Abstract
Objective
Genetic variation in six genes has been associated with elevated liver fat and nonalcoholic fatty liver disease in adults. We sought to determine the influence of these genes on liver fat and whether a genetic risk score (GRS) would improve upon the ability of common clinical risk factors to predict elevated liver fat content (ELF) in Hispanic children.
Design and Methods
223 obese Hispanic children were genotyped for six SNPs. MRI was used to measure liver fat. A GRS was tested for association with ELF using multivariate linear regression. Predictors were assessed via ROC curves and pair-wise analysis was used to determine significance alone and combined with clinical markers.
Results
Only variants in PNPLA3 and APOC3 genes were associated with liver fat (p<0.001, p=0.01, respectively). Subjects with a GRS=4 had ~3-fold higher liver fat content than subjects with GRS of 0 (15.1±12.7% vs. 5.1±3.7%, p=0.03). While the addition of the GRS to a model containing BMI and liver enzymes increased ROC AUC from 0.83 to 0.85 [95% CI, 0.79-0.89], (p=0.01), it does not improve detection of ELF from a clinical perspective.
Conclusions
Only PNPLA3 and APOC3 were related to ELF and a GRS comprised of these susceptibility alleles did not add to the discriminatory power of traditional biomarkers for clinical assessment of liver fat.
Keywords: NAFLD, liver fat, Hispanic, obesity, genetic risk
INTRODUCTION
As national rates of overweight and obesity continue to increase, so does the prevalence of nonalcoholic fatty liver disease (NAFLD). This trajectory, in terms of both increasing obesity and NAFLD, is also present in the pediatric population (1, 2). NAFLD, a condition characterized by an elevated liver fat content (ELF) of greater than 5.5 percent, which can be determined by imaging and/or histology (3), represents the proximal end of a broad spectrum of liver disease including nonalcoholic steatohepatitis (NASH), cirrhosis, and ultimately hepatocellular carcinoma. NAFLD represents the leading cause of chronic liver disease amongst all children in the United States (4, 5) and Hispanics are at an elevated risk of developing NAFLD, due in part to high obesity rates and potentially genetic factors.
Early clinical detection of ELF can be difficult, as the condition is usually asymptomatic. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as well as obesity status are currently used in the clinical evaluation of ELF and NAFLD (6, 7). MRI and magnetic resonance spectroscopy (MRS) can quantitatively assess, with a high degree of accuracy, hepatic fat content but are expensive clinical tests and not used routinely. By comparison, ultrasound is commonly used to assess liver fat content for clinical purposes and has limited utility due to the qualitative nature of the measure. While these three imaging techniques are commonly used, the “gold standard” remains liver biopsy, which can present an added risk for complications, particularly in the pediatric population. These current assessment methods represent a significant added burden and expense when compared to anthropometric and plasma assay measures in combination with genetic testing.
Recent genome-wide association studies (GWAS) and candidate gene approaches in adults have implicated six genes in the development of NAFLD and/or NASH, including patatin-like phospholipase 3 (PNPLA3), glycogen binding subunit of protein phosphatase1 (PPP1R3B), neurocan (NCAN), glucokinase regulatory protein (GCKR), lysophospholipase-like 1 (LYPLAL1), and apolipoprotein C3 (APOC3) (8-10). Previously, we validated the effect of PNPLA3 on hepatic triglyceride levels in 188 Hispanic children and demonstrated that this effect manifests as early as 8 years of age. However, the independent and combined effects of the five other variants on hepatic fat content in this pediatric population are not known. Therefore we sought to evaluate the association of these six genetic variants on hepatic fat content in obese Hispanic children and determine whether cumulative genetic burden improves the prediction of ELF beyond traditional clinical risk factors.
METHODS
Participants
This study was a cross-sectional analysis of 223 overweight and obese (BMI of 25-45 kg/m2), Hispanic children (41% male) between the ages of 8-17 years old who were recruited from several Districts in Los Angeles County. Participants were defined as Hispanic if they reported both parents and all four grandparents as Hispanic. All participants had medical and family history screening to ensure eligibility criteria were met. Patients were not eligible for the study if the following conditions were indicated: i) met any diagnostic criteria for diabetes; ii) the use of medications or supplements or the past or present diagnosis of other syndromes or diseases known to influence liver function, insulin action or lipid levels; iii) previous diagnosis of any major illness since birth; or iv) smoking (currently smoked or had smoked greater than 100 cigarettes in their lifetime) or drinking alcohol on a regular basis (in excess of 2 drinks per week as determined by questionnaire. Participants were not eligible for the study if they had current or past involvement with any weight loss/exercise/sports program in the six months prior to participation. Informed written child assent and parental consent were obtained from all patients. The Institutional Review Board of the USC Keck School of Medicine approved the study.
Anthropometry and Fasting Glucose
Height and weight were measured in triplicate for all patients, and BMI was expressed as kg body weight per m2 height. Fasting glucose was screened at the first outpatient visit, as previously described (11). If fasting blood glucose measured ≥126 mg/dl, an additional sample was immediately drawn to confirm the value and an additional visit was scheduled to confirm the diagnosis of diabetes. Diagnosis of diabetes resulted in exclusion from the study and referral to an endocrinologist.
