Abstract
Background
The corticotropin releasing hormone (CRH) system has been implicated in a variety of anxiety and mood-based symptoms and disorders. CRH receptor-2 (CRHR-2) plays a role in attenuating biological responses to stressful life events and trauma, making the CRHR-2 gene a strong candidate to study in relationship to posttraumatic stress disorder (PTSD).
Methods
The sample was 491 trauma-exposed white non-Hispanic veterans and their cohabitating intimate partners assessed via structured interview for lifetime DSM-IV PTSD; just over 60% met criteria for the disorder. Thirty-one single nucleotide polymorphisms (SNPs) in and near CRHR-2, obtained from an array of 2.5 million markers, were tested for association with PTSD diagnosis and symptom severity in the whole sample and in men and women separately.
Results
Ten SNPs showed nominally significant evidence of association with PTSD in the full sample and two SNPs (rs8192496 and rs2190242) were significant after permutation-based multiple testing correction (uncorrected ps = .0004 and .0005, odds ratios = .60 and .58, respectively). Analyses stratified by sex revealed that the effect was specific to women, who comprised 35% of the sample (uncorrected ps = .0003 and .0002, odds ratios = .41 and .35, respectively). Two additional SNPs (rs2267715 and rs2284218) also showed significant association with PTSD in women (both uncorrected ps = .001, both odds ratios = .48).
Conclusions
Results suggest that CRHR-2 variants may affect risk for PTSD in women by attenuating the stress response and reducing symptoms of the disorder.
Keywords: PTSD, single nucleotide polymorphisms, rs8192496, rs2284218, women, veterans, latent variable
Introduction
Corticotropin-releasing hormone (CRH) is a neuropeptide released by the hypothalamus following exposure to a stressor. CRH initiates activity of the hypothalamic-pituitary-adrenal axis, modulates the dopamine, serotonin, glutamate, and norepinephrine systems,[1–3] exerts effects on immune and autonomic processes,[4] and plays an important role in coordinating the physiological and behavioral response to stressors.[5,6] This is important for overall adaption to the environment and for returning the body to a state of homeostasis once the threat has passed.[6] However, CRH dysregulation has been linked to anxious and depressed behavior in animal models[7,8] and implicated in various psychiatric disorders, including anxiety, depression,[9–12] and posttraumatic stress disorder (PTSD).[13–15]
The CRH system has two receptors: CRHR-1 and CRHR-2. CRHR-1 is distributed throughout the brain, including the pituitary and neocortex,[16–19] and plays a role in stress responding[6] and anxious arousal.[20] CRHR-2 is densely populated in brain structures subserving fear, anxiety, and arousal including the septum, hypothalamus, amygdala, and bed nucleus of the stria terminalis, as well as in the dorsal raphe nucleus and choroid plexus.[16–19] Urocortins bind to CRHR-2,[21] and the receptor is thought to be responsible for dampening the stress response initiated by activation of CRHR-1. CRHR-2 expression has been shown to be associated with reduced anxious, aroused, and depressed behaviors,[22–25] although a recent animal study raises the possibility that chronic activation of the receptor by urocortin 3 may lead to increased baseline anxiety with reduced behavioral and biological responses to acute stress.[26]
Prior psychiatric genetic studies that have examined CRHR-2 have yielded mixed results. Some studies have found associations between this gene and various psychiatric phenotypes, including depression,[20] panic disorder,[20] bipolar disorder,[27] and response to anti-depressant drug treatment.[28,29] However, other studies have found no evidence for association between the gene and major depression,[30] suicidal behavior,[31] panic disorder,[32,33] and obsessive-compulsive disorder.[34]
Animal studies suggest that the expression and functioning of CRHR-2 varies in response to stressors and early life trauma such that receptor expression is up-regulated in some brain regions and down-regulated in others.[35,36] Given that the function of the receptor appears to be related to both genotype and environmental exposure, this raises the possibility that the gene plays a role in moderating the effects of life stress on psychiatric symptoms. To our knowledge, no prior study has evaluated the interaction between CRHR-2 and trauma in risk for psychiatric disorders among humans, though one study showed evidence of an interaction between CRHR-1 and trauma exposure on risk for depression.[37]
Given these initial indications that CRHR-2 may be involved in the etiology of psychiatric disorders, and its putative role in mitigating the stress response, we hypothesized that this gene might also be associated with PTSD, a disorder defined, in part, by exposure to traumatic stress. The Diagnostic and Statistical Manual-IV (DSM-IV)[38] PTSD diagnosis requires that individuals report at least one reexperiencing symptom (e.g., flashbacks, nightmares), three avoidance and numbing symptoms (e.g., avoidance of activities reminiscent of trauma, emotional numbing, social estrangement), and two hyperarousal symptoms (e.g., hypervigilance, exaggerated startle response) concurrently for a period of a least one month. While PTSD is conditional on trauma exposure, the observation that approximately 50–90% of the population has been exposed to trauma while only 8–14% develop the disorder[39,40] suggests that individual differences in biological risk may increase or decrease the likelihood of PTSD following exposure. Twin studies suggest that approximately 30–46% of the variance among veterans and other adult samples is heritable,[41–43] suggesting the potential value of searching for the specific genetic variants that comprise this overall genetic risk. Two recent genome-wide association studies have suggested that variants in the retinoid-related orphan receptor alpha (RORA)[44] and tolloid-Like 1 (TLL1)[45] genes are implicated in the disorder and a number of candidate gene studies suggest involvement of genes relevant to a variety of neurohormones and neurotransmitters (see Cornelis et al.[46] for a recent review.)
