflammatory Pathogenesis of Alzheimer's Disease. Curr Alzheimer Res 8: 67–80. [DOI] [PubMed] [Google Scholar]
- 15. Vezzani A, Aronica E, Mazarati A, Pittman QJ (2011) Epilepsy and brain inflammation. Experimental Neurology [DOI] [PubMed] [Google Scholar]
- 16. Pardridge W, Yang J, Buciak J, Tourtellotte WW (1989) Human brain microvascular DR-antigen. J Neurosci Res 23: 337–341. [DOI] [PubMed] [Google Scholar]
- 17. Dore-Duffy P (2008) Pericytes: Pluripotent Cells of the Blood Brain Barrier. Curr Pharm Des 14: 1581–1593. [DOI] [PubMed] [Google Scholar]
- 18. Kovac A, Erickson M, Banks W (2011) Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. Journal of Neuroinflammation 8: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, et al. (2012) The Adult Human Brain Harbors Multipotent Perivascular Mesenchymal Stem Cells. PLoS ONE 7: e35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dragunow M (2013) Meningeal and choroid plexus cells—Novel drug targets for CNS disorders. Brain Research 1501: 32–55. [DOI] [PubMed] [Google Scholar]
- 21. Gibbons HM, Hughes SM, Van Roon-Mom W, Greenwood JM, Narayan PJ, et al. (2007) Cellular composition of human glial cultures from adult biopsy brain tissue. Journal of Neuroscience Methods 166: 89–98. [DOI] [PubMed] [Google Scholar]
- 22. Park TI-H, Monzo H, Mee EW, Bergin PS, Teoh HH, et al. (2012) Adult Human Brain Neural Progenitor Cells (NPCs) and Fibroblast-Like Cells Have Similar Properties In Vitro but Only NPCs Differentiate into Neurons. PLoS ONE 7: e37742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGeer P, Itagaki S, McGeer E (1988) Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol 76: 550–557. [DOI] [PubMed] [Google Scholar]
- 24. Beach TG, Woodhurst WB, MacDonald DB, Jones MW (1995) Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neuroscience Letters 191: 27–30. [DOI] [PubMed] [Google Scholar]
- 25. Sapp E, Kegel KB, Aronin N, Hashikawa T, et al. (2001) Early and progrssive accumulation of reactive microglia in the Huntington disease brain. Journal of Neuropathology and Experimental Neurology 60: 161. [DOI] [PubMed] [Google Scholar]
- 26. Serrano-Pozo A, Gómez-Isla T, Growdon JH, Frosch MP, Hyman BT (2013) A Phenotypic Change But Not Proliferation Underlies Glial Responses in Alzheimer Disease. The American Journal of Pathology 182: 2332–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumann H, Boucraut J, Hahnel C, Misgeld T, Wekerle H (1996) Neuronal control of MHC class II inducibility in rat astrocytes and microglia. Eur J Neurosci 8: 2582–2590. [DOI] [PubMed] [Google Scholar]
- 28. Styren SD, Civin WH, Rogers J (1990) Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer's disease brain. Experimental Neurology 110: 93–104. [DOI] [PubMed] [Google Scholar]
- 29. O'Keefe GM, Nguyen VT, Benveniste EN (2002) Regulation and Function of Class II Major Histocompatibility Complex, CD40, and B7 Expression in Macrophages and Microglia: Implications in Neurological Diseases. Journal of Neurovirology 8: 496–512. [DOI] [PubMed] [Google Scholar]
- 30. O'Keefe GM, Nguyen VT, Benveniste EN (1999) Class II transactivator and class II MHC gene expression in microglia: modulation by the cytokines TGF-β, IL-4, IL-13 and IL-10. European Journal of Immunology 29: 1275–1285. [DOI] [PubMed] [Google Scholar]
- 31. Piskurich J, Linhoff M, Wang Y, Ting J (1999) Two distinct gamma interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor beta. Mol Cell Biol 19: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pazmany T, Tomasi TB (2006) The major histocompatibility complex class II transactivator is differentially regulated by interferon-y and transforming growth factor-b in microglial cells. Journal of Neuroimmunology 172: 18–26. [DOI] [PubMed] [Google Scholar]
- 33. Lee YJ, Han Y, Lu HT, Nguyen V, Qin H, et al. (1997) TGF-beta suppresses IFN-gamma induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. The Journal of Immunology 158: 2065–2075. [PubMed] [Google Scholar]