Body Composition and Liver Fat
Whole body fat and soft lean tissue mass was estimated by dual energy x-ray absorptiometry (DEXA) using a Hologic QDR 4500W (Hologic, Bedford, MA). MRI was carried out on a General Electric 1.5-Tesla magnet (GE Healthcare, Waukesha, WI) to assess subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT) and hepatic fat fraction using a modification of the Dixon 3-point technique as previously described (12). ELF was defined as >5.5% hepatic fat fraction as determined by MRI, a value commonly attributed to likely NAFLD (12) and used in previous genetic studies with adults.
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measurements
ALT and AST levels were measured in plasma samples from 208 participants via the kinetic rate method using a Synchron CX analyzer (Beckman Coulter Inc. Fullerton, CA) (13). All samples were prepared in accordance with manufacturer specifications and analyzed in duplicate. The analytical range for both ALT and AST was 5-400 IU/L. ALT levels were considered abnormal when they exceeded recently established population-based upper limits of normal (ULN) values (14). An ALT normal reference range of 5-23.5 U/L was utilized in this study, with 23.5 representing the mean of the following ULN cutoff values established from NHANES data in boys (>22 U/L) and girls (>25 U/L) (14).
Molecular Genetic Analyses
Genomic DNA was extracted from whole blood. SNPs were selected based upon previously identified associations with NAFLD and/or hepatic steatosis from GWAS (9) and other association studies. The following 8 SNPs were genotyped in the study population: PNPLA3 (rs738409), APOC3 (rs2854116 and rs2854117), GCKR (rs780094 and rs1260326), NCAN (rs2228603), LYPLAL1 (rs12137855) and PPP1R3B (rs4240624). Initial genotyping for the two variants in APOC3 (rs2854116 and rs2854117) and GCKR (rs780094 and rs1260326) showed these SNPs to be in high linkage disequilibrium (r2>0.8, data not shown). Therefore, subsequent analyses were only conducted with rs2854117 for APOC3 and rs780094 for GCKR. Genotyping was performed with the TaqMan Allelic Discrimination System. Each 96-well DNA plate contained the same four control DNA samples, two from International HAPMAP Project and two randomly chosen DNAs from our Hispanic study cohort. Replicate quality control samples yielded 99% concordance, and the overall call rate was 95%.
Calculations and Statistical Analyses
All variants were first tested for Hardy-Weinberg equilibrium (HWE) using a χ2 test prior to analysis. General linear models were used to compare mean values of quantitative traits across groups, with adjustment for covariates. Liver fat values were natural log-transformed for analyses. A genetic risk score (GRS) was constructed for each participant based upon the sum of the PNPLA3 and APOC3 risk alleles that individually showed a significant association with elevated liver fat fraction. A weighted GRS (wGRS) was also calculated by multiplying the effect estimate (beta) on liver fat, obtained from separate linear regression analyses of each included SNP in the present study, adjusting for age and gender, by the number of risk alleles for that corresponding variant (0,1, or 2), and summing these values. GRS groups were defined as 1 (0-1 risk alleles), 2 (2-3 risk alleles) and 3 (4 risk alleles). Analysis of variance (ANOVA), controlling for age and gender, was used to determine between group effects with post hoc Bonferroni adjustment for multiple tests. Logistic regression was used to determine the impact of a composite score comprised of BMI percent and ALT (model A) or BMI percent, ALT and GRS (model B) on the prediction of ELF, controlling for age and gender. Resultant classification tables were used to calculate model performance. Receiver Operating Characteristic (ROC) curves were constructed for the models that included BMI and ALT/AST as conventional predictors with and without the GRS. The state variable for the test was defined by ELF status (liver fat ≥ 5.5%). The resultant area under the curve (AUC) for each ROC was obtained and pair wise AUC comparisons were conducted. Data analysis was carried out using SAS software (version 9.2 of the SAS System, Cary, NC. USA) and SPSS software (version 18, SPSS Inc., Chicago, IL. USA).
RESULTS
Clinical Characteristics of the Study Population
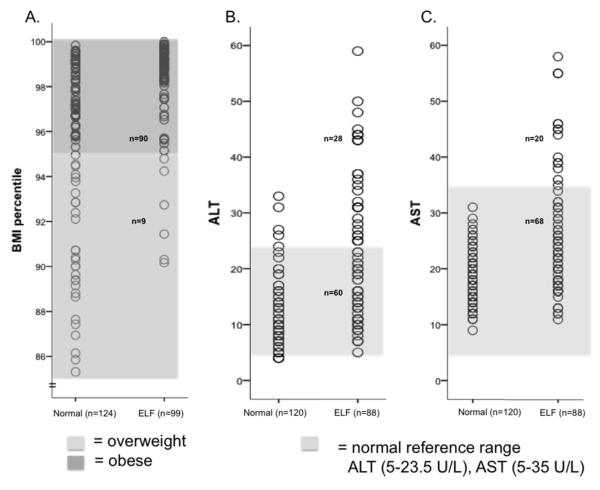
The clinical characteristics of the participants are shown in Table 1. Subjects with ELF, as defined by greater than 5.5% liver fat, were more likely to be male and had nearly four-fold higher liver fat content compared to normal liver fat participants (15.2 ±9.4 vs.3.7±1.1, p=5.4×10−32). Similarly, measures of adiposity were elevated in ELF subjects, most notably BMI percentile (97.9±3.6 vs. 92.3±10.8, p=1.5×10−6) (Table 1). The association between BMI percentile and ELF is further illustrated in Figure 1A where 90% of subjects with liver fat over 5.5% met the criteria for obesity, as defined by BMI ≥95th percentile, compared to 64% of the normal liver fat content participants. Subjects with ELF also had significantly higher serum ALT and AST levels than normal liver fat content subjects (ALT: 20.7±12.3 vs. 10.8±5.4 IU/L, p=2.2×10−13; AST: 24.8±10.3 vs. 17.9±4.4 IU/L, p=4.4×10−10) (Table 1). However, 69% and 77% of ELF subjects had ALT and AST levels, respectively, that were within the accepted normal reference ranges for these analytes (Figure 1B and C). When standard ALT cutoffs (5-35 U/L) for ULN were utilized, 84% of ELF subjects fell within the normal reference range. Taken together, these data indicate that while BMI percentile-defined obesity accurately reflects the presence of ELF, a cutoff using the clinical definition of elevated liver enzymes does not provide similar discrimination.