In this study, we examined an array of single nucleotide polymorphisms (SNPs) in CRHR-2 for evidence of an association with both lifetime PTSD diagnosis and lifetime PTSD symptom severity. These analyses were conducted in a sample of trauma-exposed participants, which permitted examination of the role of genotype in individuals exposed to a major environmental risk factor. We also tested whether degree and diversity of trauma exposure (as defined by the number of different traumatic events an individual was exposed to) interacted with CRHR-2 to predict PTSD. We hypothesized that variants within CRHR-2 would affect the probability of developing PTSD in trauma-exposed participants and that genotype would moderate the effects of trauma exposure. Finally, given preliminary evidence for sex differences in the role of CRHR-2 in stress responding,[24,36,47] our third aim was to examine possible sex × genotype interactions in predicting PTSD.
Methods and Materials
Participants
The full sample (described in Logue et al. [44]) included 852 veterans and their intimate partners who participated in one of two research studies with identical diagnostic assessment procedures, allowing the data from the two studies to be combined. One study enrolled veterans who screened positive for PTSD and the second study enrolled trauma-exposed veterans and their cohabitating intimate partners (see below). The present study focused on white non-Hispanic participants--the largest racially homogenous subsample of participants. Ancestry was determined with the program STRUCTURE using 10,000 randomly chosen markers with minor allele frequency (MAF) > .05 and a Bayesian clustering analysis to assign subjects to ancestry groups.[48,49] Through this method, we identified a subgroup of 540 white non-Hispanic participants. The possibility of PTSD-associated population substructure within the Caucasian sample was examined by using principal components (PC) analysis of 10,000 randomly chosen markers with MAF > .05 in the program EIGENSTRAT.[50] The top 10 PCs from that analysis were then entered into a multiple regression predicting PTSD diagnosis, and separately, PTSD severity. We found no evidence for PTSD-associated population substructure when evaluating either PTSD diagnosis or severity using multiple regression (overall model p-values > .05), so the PCs were not included in subsequent analyses.
The current study was based on 491 of these individuals who reported lifetime exposure to a traumatic event and had valid lifetime PTSD interview data. Of these, 155 had participated in the veteran-only study and 336 had participated in the study on couples; 364 were veterans and 127 were partners (41 “partners” from the couples study were also veterans and are included in the veteran total). The majority of participants were male (65%, n = 319), and the overall mean age was 51.95 years (range: 21 – 75, SD: 11.06; mean age among males: 52.39; mean age among females: 51.13). The majority of the veterans were male (86.5% or n = 315) and the majority of partners were female (96.9% or n = 123).
A total of 60.29% (n = 296) met DSM-IV diagnostic criteria for a lifetime diagnosis of PTSD (comprised of 251 veterans and 45 partners or 217 males and 79 females). The mean number of distinct types of lifetime traumatic experiences meeting the DSM-IV PTSD A1 and A2 definition of a traumatic event did not differ in men (M = 10.12, SD = 3.66) compared to women (M = 9.56, SD = 4.85), t (489) = 1.45 p = .15. The most prevalent type of trauma among men was the sudden death of a friend or loved one, occurring in 57.1% of the men, followed by combat, reported by 53.3% of the men. The most prevalent type of trauma among women was sudden death of a friend or loved one occurring in 64% of the women, followed by sexual assault at any age, occurring in 57.9% of the women. The total number of distinct lifetime traumatic events meeting the DSM-IV PTSD A1 and A2 definition of trauma was higher among veterans (M = 10.51, SD = 3.94) compared to non-veterans (M = 8.25, SD = 4.18), t (489) = 5.46, p < .001. The most common type of trauma among the veterans was the sudden death of a friend or loved one (58.8% of the veterans), followed by combat exposure (48.4%); the sudden death of a friend or loved one was the most common trauma type among the non-veterans (occurring in 61.4% of this group), followed by sexual assault at any age (51.1%).