- 34. Lee SC, Liu W, Roth P, Dickson DW, Berman JW, et al. (1993) Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. The Journal of Immunology 150: 594–604. [PubMed] [Google Scholar]
- 35. de Haas AH, van Weering HRJ, de Jong EK, Boddeke HWGM, Biber KPH (2007) Neuronal Chemokines: Versatile Messengers In Central Nervous System Cell Interaction. Molecular Neurobiology 36: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, et al. (1998) Binding and Functional Properties of Recombinant and Endogenous CXCR3 Chemokine Receptors. Journal of Biological Chemistry 273: 18288–18291. [DOI] [PubMed] [Google Scholar]
- 37. Van Der Meer P, Goldberg S, Fung K, Sharer L, Gonzalez-Scarano F, et al. (2001) Expression pattern of CXCR3, CXCR4, and CCR3 chemokine receptors in the developing human brain. J Neuropathol Exp Neurol 60: 25–32. [DOI] [PubMed] [Google Scholar]
- 38. Biber K, Dijkstra I, Trebst C, De Groot CJA, Ransohoff RM, et al. (2002) Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience 112: 487–497. [DOI] [PubMed] [Google Scholar]
- 39. Flynn G, Maru S, Loughlin J, Romero IA, Male D (2003) Regulation of chemokine receptor expression in human microglia and astrocytes. Journal of Neuroimmunology 136: 84–93. [DOI] [PubMed] [Google Scholar]
- 40. Sorensen T (2004) Targeting the Chemokine Receptor CXCR3 and Its Ligand CXCL10 in the Central Nervous System: Potential Therapy for Inflammatory Demyelinating Disease? Curr Neurovasc Res 1: 183–190. [DOI] [PubMed] [Google Scholar]
- 41. Maingat F, Viappiani S, Zhu Y, Vivithanaporn P, Ellestad KK, et al. (2010) Regulation of Lentivirus Neurovirulence by Lipopolysaccharide Conditioning: Suppression of CXCL10 in the Brain by IL-10. The Journal of Immunology 184: 1566–1574. [DOI] [PubMed] [Google Scholar]
- 42. Balashov KE, Rottman JB, Weiner HL, Hancock WW (1999) CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1a and IP-10 are expressed in demyelinating brain lesions. Proceedings of the National Academy of Sciences 96: 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN (2000) Expression of the interferon-γ-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathology and Applied Neurobiology 26: 133–142. [DOI] [PubMed] [Google Scholar]
- 44. Xia MQ, Bacskai BJ, Knowles RB, Qin SX, Hyman BT (2000) Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. Journal of Neuroimmunology 108: 227–235. [DOI] [PubMed] [Google Scholar]
- 45. Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, et al. (2008) Chemokine production and chemokine receptor expression by human glioma cells: Role of CXCL10 in tumour cell proliferation. Journal of Neuroimmunology 199: 35–45. [DOI] [PubMed] [Google Scholar]
- 46. Gibbons HM, Smith AM, Teoh HH, Bergin PM, Mee EW, et al. (2011) Valproic acid induces microglial dysfunction, not apoptosis, in human glial cultures. Neurobiology of Disease 41: 96–103. [DOI] [PubMed] [Google Scholar]
- 47.Smith A, Gibbons H, Lill C, Faull RM, Dragunow M (2013) Isolation and Culture of Adult Human Microglia Within Mixed Glial Cultures for Functional Experimentation and High-Content Analysis. In: Joseph B, Venero JL, editors. Microglia. New York: Humana Press. pp. 41–51. [DOI] [PubMed] [Google Scholar]
- 48. Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, et al. (2013) The transcription factor PU.1 is critical for viability and function of human brain microglia. Glia 61: 929–942. [DOI] [PubMed] [Google Scholar]
- 49. Smith A, Gibbons H, Oldfield R, Bergin P, Mee E, et al. (2013) M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. Journal of Neuroinflammation 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith AM, Gibbons HM, Dragunow M (2010) Valproic acid enhances microglial phagocytosis of amyloid-b1-42. Neuroscience 169: 505–515. [DOI] [PubMed] [Google Scholar]
- 51. Dragunow M (2008) High-content analysis in neuroscience. Nature Reviews Neuroscience 9: 779–788. [DOI] [PubMed] [Google Scholar]