Table 1.
The Clinical Characteristics of the Study Population
| Trait | All Participants (n=223) |
Normal Liver fat (n=126) |
ELF (n=97) |
p-value |
|---|---|---|---|---|
| Age (year) | 13.5 ± 2.9 | 13.5 ± 3.1 | 13.5 ± 2.8 | NS |
| Male/Female (n) | 93/130 | 43/83 | 50/47 | 0.005 |
| Height (cm) | 157.3 ± 16.6 | 157.4 ± 18.2 | 157.2 ± 11.6 | NS |
| Weight (kg) | 77.9 ± 28.2 | 72.1 ± 27.4 | 85.4 ± 27.6 | <0.001 |
| BMI (kg/m2) | 30.5 ± 7.6 | 28.2 ± 7.1 | 33.4 ± 7.4 | <0.001 |
| BMI percentile | 94.5 ± 10.3 | 92.3 ± 10.8 | 97.9 ± 3.6 | <0.001 |
| SAT (L) | 12.1 ± 7.1 | 10.4 ± 6.6 | 14.2 ± 7.2 | <0.001 |
| VAT (L) | 1.8 ± 1.3 | 1.4 ± 0.9 | 2.2 ± 1.4 | <0.001 |
| Total Fat (kg) | 29.1 ± 12.1 | 26.1 ± 11.9 | 32.9 ± 11.5 | <0.001 |
| Liver fat (%) | 8.8 ± 8.5 | 3.7 ± 1.1 | 15.2 ± 9.4 | <0.001 |
| ALT (IU/L)‡ | 14.9 ± 10.2 | 10.8 ± 5.4 | 20.7 ± 12.3 | <0.001 |
| AST (IU/L)‡ | 20.8 ± 8.2 | 17.9 ± 4.4 | 24.8 ± 10.3 | <0.001 |
| TAG (mg/dL)† | 107.6 ± 52.7 | 96.6 ± 45.5 | 121.8 ± 58.1 | 0.001 |
| Total Cholesterol (mg/dL)† | 140.9 ± 29.6 | 138.4 ± 28.6 | 143.9 ± 30.7 | NS |
| HDL (mg/dL)† | 37.7 ± 9.4 | 39.0 ± 10.1 | 35.9 ± 8.3 | 0.024 |
| LDL (mg/dL)† | 85.2 ± 28.4 | 84.4 ± 28.8 | 86.2 ± 28.1 | NS |
Data are shown as mean ± SD. ELF was defined as liver fat content greater than 5.5% by MRI. NS=not significant. p-values are given for comparison between ELF and normal liver fat content groups and were obtained via independent t-tests. SAT = subcutaneous adipose tissue, VAT = visceral adipose tissue. ELF = elevated liver fat content, TAG = triacylglycerol.
n=208,
n=183.
Fig. 1. Distribution of BMI percentile, ALT and AST by liver fat content.
90% of subjects with ELF had a BMI ≥95th percentile (dark shaded box), compared to 64% of participants with normal liver fat content (A). 69% and 77% of ELF subjects had ALT (B) and AST (C) levels, respectively, that were within the normal reference ranges (ALT = 5-23.5 U/L, AST = 5-35 U/L) indicated by the light shaded box. Normal = normal liver fat content, Yes= ELF. ELF = elevated liver fat content defined as ≥ 5.5%.
Genetic Effects on Liver Fat Content
To explore the genetic determinants of liver fat content in this pediatric population, we genotyped six previously reported variants in PNPLA3, PPP1R3B, NCAN, GCKR, LYPLAL1, and APOC3. All SNPs were in HWE and, with the exception of PNPLA3, LYPLAL1, and PPP1R3B, the effect allele frequencies (EAF) of the SNPs in APOC3, GCKR, and NCAN in this Hispanic cohort were similar to those previously reported in Caucasians (Table 2).
Table 2.