Measures
Clinician Administered PTSD Scale (CAPS). [51]
The CAPS is the gold-standard structured diagnostic interview for the assessment of PTSD, its associated features, and functional impairment. Each of the 17 DSM-IV PTSD symptoms is assessed using frequency and intensity scales (each ranging from 0–4), which are then summed across symptoms to form a single severity score. The PTSD diagnosis was calculated using a validated scoring rule[52] which required endorsement of at least 1 reexperiencing, 3 avoidance and numbing, and 2 hyperarousal symptoms, each at a frequency of 1 or greater and an intensity of 2 or greater. Cronbach’s alpha in this sample was .90 for lifetime symptoms. Inter-rater reliability, as determined by a second rater making independent ratings from videotaped recordings of approximately 25% of the total participant interviews, was excellent for both the lifetime diagnosis (κ = .87) and the dimensional lifetime severity scores (intraclass correlation coefficient = .97).
Traumatic Life Events Questionnaire (TLEQ). [53]
The TLEQ is a self-report instrument that assesses history of exposure to 22 different types of traumatic experiences that meet the DSM-IV PTSD Criterion A1 definition of trauma. Events include childhood physical and sexual abuse, combat exposure, motor vehicle accidents, natural disaster, and adult sexual and physical assault, among others. The measure also assesses whether the experience met DSM-IV PTSD Criterion A2 (i.e., the emotional response to the trauma included intense fear, helplessness, or horror) and asks the respondent to indicate the number of times each event occurred on a 7-point scale ranging from “never” to “more than five times.” Participants also identified their “worst” trauma from the list of trauma types endorsed on the TLEQ. The TLEQ has shown good test-retest reliability and predictive validity with respect to PTSD diagnoses.[53]
Procedure
Data from two studies were combined for these analyses. Study 1 recruited trauma-exposed military veterans who screened positive for PTSD over the telephone; Study 2 recruited trauma-exposed military veterans and their cohabitating intimate partners. Both groups were recruited via flyers posted in local VA hospitals, clinician referrals, and through our Center’s participant recruitment database of individuals who have expressed interest in participating in research studies; couples were also recruited by mail. Both studies included comprehensive structured diagnostic interviews that were digitally videotaped for purposes of quality control and evaluating inter-rater reliability. For Study 2, both members of the couple underwent the same diagnostic interviews (separately), and both provided blood samples for genetic analyses. The PTSD assessment was linked to a single index traumatic event meeting the DSM-IV A1 and A2 definition of a traumatic event. To do so, the clinician administering the PTSD interview reviewed responses to the TLEQ and as part of the CAPS interview, requested greater detail on the self-identified worst traumatic experience in order to ensure that the index event met the DSM-IV definition of a traumatic experience. The studies were approved and reviewed annually by the appropriate institutional review boards (IRBs) and scientific review panels.
Genotyping
DNA was isolated from peripheral blood samples on a Qiagen AutoPure instrument with Qiagen reagents and samples normalized using PicoGreen assays (Invitrogen). Samples were run on an Illumina OMNI 2.5–8 array and scanned using an Illumina HiScan System according to the manufacturer’s protocol. Details on call rates, elimination of participants, and evaluation of biological sex using X-chromosome homozygosity are described in detail elsewhere[44]. All SNP bp locations were derived from the hg19 human-genome assembly (February, 2009). We restricted our analyses to the 31SNPs on the genotyping array within 5 kb of CRHR-2 with a minor allele frequency (MAF) in this sample greater than 5%. None of the SNPs failed a test of Hardy-Weinberg Equilibrium (i.e., all p > .01).
Statistical Analyses
Data were combined across the two research studies and across veterans and their partners (as noted above, “cases” as well as “controls” included both veterans and partners). We ran parallel analyses predicting PTSD diagnosis and PTSD severity. We used the standard case/control association test (i.e., χ2) in PLINK[54] to evaluate the association between SNPs in CRHR-2 and lifetime PTSD diagnoses. The PLINK max(T) permutation procedure with 5000 replications was used to correct for multiple testing across the examined SNPs. We evaluated interactions between each SNP and total number of lifetime different traumatic experiences meeting PTSD Criterion A1 and A2, and, separately, biological sex, using the logistic regression function in PLINK. After identifying SNPs that showed associations with lifetime PTSD diagnosis, we then evaluated the association between these SNPs and lifetime PTSD severity using the latent variable modeling software Mplus 6.12.[55] The dimensional latent variable model offers several potential statistical advantages over a dichotomous representation of the diagnosis for genetic association analysis, including improved reliability and construct validity and enhanced statistical power, and it avoids the loss of information surrounding arbitrary diagnostic cut-points by use of the full range of symptom severity information. Linkage disequilibrium (LD) was evaluated using Haploview.[56]
PTSD symptom severity was defined (i.e., indicated) by summary scores on four symptom clusters (reexperiencing, avoidance, emotional numbing, and hyperarousal; cf., King et al.[57]), and the SNPs were included in a structural equation model predicting this latent variable. We also evaluated the moderating effect of lifetime trauma exposure (as indicated by the number of different types of trauma an individual was exposed to) and, in a separate model, sex, in predicting PTSD severity. Throughout these analyses, we used additive coding of the CRHR-2 SNPs, i.e., 0, 1, or 2 based on the number of minor alleles.