- 52. Burkert K, Moodley K, Angel CE, Brooks A, Graham ES (2012) Detailed analysis of inflammatory and neuromodulatory cytokine secretion from human NT2 astrocytes using multiplex bead array. Neurochemistry International 60: 573–580. [DOI] [PubMed] [Google Scholar]
- 53. Graeber MB (2010) Changing Face of Microglia. Science 330: 783–788. [DOI] [PubMed] [Google Scholar]
- 54. Delvig AA, Lee JJ, Chrzanowska-Lightowlers ZMA, Robinson JH (2002) TGF-B1 and IFN-y cross-regulate antigen presentation to CD4 T cells by macrophages. Journal of Leukocyte Biology 72: 163–166. [PubMed] [Google Scholar]
- 55. Nandan D, Reiner NE (1997) TGF-beta attenuates the class II transactivator and reveals an accessory pathway of IFN-gamma action. The Journal of Immunology 158: 1095–1101. [PubMed] [Google Scholar]
- 56. Yoo S-W, Chang D-Y, Lee H-S, Kim G-H, Park J-S, et al. (2013) Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiology of Disease 58: 249–257. [DOI] [PubMed] [Google Scholar]
- 57. Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, et al. (2005) Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia 51: 279–285. [DOI] [PubMed] [Google Scholar]
- 58. Papavasiliou A, Mehler M, Mabie P, Marmur R, Qingbin S, et al. (1997) Paracrine regulation of colony-stimulating factor-1 in medulloblastoma: implications for pathogenesis and therapeutic interventions. Neurosurgery 41: 916–923. [DOI] [PubMed] [Google Scholar]
- 59. Alterman R, Stanley E (1994) Colony stimulating factor-1 expression in human glioma. Mol Chem Neuropathol 21: 177–188. [DOI] [PubMed] [Google Scholar]
- 60. Jones LL, Kreutzberg GW, Raivich G (1998) Transforming growth factor beta's 1, 2 and 3 inhibit proliferation of ramified microglia on an astrocyte monolayer. Brain Research 795: 301–306. [DOI] [PubMed] [Google Scholar]
- 61. Suzumura A, Sawada M, Yamamoto H, Marunouchi T (1993) Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. The Journal of Immunology 151: 2150–2158. [PubMed] [Google Scholar]
- 62. Ulvestad E, Williams K, Bo L, Trapp B, Antel JP, et al. (1994) HLA class II molecules (HLA-DR, -DP, -DQ) on cells in the human CNS studied in situ and in vitro. Immunology 82: 535–541. [PMC free article] [PubMed] [Google Scholar]
- 63. Wierzba-Bobrowicz T, Gwiazda E, Kosno-Kruszewska E, Lewandowska E, Lechowicz W, et al. (2002) Morphological analysis of active microglia - rod and ramified microglia in human brains affected by some neurological diseases (SSPE, Alzheimer's disease and Wilson's disease). Folia Neuropathologica 40: 125–131. [PubMed] [Google Scholar]
- 64. Yong V, Yong F, Ruijs T, Antel JP, Kim S (1991) Expression and modulation of HLA-DR on cultured human adult astrocytes. J Neuropathol Exp Neurol 50: 16–28. [DOI] [PubMed] [Google Scholar]
- 65. Weiss R, Lifshitz V, Frenkel D (2011) TGF-b1 affects endothelial cell interaction with macrophages and T cells leading to the development of cerebrovascular amyloidosis. Brain, Behavior, and Immunity 25: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 66. Hashioka S, Klegeris A, Schwab C, Yu S, McGeer PL (2010) Differential expression of interferon-y receptor on human glial cells in vivo and in vitro. Journal of Neuroimmunology 225: 91–99. [DOI] [PubMed] [Google Scholar]
- 67. Armendariz-Borunda J, Endres RO, Ballou LR, Postlethwaite AE (1996) Transforming growth factor-beta inhibits interferon-gamma-induced HLA-DR expression by cultured human fibroblasts. The International Journal of Biochemistry & Cell Biology 28: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 69. Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, et al. (1993) Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol 85: 628–634. [DOI] [PubMed] [Google Scholar]
- 70. Nakahara H, Konishi Y, Beach T, Yamada N, Makino S, et al. (2010) Infiltration of T Lymphocytes and Expression of ICAM-1 in the Hippocampus of Patients with Hippocampal Sclerosis. Acta Histochem Cytochem 43: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kivisäkk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, et al. (2009) Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Annals of Neurology 65: 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, et al. (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462: 94–98. [DOI] [PubMed] [Google Scholar]