Individual Effects of SNPs on Liver Fat Content.
| Effect Allele Copy Number | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gene | SNP | Allelesa | EAFb | Reported EAFc |
0 | 1 | 2 | p-valued |
| PNPLA3 | rs738409 | G/C | 0.52 | 0.239 | 5.6±4.3 (n=53) |
8.0±8.3 (n=109) |
12.3±9.8 (n=60) |
4.2×10−6 |
| APOC3 | rs2854117 | A/G | 0.35 | 0.302 | 7.1±7.6 (n=91) |
9.4±8.4 (n=103) |
11.3±10.5 (n=26) |
0.01 |
| GCKR | rs780094 | A/G | 0.32 | 0.391 | 8.6±9.0 (n=101) |
8.5±7.4 (n=102) |
10.7±9.9 (n=22) |
0.44 |
| NCAN | rs2228603 | T/C | 0.03 | 0.09 | 8.7±8.5 (n=212) |
9.2±7.8 (n=11) |
NA | 0.96 |
| LYPLAL1 | rs12137855 | C/T | 0.92 | 0.79 | 9.7±0 (n=1) |
7.8±8.4 (n=36) |
8.9±8.5 (n=184) |
0.50 |
| PPP1R3B | rs4240624 | A/G | 0.67 | 0.92 | 8.7±7.9 (n=101) |
8.8±9.6 (n=88) |
8.5±7.8 (n=28) |
0.38 |
Data are shown as mean liver fat content (%) ± SD as a function of carrying 0, 1, or 2 copies of the effect alleles for selected SNPs.
Effect/Other allele.
EAF, effect allele frequency in Hispanics.
Reported effect allele frequency in Caucasians (HapMap-CEU).
p-values are obtained from multiple linear regression using natural log-transformed values, adjusted for age, sex and VAT.
Of the variants tested, those in PNPLA3 and APOC3 demonstrated significant dose-dependent associations with liver fat content even after adjustment for age, sex, and VAT (Table 2). For example, each copy of the risk allele for PNPLA3 rs738409 and APOC3 rs2854117 increased liver fat by ~40-50% (ptrend=4.2×10−6) and 20-30% (ptrend=0.01), respectively. In addition, the association of APOC3 with liver fat remained significant (p=0.05) after conditioning on the effect of PNPLA3, but there was no was no evidence for an interaction between the two genes (pinteraction=0.40). However, it should be noted that p-value we obtained for the association of APOC3 with liver fat is slightly above the threshold for significance based on a Bonferroni correction for multiple tests (0.05/6=0.008).
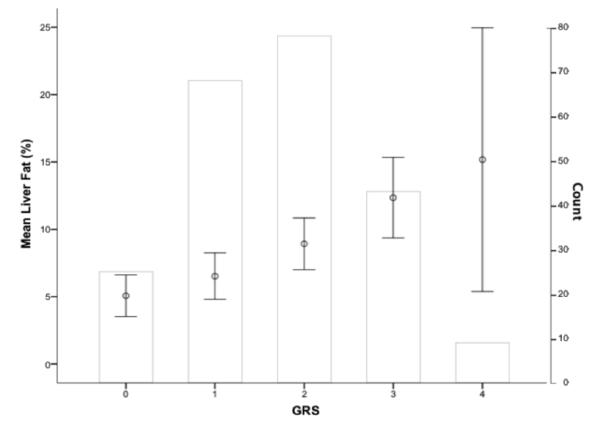
We next evaluated the combined effects of the PNPLA3 and APOC3 SNPs on liver fat content. For each subject, an unweighted GRS was calculated for rs738409 and rs2854117. As shown in Figure 2, the GRS was normally distributed in this study population and there was a significant stepwise increase in liver fat content as a function of the number of risk alleles carried (ptrend=3.12×10−5). Participants with 4 risk alleles had 3-fold higher liver fat than those with a GRS of 0 (15.1±12.7% vs. 5.1±3.7%, p=0.03). These associations remained significant when controlling for age, sex, and VAT. An analysis using the wGRS yielded similar results (ptrend=1.3×10−4; data not shown). We also constructed a comprehensive GRS with all 6 SNPs. In this analysis, the maximum number of risk alleles carried by any one individual did not exceed 9 in our population, Using tertiles of risk alleles (T1=0-3, n=22; T2=4-6, n=143; and T3=7-9, n=44), a clear, stepwise increase in liver fat was observed by GRS tertile (Supplemental Figure 1), but the overall association did not reach significance (ptrend=0.08).
Fig. 2. Combined genetic effects on liver fat content.
GRS category (0-4) is shown along the x-axis and mean liver fat ± 95% CI (open circles with bars) is plotted on the left y-axis. The right y-axis denotes the number of subjects in each GRS category, represented by the non-shaded bars. The distribution of GRS in the population was normal. Mean liver fat significantly increased as a function of GRS (ptrend=3.12 × 10−5).
Discriminatory Analysis
We next determined whether cumulative genetic burden could improve upon the discriminatory ability of common clinical risk factors to detect ELF. Based on our observed clinical associations described above, and high correlation between ALT and AST levels (r=0.72, p<0.001), BMI percentile and ALT were chosen as representative conventional risk factors in these analyses. The model including GRS had higher discriminatory ability than BMI and ALT alone, (χ2=80.6, p<0.001 with df=2 vs. χ2=72.7, p<0.001 with df=1, respectively) and overall prediction success was marginally, but significantly, improved in the full model (0.76 vs. 0.75; p<0.01).