Structural equation models were conducted using a maximum likelihood estimator that is robust to non-normality (MLR). The nested structure of the data (some individuals clustered within couples) was specified, and the standard errors of the parameter estimates were adjusted to reduce the risk of type I error associated with non-independence of observations. Model evaluation was based on a several fit indices,[58] including the overall model χ2, the root mean square error of approximation (RMSEA; values ≤ .06 are preferred), the standardized root mean square residual (SRMR; values ≤ .08 are preferred), and the confirmatory and Tucker-Lewis fit indices (CFI and TLI, respectively; values ≥ .90 indicate adequate fit while values greater ≥ .95 indicate excellent fit).
Results
Association between SNPs in CRHR-2 and Lifetime PTSD Diagnoses
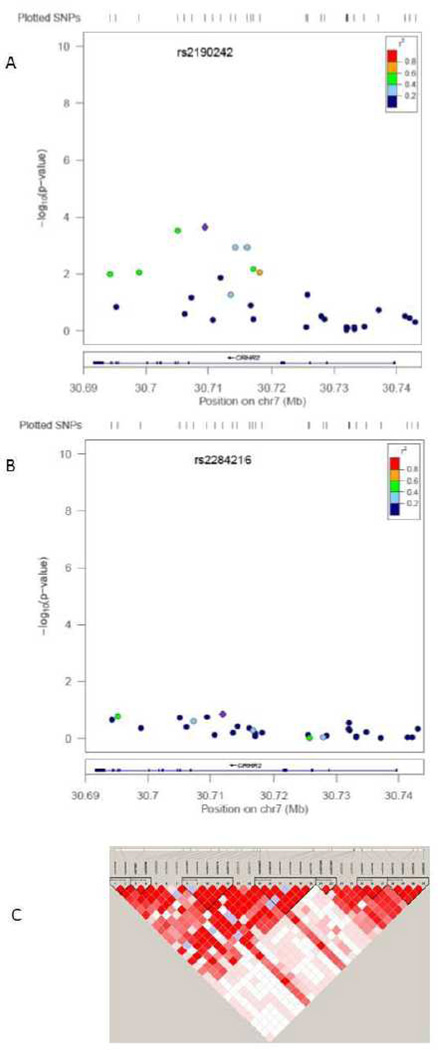
Ten SNPs in CRHR-2 showed nominally significant associations with lifetime PTSD diagnosis (see Table 1). The p-values for two SNPs remained significant after correction for multiple testing based on the 31 SNPs examined. Specifically, the minor allele (G) of rs8192496 was associated with reduced risk for PTSD (χ2 = 12.55, uncorrected p = .0004, corrected p = .01; OR = 0.60). The MAF was 29% (23% in cases and 33% in controls, see Table 1). This SNP is located at 30,705,105 base pair (bp)--13,536 bp from the start of the gene in an intronic region. In addition, the minor allele (C) of rs2190242 was associated with reduced risk for PTSD (χ2 = 12.06, uncorrected p-value = .0005, corrected p-value = .01; OR = 0.58). The MAF was 21% (17% in cases and 26% in controls; see Table 1). This SNP is located at 30,709,475 bp, which is 17,196 bp from the start of the gene and 4,370 bp from rs8192496; it is also located in an intronic region. These two SNPs are in relatively high LD with each other (D’ = .92, R2 = .57). LD across the region is shown in Figure 1, Panel C.
Table 1.
CRHR-2 SNPs Showing Nominal Associations with Lifetime PTSD Diagnosis
| SNP | bp | Allele | Freq affected |
Freq unaffected |
OR (95% CI) |
p uncorrected | p corrected |
|---|---|---|---|---|---|---|---|
| rs3779250 | 30694260 | G | 0.29 | 0.37 | 0.69 (.52 – .90) | 0.006 | 0.10 |
| rs973002 | 30698904 | G | 0.13 | 0.19 | 0.65 (.46 – .92) | 0.01 | 0.21 |
| rs8192496 | 30705105 | G | 0.23 | 0.33 | 0.60 (.45 – .80) | 0.0004 | 0.01 |
| rs2240404 | 30707302 | A | 0.1 | 0.15 | 0.65 (.44 – .95) | 0.03 | 0.33 |
| rs2190242 | 30709475 | C | 0.17 | 0.26 | 0.58 (.42 – .79) | 0.0005 | 0.01 |
| rs2284216 | 30711961 | A | 0.06 | 0.11 | 0.54 (.34 – .85) | 0.007 | 0.11 |
| rs2284218 | 30714333 | G | 0.32 | 0.41 | 0.67 (.52 – .88) | 0.003 | 0.05 |
| rs2267715 | 30716087 | G | 0.33 | 0.41 | 0.68 (.52 – .89) | 0.004 | 0.07 |
| rs2267717 | 30717043 | A | 0.09 | 0.14 | 0.64 (.43 – .95) | 0.03 | 0.34 |
| rs2284220 | 30718103 | G | 0.11 | 0.16 | 0.64 (.44 – .93) | 0.02 | 0.24 |
Note. The sample was comprised of 319 men and 172 women; 296 met criteria for PTSD. SNPs are ordered by bp locations and p-values are rounded to the first non-zero integer or to two decimal places (for non-significant results). CRHR-2 = corticotropin releasing hormone receptor 2; SNP = single nucleotide polymorphism; CI = confidence interval; PTSD = posttraumatic stress disorder; CHR = chromosome; bp = base pair; Freq = frequency; OR = odds ratio.