- 73. Adler M, Geller E, Chen X, Rogers T (2005) Viewing chemokines as a third major system of communication in the brain. The AAPS Journal 7: E865–E870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Croitoru-Lamoury J, Guillemin GJ, Boussin FD, Mognetti B, Gigout LI, et al. (2003) Expression of chemokines and their receptors in human and simian astrocytes: Evidence for a central role of TNFα and IFNγ in CXCR4 and CCR5 modulation. Glia 41: 354–370. [DOI] [PubMed] [Google Scholar]
- 75. Uddin J, Garcia HH, Gilman RH, Gonzalez AE, Friedland JS (2005) Monocyte-Astrocyte Networks and the Regulation of Chemokine Secretion in Neurocysticercosis. The Journal of Immunology 175: 3273–3281. [DOI] [PubMed] [Google Scholar]
- 76. Wang X, Ellison JA, Siren A-L, Lysko PG, Yue T-L, et al. (1998) Prolonged Expression of Interferon-Inducible Protein-10 in Ischemic Cortex After Permanent Occlusion of the Middle Cerebral Artery in Rat. Journal of Neurochemistry 71: 1194–1204. [DOI] [PubMed] [Google Scholar]
- 77. Kremlev SG, Roberts RL, Palmer C (2004) Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. Journal of Neuroimmunology 149: 1–9. [DOI] [PubMed] [Google Scholar]
- 78. Klein RS, Lin E, Zhang B, Luster AD, Tollett J, et al. (2005) Neuronal CXCL10 Directs CD8+ T-Cell Recruitment and Control of West Nile Virus Encephalitis. Journal of Virology 79: 11457–11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Salmaggi A, Gelati M, Dufour A, Corsini E, Pagano S, et al. (2002) Expression and Modulation of IFN-g-Inducible Chemokines (IP-10, Mig, and I-TAC) in Human Brain Endothelium and Astrocytes: Possible Relevance for the Immune Invasion of the Central Nervous System and the Pathogenesis of Multiple Sclerosis. J Interferon Cytokine Res 22: 631–640. [DOI] [PubMed] [Google Scholar]
- 80. Wu Z, Zhang J, Nakanishi H (2005) Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. Journal of Neuroimmunology 167: 90–98. [DOI] [PubMed] [Google Scholar]
- 81. Dionne KR, Leser JS, Lorenzen KA, Beckham JD, Tyler KL (2011) A brain slice culture model of viral encephalitis reveals an innate CNS cytokine response profile and the therapeutic potential of caspase inhibition. Experimental Neurology 228: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, et al. (2012) Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 60: 717–727. [DOI] [PubMed] [Google Scholar]
- 83. Sui Y, Potula R, Dhillon N, Pinson D, Li S, et al. (2004) Neuronal Apoptosis Is Mediated by CXCL10 Overexpression in Simian Human Immunodeficiency Virus Encephalitis. The American Journal of Pathology 164: 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. van Weering HRJ, Jong APHd, Haas AHd, Biber KPH, Boddeke HWGM (2010) CCL21-induced calcium transients and proliferation in primary mouse astrocytes: CXCR3-dependent and independent responses. Brain, Behavior, and Immunity 24: 768–775. [DOI] [PubMed] [Google Scholar]
- 85. Nelson TE, Gruol DL (2004) The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. Journal of Neuroimmunology 156: 74–87. [DOI] [PubMed] [Google Scholar]
- 86. Dijkstra IM, Hulshof S, van der Valk P, Boddeke HWGM, Biber K (2004) Cutting Edge: Activity of Human Adult Microglia in Response to CC Chemokine Ligand 21. J Immunol 172: 2744–2747. [DOI] [PubMed] [Google Scholar]
- 87. Tanuma N, Sakuma H, Sasaki A, Matsumoto Y (2006) Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathologica 112: 195–204. [DOI] [PubMed] [Google Scholar]
- 88. Israelsson C, Bengtsson H, Lobell A, Nilsson LNG, Kylberg A, et al. (2010) Appearance of Cxcl10-expressing cell clusters is common for traumatic brain injury and neurodegenerative disorders. European Journal of Neuroscience 31: 852–863. [DOI] [PubMed] [Google Scholar]