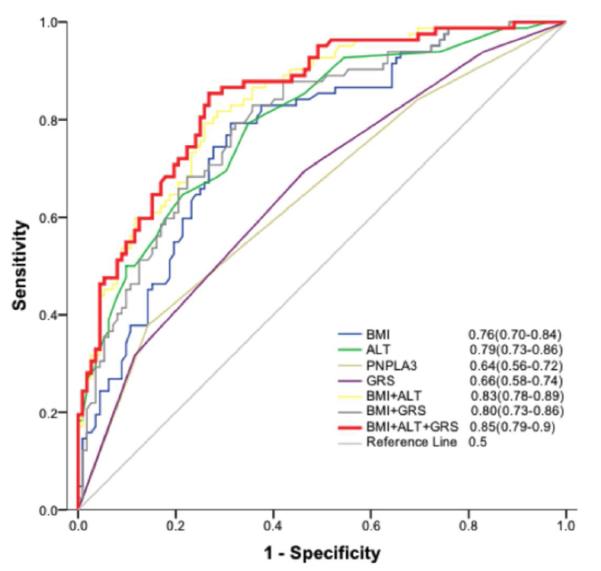
We next carried out ROC curves for the predictors independently and in combination (Figure 3). Compared to BMI percentile alone, the AUC for the model with BMI + ALT was significantly higher (0.77, 95% CI, 0.70-0.82 vs. 0.84, 95% CI, 0.78-0.89; p=0.03), indicating that ALT levels significantly improve discrimination of ELF. A model that included BMI percentile and the GRS also significantly improved discrimination (0.80 95% CI 0.73-0.86) compared to BMI percentile alone, although not to the same extent as the addition of ALT. PNPLA3 alone demonstrated a similar ability to discriminate ELF (0.64 95% CI, 0.56-0.72) compared to the GRS, however addition of APOC3 to the model did not improve AUC values over PNPLA3 alone. Finally, inclusion of all three risk factors (BMI percentile, ALT, and GRS) slightly increased the discriminatory power to detect ELF to 0.85 (95% CI, 0.79-0.89; p=0.01). The performance of the wGRS for ELF discriminatory ability was nearly identical to that of the GRS (data not shown). As shown in Table 3, the addition of GRS to BMI percentile and ALT improved sensitivity and specificity parameters. Taken together, these data indicate that the addition of GRS marginally, but significantly, improves overall ELF discrimination (15).
Fig. 3. Utility of a GRS to predict Elevated Liver Fat Content.
The ROC curves for the different models are shown where the state variable was defined by ELF. Test variables were BMI percentile, ALT, GRS and combinations thereof (listed next to line legend with AUC [CI]). BMI+ALT+GRS (solid red line) was a significantly better discriminator of ELF than BMI+ALT (AUC=0.85; 95% CI, 0.79-0.89 vs. AUC=0.84; 95% CI, 0.78-0.89, respectively [p=0.01]). The reference line (AUC=0.5) represents no predictive ability. ELF = elevated liver fat content ≥ 5.5%.
Table 3.
Performance Parameters of Clinical and Genetic Risk Factors on ELF Prediction.
| Parameter | GRS | ALT | BMI | BMI+ALT | BMI+GRS | BMI+ALT+GRS |
|---|---|---|---|---|---|---|
| Sens | 0.69 | 0.76 | 0.65 | 0.75 | 0.66 | 0.76 |
| Spec | 0.60 | 0.68 | 0.78 | 0.75 | 0.73 | 0.75 |
| Prev | 0.23 | 0.30 | 0.58 | 0.40 | 0.52 | 0.40 |
| PPV | 0.34 | 0.51 | 0.80 | 0.67 | 0.73 | 0.68 |
| NPV | 0.86 | 0.87 | 0.61 | 0.81 | 0.67 | 0.82 |
| DLR+ | 1.71 | 2.35 | 2.91 | 2.94 | 2.48 | 3.11 |
| DLR− | 0.52 | 0.36 | 0.46 | 0.34 | 0.46 | 0.31 |
The estimated sensitivity (Sens), specificity (Spec), prevalence (Prev), positive and negative predictive values (PPV and NPV, respectively) and diagnostic likelihood ratio (DLR+ and DLR−) values for the predicted probability of ELF are listed by different models (columns). ELF = elevated liver fat content, GRS = genetic risk score, ALT = alanine aminotransferase, BMI = body mass index.
To test the ability of ALT, BMI percentile and GRS to predict liver fat on a continuous scale, we conducted linear regression analyses. All three variables were significant, independent predictors of liver fat percent, with ALT explaining the most variance in liver fat (39%). A per-unit increase in ALT, BMI and GRS corresponded to a significant increase in liver fat of 0.39, 0.81 and 2.1%, respectively. In multiple regression analyses, ALT, BMI percentile and GRS were tested as predictors of liver fat percent in a stepwise model of regression. The full prediction model explained the most variance in liver fat percent (48%), with an overall model significance of p=0.001 (Table 4).
Table 4.
ALT, BMI Percentile and GRS as Predictors of Liver Fat Content.
| Model | Predictor(s) | r2 | SEE | β | p |
|---|---|---|---|---|---|
| 1 | ALT | 0.39 | 6.9 | 0.39 | <0.001 |
| 2 | GRS | 0.1 | 8.3 | 2.07 | <0.001 |
| 3 | BMI | 0.09 | 8.2 | 0.81 | <0.001 |
| 4 | ALT + GRS | 0.44 | 6.7 | - | <0.001 |
| 5 | ALT + GRS + BMI | 0.48 | 6.5 | - | 0.001 |
Output values from multiple regression analyses. ALT, GRS and BMI percentile were tested as predictors of liver fat content (expressed as a percentile) independently and in stepwise models of regression. All variables were tested for collinearity and outliers. The full prediction model explained the most variance in liver fat content (48%), with an overall model significance of p=0.001. GRS = genetic risk score, ALT = alanine aminotransferase, BMI = body mass index, SEE = standard error of the estimate, β = beta regression coefficient, p = model significance.