Figure 1.
shows the association between the 31 CRHR-2 SNPs and PTSD in 172 women (Panel A) and 319 men (Panel B). The R2 in reference to the peak SNP in each subsample is also shown in Panels A and B, as is the genomic structure of the region; the peak SNP in each subsample is listed at the top of Panels A and B. Panel C shows the LD (D’) of the SNPs that were evaluated; darker cells indicate higher D’ coefficients.
Interactions with trauma exposure and sex
The number of distinct traumatic experiences was a significant predictor of PTSD (OR: 1.15, 95% CI: 1.09 – 1.21, p = .0000001), but we found no evidence it interacted with any of the SNPs to predict PTSD diagnosis (all p >.05). Sex was a significant predictor of PTSD (OR= 2.48, 95% CI: 1.69 – 3.64, p =.000004), with men at greater risk for the disorder. A sex by SNP interaction term was nominally significant for three of the examined variants: rs8192496 (interaction OR = 1.91, 95% CI: 1.03 – 3.56, p = .04), rs2190242 (interaction OR = 2.15, 95% CI: 1.06 – 4.38, p = .03) and rs2267715 (interaction OR = 1.79, 95% CI: 1.01 – 3.18, p = .04). Analysis of the association between these SNPs and PTSD diagnosis in male and female participants separately revealed these associations to be significant in women (for rs8192496: OR = 0.41, 95% CI: .25 - .67, p = .0003, corrected p = .006; for rs2190242: OR = 0.35, 95% CI: .20 - .62, p = .0002, corrected p = .004; for rs2267715: OR = 0.48, 95% CI: .30 - .74, p = 0.001, corrected p = .02) but not men (for rs8192496: OR = 0.78, 95% CI: .54 – 1.13, p = .19; for rs2190242: OR = 0.75, 95% CI: .50 – 1.13, p = .17; for rs2267715: OR = 0.87, 95% CI: .62 – 1.24, p = .44). In addition, rs2284218 showed a female-specific association with PTSD that withstood permutation testing (OR = 0.48, 95% CI: .31 – .75, p = .001, corrected p = .02); rs2284218 was in perfect LD with rs2267715 (D’ = 1, R2 = 1). These two SNPs (rs2267715 and rs2284218) were in high LD with rs8192496 (D’ = .78, R2 = .40) and rs2190242 (D’ = .92, R2 = .38), respectively. Panels A and B of Figure 1 show the association results for women and men, respectively. In sum, there were four SNPs which showed significant association with PTSD diagnostic status in women; these four SNPs were also the four most significant when evaluated in the full sample, and two of them withstood correction for multiple testing in the full sample (see Table 1).1 The results of follow-up analyses in which we controlled for the number of different traumatic events in the sex-stratified models did not differ meaningfully from those reported above, although trauma exposure was a significant predictor of PTSD in both men and women.
Association between SNPs in CRHR-2 and Latent PTSD Severity
Next, we used structural equation modeling to evaluate the association between the two SNPs that were significant in the full sample and PTSD severity, using a single factor model of PTSD2. Given the LD between them (and hence the potential for multicollinearity), we evaluated each SNP in separate analyses. The model examining rs8192496 and PTSD severity fit the data well (see Table 2, Model B1) and the SNP showed a significant association with PTSD severity (unstandardized β = −1.78, p = .01). The model evaluating rs2190242 also fit the data well (see Table 2, Model C1; unstandardized β = −1.96, p = .01). Each additional copy of the minor allele of rs8192496 and rs2190242 was associated with a .19 and .21 standard score (i.e., SD) decrease, respectively, in mean PTSD symptom severity (i.e., these are the parameter estimates when standardized according to the dependent variable only).
Table 2.
Fit Statistics for Structural Equation Models
| Model | χ2 (df), p-value | RMSEA | SRMR | CFI | TLI |
|---|---|---|---|---|---|
| A. Measurement Model | |||||
| 1. One Factor | 18.39 (2), p < .0001 |
.13 | .02 | .98 | .94 |
| B. SEMs for rs8192496 | |||||
| 1. Main effect | 19.97 (5), p = .001 |
.08 | .02 | .98 | .96 |
| 2. Interaction with Trauma | .21.56 (11), p = .03 |
.04 | .02 | .99 | .98 |
| 3. Interaction with Sex | 27.94 (11), p = .003 |
.06 | .02 | .98 | .97 |
| C. SEMs for rs2190242 | |||||
| 1. Main effect | 19.58 (5), p = .002 |
.08 | .02 | .98 | .96 |
| 2. Interaction with Trauma | 21.50 (11), p = .03 |
.04 | .02 | .99 | .98 |
| 3. Interaction with Sex | 26.71 (11), p = .005 |
.05 | .02 | .98 | .97 |
| D. SEM for rs2267715 | |||||
| 1. Interaction with Sex | 33.26 (11), p = .0005 |
.07 | .03 | .97 | .96 |
| E. SEM for rs2284218 | |||||
| 1. Interaction with Sex | 33.44 (11), p = .0004 |
.07 | .02 | .97 | .96 |
Note. The sample was comprised of 319 men and 172 women; 296 met criteria for PTSD. df = degrees of freedom; RMSEA = root mean square error of approximation; SRMR = standardized root mean square residual, CFI = confirmatory fit index; TLI = Tucker-Lewis index; SEM = structural equation model.
Interaction with trauma
We next evaluated whether the association between these SNPs and PTSD severity was moderated by lifetime trauma exposure. These models fit the data well (see Table 2, Models B3 and C3). Trauma exposure was a significant predictor of PTSD severity (unstandardized β = .88, p < .001), but did not interact with rs8192496 (unstandardized β = .22, p = .09) or rs2190242 (unstandardized β = .15, p = .31) to predict PTSD severity.
Interaction with sex
We also ran four models, based on the results obtained for PTSD diagnosis, that included sex × SNP interactions in the prediction of PTSD severity. The fit of these models is listed in Table 2 (models B4, C4, D1, and E1). As before, sex was a significant predictor of PTSD severity, with men showing higher mean levels of PTSD than women. Sex did not interact with rs8192496 (unstandardized β = 1.93, p = .16), rs2267715 (unstandardized β = 2.14, p = .10), or rs2284218 (unstandardized β = 1.79, p = .17). However, it did interact with rs2190242 (unstandardized β = 3.62, p = .02). Given this pattern of results, we then stratified the sample by sex and evaluated the effect of each of these SNPs on PTSD severity, separately, for men and women. All of these SNPs showed significant association with PTSD severity in women (for rs8192496: unstandardized β = −2.86, p = .01; for rs2190242: unstandardized β = −4.26, p = .001; for rs2267715: unstandardized β = −3.07, p = .003; for rs2284218: unstandardized β = −2.89, p = .005). None of the SNPs were significantly associated with PTSD severity in men (for rs8192496: unstandardized β = −.86, p = .30; for rs2190242: unstandardized β = −.50, p = .57; for rs2267715: unstandardized β = −.78, p = .35; for rs2284218: unstandardized β = −.95, p = .25). In women, each additional copy of the minor allele of rs8192496, rs2190242, rs2267715, or rs2284218 was associated with a .29, .43, .31, or .29, respectively, standard score decrease in mean PTSD symptom severity.
Discussion
We evaluated the association between SNPs in CRHR-2 and PTSD diagnostic status and severity in a sample of trauma-exposed veterans and their partners with a high prevalence of PTSD. We focused on CRHR-2 because prior studies suggest that it plays a role in moderating the release of stress hormones, thereby reducing symptoms of anxiety and depression. Ten SNPs in CRHR-2 showed nominally significant evidence of association with lifetime PTSD diagnostic status. Two SNPs, rs8192496 and rs2190242 (in high LD), withstood correction for multiple testing. Interaction analyses revealed that these effects were specific to women and that rs2267715 and rs2284218 also evidenced association with PTSD in women only. The minor allele of these SNPs was associated with reduced risk and severity of PTSD symptoms.
These findings provide initial evidence that the relationship of the CRHR-2 genotype to PTSD may be specific to women. This is consistent with prior observations that women are more likely to exhibit anxiety and unipolar depressive disorders[59] and PTSD.[39] The animal literature also suggests that CRHR-2 may differentially mediate anxiety and depressive behaviors in females compared to males. Specifically, Bale and Vale[47] showed that female, but not male, CRHR-2 knockout mice spent less time climbing during a forced swim task (interpreted as an indicator of depressed behavior) compared to wild mice. Further, a study of rats found that prenatal stress exposure was selectively associated with reduced CRHR-2 mRNA expression in the amygdala among female but not male offspring, suggesting that stress and trauma may interact with sex in the functioning of CRHR-2.[36] However, this pattern of results is not unequivocal, as other research with CRHR-2-deficient mice showed increased anxious behavior in male, but not female, mice.[24] Finally, there is also evidence that male sex hormones affect CRHR-2 mRNA expression in the lateral septum in rats,[60] suggesting a possible link between functioning of CRHR-2 and sex.