DISCUSSION
In the present study, we evaluated the association of commonly used clinical measures with ELF and examined the contribution of genetic variants to hepatic lipid content in a Hispanic pediatric population. We further assessed whether the combination of BMI, plasma liver enzyme levels, and cumulative genetic burden could serve as potential clinical tool for re-classification of ELF.
Our data show that almost 90% of children with MRI-defined ELF fell above the 95th percentile for BMI, illustrating the strong effect of obesity on hepatic fat content. However, 64% of the subjects with normal liver fat content were also above the 95th percentile for BMI, suggesting that this measure alone is not sufficient for indicating ELF. Similarly, ALT levels correlated significantly with liver fat, but 69% of ELF subjects fell within the recently established pediatric clinical reference range considered to be normal (5-23.5 IU/L). This high false negative rate could be problematic for diagnosing NAFLD in larger populations, as ALT is a commonly used primary indicator of potential NAFLD in children (16). It is established that the upper limits of normal levels for ALT vary between populations (17, 18) and abnormal ALT levels have been shown to occur in about 36% of obese Hispanics (19). In this regard, Ruhl et al. suggest that ALT upper limit ranges should be both ethnic specific and adjusted to reflect the growing prevalence of NAFLD in the adult population (20). Our data support this recommendation. Reducing the current normal reference range for ALT could also potentially decrease false negative diagnoses and improve the sensitivity of ALT as a predictor of ELF in obese Hispanic children.
Of the genes tested, only PNPLA3 and APOC3 demonstrated significant associations with liver fat content. Since being identified (8), PNPLA3 has been reproducibly associated with NAFLD and related phenotypes in numerous studies with both adults and children (5, 21-25), including one by our group where the effect of PNPLA3 was observed in Hispanic children as young as 8 years of age (5). Although the underlying molecular mechanism for how PNPLA3 promotes triglyceride accumulation in the liver is not entirely known, it is clear that PNPLA3 rs738409 represents the strongest validated genetic risk factor for NAFLD identified thus far. By comparison, we did not observe any evidence for association of GCKR, NCAN, PPP1R3B and LYPLAL1 with ELF in this study sample. Given that these genes were identified through a GWAS of over 7000 Caucasian adults (9), it is likely that our study was underpowered to detect their smaller effects on liver fat. To formally evaluate this possibility, we used effect sizes recently reported in Hispanic-American adults for 5 SNPs that were associated with liver fat content, as measured by computed tomographySupplemental Reference, to conduct a post hoc power analysis (Quanto V1.2.4). Assuming 80% power, an α-error of 0.05, and the allele frequencies observed in our Hispanic study population, we had sufficient power to detect a significant association with PNPLA3 rs738409, which is not surprising given the robust effect of this variant on liver fat observed in numerous studies. However, our study sample of 223 subjects did not provide sufficient power to detect associations with the GCKR, LYPLAL1, NCAN, and PPP1R3B variants (Supplemental Table 1). Interestingly, GCKR was associated with liver fat fraction in Chinese adults (26) as well as in a recent study of Caucasian, African American, and Hispanic children, in which an effect was observed in all three ethnicities (27). Thus, it remains to be determined whether the effect of this gene is stronger in adults than in children.
Our study also demonstrated an association of APOC3 with liver fat content. APOC3 is transported on circulating triglyceride-rich lipoproteins and inhibits both lipoprotein lipase and hepatic lipase, thus delaying the catabolism of triglyceride-rich particles (28,29). Interestingly, over-expression of APOC3 in mice leads to diet-induced ELF and hepatic insulin resistance (30). Petersen et al. identified two tightly linked promoter polymorphisms in APOC3 that were associated with NAFLD in a relatively small candidate gene study of Asian-Indian men (10). Of note, the association of APOC3 with liver fat content in our Hispanic pediatric population was observed even after controlling for the strong effect of PNPLA3. However, other studies in different ethnicities have failed to detect an association with APOC3 (26, 31-34), including two in children (26,34). Such discrepancies could be due to ethnic and age-specific genetic effects (35), differences in the study populations, and/or the methods used to quantitate liver fat. For example, to our knowledge there are no published studies reporting the effect of APOC3 on quantitative measures of liver fat content, thus precluding a more direct comparison with our results. Additional studies, particularly in larger samples sizes and multiple ethnicities, will be needed in order to clarify the genetic contribution of APOC3 to hepatic lipid content.
Using a GRS comprised of PNPLA3 and APOC3 susceptibility alleles, we showed that individuals carrying all 4 risk alleles have almost 3-fold higher hepatic fat content than non-variant carriers, illustrating the joint effects of PNPLA3 and APOC3 on liver fat content. Together, the additive effects of PNPLA3 rs738409 and APOC3 rs2854117 account for 12% of the variance in liver fat in our study population, although we did not obtain evidence for a genetic interaction between these variants. However, the GRS only resulted in a modest improvement in ELF discrimination beyond BMI percentile and ALT levels. A model containing ALT, BMI percentile and the GRS was able to explain 47% of the variability in liver fat, whereas BMI percentile and ALT alone explained 41%. Other studies have used similar approaches to predict NAFLD by using biomarkers and anthropometric measures, which have been extended to the prediction of NASH and fibrosis (36, 37).