Other investigators have reported associations between CRHR-2 SNPs and related psychiatric phenotypes. For example, rs3779250 (at 30,694,260 bp) was associated with depression in a Japanese sample[20] with the C variant less common in affected cases. In our sample of white non-Hispanic participants, the same variant of this SNP was also associated with reduced risk for PTSD (OR = 0.69, p = .006). That SNP was in moderately-high LD with our two most significant SNPs (D’ = .88, R2 = .61 with rs8192496 and D’ = .94, R2 = .47 with rs2190242) and is 10,845 bp and 15,215 bp away from rs8192496 and rs2190242, respectively.
These findings are consistent with prior work suggesting that the function of the CRHR-2 is to modulate neuroendocrine stress responses[6] by counteracting ACTH binding to CRHR-1. For example, CRHR-2 knockout mice evidenced greater activation of the stress response system following a stressor as evidenced by increased immediate ACTH, increased corticosteroids, and increased anxious behavior.[22–24] In another study, CRHR-2 knockout mice also showed greater depressive behavior compared to wild types.[47] Injection of a CRHR-2 agonist yielded reduced anxiety in rats following a stressor,[25] further implicating the receptor in modulation of anxiety. Lebow et al.[18] reported PTSD-like symptoms (hyperarousal, hypervigilance, attention impairment, risky behaviors, and difficulty sleeping) in rodents who exhibited CRHR-2 mRNA upregulation in the bed nucleus of the stria terminalis. Finally, mice who were deficient in urocortin 3 (a CRHR-2 ligand) evidenced poor ability to distinguish between familiar and unfamiliar mice in a social memory task, indicating that CRHR-2 plays a role in social learning and processing.[61] This may be analogous to the tendency to appraise everyday situations as potentially threatening (as reflected in hypervigilant behaviors) and to engage in social isolation and avoidance in PTSD (both DSM-defined symptoms of the disorder). Taken together, CRHR-2 appears to play a role in the sensitivity of the organism to stressors, in reducing anxiety and depressive behaviors following stressor exposure, in dampening biological stress responses, and increasing social processing and memory.[6,21,61]
Results of this study showed no evidence for a moderating role of genotype on the association between lifetime trauma exposure (number of different types of traumatic events) and PTSD. This may be because we had essentially already accounted for the interaction between genotype and trauma by evaluating CRHR-2 among trauma-exposed participants only. Our interaction analyses, evaluated in a sample with considerable heterogeneity with respect to the degree and diversity of trauma, suggested that these aspects of trauma exposure did not further interact with genotype to predict PTSD.
Limitations and Conclusions
The generalizability of these results was limited by our focus on white non-Hispanic participants. Our analyses, particularly those conducted in the subset of women, were likely under-powered to observe effects among SNPs with very low MAF. As veterans were predominantly male and partners female (and as veterans and partners differed from each other in the nature and extent of trauma exposure), it is impossible to fully disentangle effects attributable to sex from those attributable to veteran status and trauma history. This concern is not unique to this sample as sex is nearly always confounded with trauma type (e.g., women are more likely to experience sexual trauma relative to men[39]) and with other demographic and social factors as well. Given this, the findings of this study should be considered preliminary until future replication studies in larger samples are completed. However, the fact that these findings converge with other research showing association between CRHR-2 and related psychiatric phenotypes[20,27,29] should alleviate concern about potential false positive associations. Additionally, as these are tag SNPs, which are presumably not functional, additional work is needed to identify the causal variants underlying the association, the particular functional mechanism by which these variants produce their effect, and how this function may differ by sex. Strengths of this study included our use of gold standard assessment procedures and the application of modeling techniques to study the association between genes and PTSD.
To our knowledge, this was the first candidate gene study to evaluate the association between CRHR-2 and PTSD. We evaluated SNPs in the gene in a rigorously assessed clinical sample with a high prevalence of PTSD. We found that two CRHR-2 SNPs in high LD were associated with PTSD diagnosis and symptom severity, with the minor alleles of each associated with decreased risk for the development of the disorder following trauma exposure. The observed association was driven by the effect in women. This pattern of association is consistent with research suggesting that CRHR-2 is involved in attenuation of the biological stress response and concomitant attenuation of anxiety and depression symptoms.
Acknowledgments
Funding for this study was provided by RO1 MH079806 (MWM), VA CSR&D Merit Award # 5I01CX000431-02 (MWM), VA BLR&D Merit Award # 1I01BX002150-01 (MWM and MWL), as well as a VA CSR&D Career Development Award (EJW) and K01MH093750 (KSM).
Footnotes
Financial Disclosures
Mark Miller and Annemarie Reardon own stock in Illumina. All other authors of this manuscript reported no biomedical financial interests or potential conflicts of interest.
A haplotype analysis of the most significant SNPs, as well as a moving window haplotype analysis, in the whole sample and in women alone did not yield associations with PTSD that were more statistically significant than those observed for the individual SNPs, arguing against the utility of haplotype analyses in this study.
The confirmatory factor analysis evaluating the single factor model of PTSD (i.e., indicated by four symptom cluster severity scores) provided adequate fit to the data (see Table 2). The indicators loaded significantly on the latent PTSD factor (all ps < .001), and standardized factor loadings were in the range of .73 – .83. Mean total PTSD severity was 60.03 (SD = 32.03).