Hispanic children are vulnerable to the current obesogenic environment and poised to benefit the most from early ELF detection. However, clinically useful predictors of ELF or NAFLD, other than liver enzymes and BMI, are lacking in this population. One study showed that ALT and BMI-z score were both independent predictors of NAFLD and together they accounted for most of NAFLD prediction in obese Italian children (38). However this study also noted that ALT alone should not be used as a surrogate marker of NAFLD in obese children (38), supporting existing literature in adults (39) as well as our current findings. Maffeis et al. were able to develop a highly discriminatory NAFLD predictive model in obese Italian children using waist-to-height ratio, ALT, adiponectin, and insulin resistance (40). While encouraging, this would require administration of oral glucose tolerance tests and additional biomarker assays.
Our approach with genetic factors, which in previous studies have been shown to explain up to 25% of the variability in hepatic fat content (27), was statistically significant but did not result in a substantial improvement in clinical utility over two common predictors of ELF. Additionally, PNPLA3 appears to be driving the utility of the GRS in this population, which is not surprising given such a strong association of the gene variant with both NAFLD and NASH in other studies (5, 21-25). Thus, it is not clear whether such an incremental increase in ELF discrimination merits the clinical implementation of a PNPLA3 and APOC3 GRS in the detection of ELF at the present time. Furthermore, the increased discrimination we observe is specific to Hispanic children and it remains to be determined whether a GRS similarly improves risk classification in adults and/or other ethnicities. We did not detect associations of liver fat content with the four other validated NAFLD variants in PPP1R3B, NCAN, GCKR, and LYPLAL1, presumably due to our relatively small sample size. Thus, it is possible that a more comprehensive GRS could still provide additional prognostic value for the early detection of ELF, which could be addressed in the future through prospective study designs with larger numbers of subjects.
While potentially relevant from a clinical perspective, our study has limitations, including defining ELF and/or NAFLD as liver fat content greater than 5.5%. This cut-point is generally accepted in the literature but is an arbitrary level based on previously published work. Although the use of MRI to detect percent liver fat fraction correlates highly with values derived through histology, liver biopsy remains the gold standard in steatosis quantification. However, we were not able to assess the association of a GRS with the level of histological damage (i.e. NASH) in this study, which is an important consideration since steatosis often does not progress to fibrotic conditions. Therefore, further studies in populations where biopsy samples are available will be necessary to assess the utility of a GRS in predicting the degree of liver inflammation and/or fibrosis in addition to liver fat percent. In this regard, the development of an accurate and inexpensive GRS test for predicting abnormal liver fat content may still represent an alternative to costly and invasive biopsies or imaging techniques.
In conclusion, we have shown that variants of PNPLA3 and APOC3 independently and in an additive manner contribute to elevated hepatic triglyceride content in Hispanic children. Although BMI percentile and ALT were useful predictors of ELF in this population, they lack sensitivity when used alone. Moreover, risk prediction models with BMI percentile that incorporated a GRS with PNPLA3 and APOC3, either alone or in combination with ALT, marginally improved upon the ability to discriminate ELF however did not improve detection of ELF from a clinical perspective. Larger study populations may allow a more comprehensive evaluation of the clinical utility of a GRS, which could lead to early detection and treatment of ELF or NAFLD in at-risk subjects.
Supplementary Material
Supplemental Figure 1. Combined genetic effects six SNPs on liver fat content. Comprehensive 6-SNP GRS tertile category (1-3) is shown along the x-axis and mean liver fat is plotted on the y-axis. The comprehensive GRS was based on tertiles of risk alleles (T1=0-3, n=22;T2= 4-6, n=143; and T3=7-9, n=44) and tested for an association with liver fat. Mean liver fat was not significantly increased as a function of comprehensive GRS (ptrend=0.08).
Supplemental Table 1. Power calculation for six SNPs previously associated with liver fat content.
What is already known about this subject?
-The prevalence of nonalcoholic fatty liver disease (NAFLD) continues to rise in both children and adults.
-Hispanics are disproportionately affected by NAFLD, due in part to high obesity rates in this population.
-Variants in the PNPLA3 gene (rs738409) explain some of the elevated risk in Hispanics, with effects seen in children as young as 8 years old.
What does this study add?
-Liver fat significantly increased as a function of PNPLA3 (rs738409) risk allele number and subjects with higher genetic risk score (GRS) had significantly higher liver fat.
-Current clinical reference ranges for ALT produce high false negative diagnoses of elevated liver fat (ELF) in Hispanic children.
-A GRS significantly added to the ability of common clinical risk factors to predict ELF, and potential NAFLD, in obese Hispanic children.
ACKNOWLEDGMENTS
The authors would like to thank the study participants and their families, the staff at The Childhood Obesity Research Center (CORC) at USC, Susanna Vikman for her assistance with genotyping and Mark K. Hechinger for his assistance with the ALT and AST assays.
FINANCIAL SUPPORT This study was supported by: a) National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institute of Health (NIH), through Grant Award Number TL1RR031992, b) Ruth L. Kirschstein National Research Service Award NIH grant number 2T32ES013678-06, c) National Institute of Diabetes and Digestive and Kidney Diseases (R01DK059211), d) Minority Health Research Center of Excellence (P60 MD002254), e) Dr. Robert C. and Veronica Atkins Foundation, f) USC MGM Mirage Fund. A portion of this work was conducted in a facility constructed with support from the National Institute of Health Research Facilities Improvement Program (RR10600-01, CA62528-01, RR14514-01) from the National Center for Research Resource
Footnotes
CONFLICTS Competing interests: the authors have no competing interests
Contributor Information
Ryan W. Walker, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Frank Sinatra, Pediatrics. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Jaana Hartiala, University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Marc Weigensberg, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Donna Spruijt-Metz, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Tanya L. Alderete, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Michael I. Goran, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA
Hooman Allayee, Preventive Medicine. University of Southern California, Keck School of Medicine. Los Angeles, 90089. USA.
REFERENCES
- 1.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28(1):13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 2.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43(4):413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. 2004;8:549–558. doi: 10.1016/j.cld.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Walker R, Le KA, Mahurkar S, Vikman S, Davis JN, et al. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes. 2010;59(12):3127–3130. doi: 10.2337/db10-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prati D, Colli A, Conte D, Colombo M. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42(3):650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 7.Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121(3):710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 8.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7(3):e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362(12):1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasson RE, Adam TC, Davis JN, Weigensberg MJ, Ventura EE, Lane CJ, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95(8):4048–4051. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 13.Henry RJ, Chiamori N, Golub OJ, Berkman S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:1357–1364. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sackett D. Evidence-Based Medicine: How to Practice and Teach EBM. 2nd ed Churchill Livingstone; 2000. [Google Scholar]
- 16.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC, Public Policy Committee of the American Association for the Study of Liver Disease Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 17.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328(7446):983–983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):561–565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 20.Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55(2):447–454. doi: 10.1002/hep.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52(6):1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- 22.Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, et al. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010;53(2):335–338. doi: 10.1016/j.jhep.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, NASH CRN The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52(3):894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50(10):2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52(4):1274–1280. doi: 10.1002/hep.23823. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Wen J, Tao X, Lu B, Du Y, Wang M, et al. Genetic variation in the GCKR gene is associated with non-alcoholic fatty liver disease in Chinese people. Mol Biol Rep. 2011;38(2):1145–1150. doi: 10.1007/s11033-010-0212-1. [DOI] [PubMed] [Google Scholar]
- 27.Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2011;55(3):781–9. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalto-Setälä K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, Zechner R, et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992;90(5):1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249(4970):790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- 30.Hyysalo J, Stojkovic I, Kotronen A, Hakkarainen A, Sevastianova K, Makkonen J, et al. Genetic variation in PNPLA3 but not APOC3 influences liver fat in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2012;27(5):951–6. doi: 10.1111/j.1440-1746.2011.07045.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee HY, Birkenfeld AL, Jornayvaz FR, Jurczak MJ, Kanda S, Popov V, et al. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54(5):1650–1660. doi: 10.1002/hep.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlitina J, Boerwinkle E, Cohen JC, Hobbs HH. Dissociation between APOC3 variants, hepatic triglyceride content and insulin resistance. Hepatology. 2011;53(2):467–474. doi: 10.1002/hep.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sentinelli F, Romeo S, Maglio C, Incani M, Burza MA, Scano F, et al. Lack of effect of apolipoprotein C3 polymorphisms on indices of liver steatosis, lipid profile and insulin resistance in obese Southern Europeans. Lipids Health Dis. 2011;10:93–93. doi: 10.1186/1476-511X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valenti L, Nobili V, Al-Serri A, Rametta R, Leathart JB, Zappa MA, et al. The APOC3 T-455C and C-482T promoter region polymorphisms are not associated with the severity of liver damage independently of PNPLA3 I148M genotype in patients with nonalcoholic fatty liver. J Hepatol. 2011;55(6):1409–1414. doi: 10.1016/j.jhep.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 35.Duseja A, Aggarwai R. APOC3 and PNPLA3 in non-alcoholic fatty liver disease: need to clear the air. J Gastroenterol Hepatol. 2012;27(5):848–51. doi: 10.1111/j.1440-1746.2012.07103.x. [DOI] [PubMed] [Google Scholar]
- 36.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 37.Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21(4):431–9. doi: 10.1007/s11695-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 38.Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61(7):877–83. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 39.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 40.Maffeis C, Banzato C, Rigotti F, Nobili V, Valandro S, Manfredi R, et al. Biochemical parameters and anthropometry predict NAFLD in obese children. JPGN. 2011;53(9):590–3. doi: 10.1097/MPG.0b013e31822960be. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Combined genetic effects six SNPs on liver fat content. Comprehensive 6-SNP GRS tertile category (1-3) is shown along the x-axis and mean liver fat is plotted on the y-axis. The comprehensive GRS was based on tertiles of risk alleles (T1=0-3, n=22;T2= 4-6, n=143; and T3=7-9, n=44) and tested for an association with liver fat. Mean liver fat was not significantly increased as a function of comprehensive GRS (ptrend=0.08).
Supplemental Table 1. Power calculation for six SNPs previously associated with liver fat content.