References
- 1.Lavicky J, Dunn AJ. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 2.Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Kovács KJ. CRH: The link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. doi: 10.1016/j.jchemneu.2013.05.003. in press. [DOI] [PubMed] [Google Scholar]
- 5.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: A role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL, Vale WW. CFR and CFR Receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 7.Flandreau EI, Ressler KJ, Owens MJ, et al. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gaalen MM, Stenzel-Poore MP, Holsboer F, et al. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr Top Med Chem. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- 12.Ströhle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36:S207–s214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- 13.Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 14.Sautter FJ, Bissette G, Wiley J, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- 16.Arborelius L, Owens MJ, Plotsky PM, et al. The role of corticotrophin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 17.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebow M, Neufeld-Cohen A, Kuperman Y, et al. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J Neurosci. 2012;32:6906–6916. doi: 10.1523/JNEUROSCI.4012-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Ishitobi Y, Nakayama S, Yamaguchi K, et al. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am J Med Genet. 2012;159:429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- 21.Bale TL. Sensitivity to stress: Dysregulation in CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat Genet. 2000;25:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 23.Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotrophin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto T, Radulovic J, Radulovic M, et al. Deletion of CRHR2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 25.Valdez GR, Zorrilla EP, Rivier J, et al. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;8:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 26.Neufeld-Cohen A, Kelly PAT, Paul ED, et al. Chronic activation of corticotrophin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72:437–447. doi: 10.1016/j.biopsych.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotrophin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. European Psychiatry. 2007;22:282–287. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Papiol S, Arias B, Gastró C, et al. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007;104:83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Wong M-L, Dong C, Maestre-Mesa J, et al. Polymorphisms in inflammation-related genes are association with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villafuerte SM, Del-Favero J, Adolfsson R, et al. Gene-based SNP genetic association study of corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet. 2002;114:222–226. doi: 10.1002/ajmg.10179. [DOI] [PubMed] [Google Scholar]
- 31.Roy A, Gorodetsky E, Yuan Q, et al. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keck ME, Kern N, Erhardt A, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet. 2008;147:1196–1204. doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- 33.Tharmalingam S, King N, De Luca V, et al. Lack of association between the corticotrophin-releasing hormone receptor 2 gene and panic disorder. Psychiatr Genet. 2006;16:93–97. doi: 10.1097/01.ypg.0000218610.45441.c3. [DOI] [PubMed] [Google Scholar]
- 34.Kas MJH, Gelegen C, van Nieuwerburgh F, et al. Compulsivity in mouse strains homologous with chromosomes 7p and 15q linked to obsessive-compulsive disorder. Am J Med Genet. 2010;153:252–259. doi: 10.1002/ajmg.b.30994. [DOI] [PubMed] [Google Scholar]
- 35.Vázquez DM, Eskandari R, Phelka A, et al. Impact of maternal deprivation on brain corticotropin-releasing hormone circuits: Prevention of CRH receptor-2 mRNA changes by Desipramine Treatment. Neuropsychopharmacology. 2003;28:898–909. doi: 10.1038/sj.npp.1300126. [DOI] [PubMed] [Google Scholar]
- 36.Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of corticotropin-releasing hormone and its receptors of the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]
- 37.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: Author; 1994. [Google Scholar]
- 39.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 40.Breslau N, Peterson EL, Poisson LM, Schultz LR, Lucia VC. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol Med. 2004;24:889–898. doi: 10.1017/s0033291703001612. [DOI] [PubMed] [Google Scholar]
- 41.True WJ, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 42.Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 43.Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Bucholz KK, Madden PAF, Heath AC, Martin NG, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Logue MW, Baldwin C, Guffanti G, et al. A genome-wide association study of posttraumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. doi: 10.1038/mp.2012.113. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelertner J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Bio Psychiatry. doi: 10.1016/j.biopsych.2013.04.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatr Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: Sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 51.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 52.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assessment. 1999;11:124–133. [Google Scholar]
- 53.Kubany ES, Leisen MB, Kaplan AS, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychol Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 54.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muthén LK, Muthén BO. Mplus User’s Guide. 6th ed. Los Angeles, CA: Muthén & Muthén; pp. 1998–2010. [Google Scholar]
- 56.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 57.King DW, Leskin GA, King LA, et al. Confirmatory factor analysis of the clinician-administered PTSD scale: Evidence for the dimensionality of posttraumatic stress disorder. Psychol Assessment. 1998;10:90–96. [Google Scholar]
- 58.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 59.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiser MJ, Goel N, Sandau US, et al. Androgen regulation of corticotropin-releasing hormone receptor 2 (CRHR2) mRNA expression and receptor binding in the rat brain. Exp Neurol. 2008;214:62–68. doi: 10.1016/j.expneurol.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deussing JM, Breu J, Kuhne C, et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J Neurosci. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]