Abstract
Objective
To evaluate the structure-function relationship with RTVue spectral domain optical coherence tomograph-derived structural measurements, and to evaluate the relationship using a linear model.
Methods
In a cross-sectional study, structure-function relationships were determined for all the participants of Diagnostic Innovations in Glaucoma Study (DIGS) and African Descent and Glaucoma Evaluation Study (ADAGES) who had undergone standard automated perimetry (SAP) and RTVue testing within 6 months of each other. Strength of relationship was reported as coefficient of determination (R2). The relationship was also evaluated using a linear model described by Hood and Kardon.
Results
A total of 579 examinations from 80 eyes of 47 normal subjects, 199 eyes of 130 suspects and 213 eyes of 146 glaucoma patients with SAP and RTVue were analyzed. R2 for the association between SAP total deviation and RTVue parameters ranged from 0.01 (p=0.02) for the nasal rim area to 0.30 (p<0.001) for the inferior inner macular thickness. The linear model proposed by Hood and Kardon fitted our data well.
Conclusions
Strongest structure-function associations using RTVue were found for RNFL measurements at arcuate areas and inner macular thickness measurements. The linear model proposed by Hood and Kardon is useful to study the structure-function relationship in glaucoma.
INTRODUCTION
Evaluation of the relationship between anatomic structure (optic nerve and retinal nerve fiber layer) and function (visual sensitivity) in glaucoma can aid in assessing the relative efficacy of structural and functional tests in detecting glaucomatous damage as well as in their optimal use.1 Though a significant relationship is expected between structure and function as both provide related information, studies with the available measurement methods have shown that this relationship is modest at best.2–7 One of the reasons for this imperfect relationship may be the variability associated with measurements obtained with currently available methods.
The introduction of spectral domain optical coherence tomography (SDOCT) devices has enabled imaging of the anatomic structures at the posterior pole of the eye with better resolution and with a much faster scan rate compared to earlier versions of this technology.8–9 The greater scanning speed enables the acquisition of a larger number of scans in a single imaging session, thereby reducing the need for data interpolation. The RTVue (Optovue Inc, Fremont, CA) is one such SDOCT device with a scan rate of 26,000 A scans per second and an axial resolution of 5μm compared to its predecessor - the time domain OCT (Stratus OCT, Carl Zeiss Meditec, Dublin, CA), which has a scan rate of 400 scans per second and an axial resolution of 8–10μ. Variability of the macular10–11 and retinal nerve fiber layer (RNFL) thickness measurements12–16 with SDOCT has been shown to be less than that with Stratus OCT. Though it is expected that the structure and function relationship with SDOCT would be better than that with the previous methods used to evaluate the anatomic structure, a recent study by Leung et al.14 showed that the structure and function relationship with SDOCT was similar to that with Stratus OCT. However, this study only evaluated the global structural measure (average RNFL thickness measurement) with the global visual field index (mean deviation) without evaluating the sectors separately. As RNFL thickness varies around the disc and glaucoma preferentially affects the poles of the disc, comparing sectors of RNFL to corresponding sectors of visual field is likely to give better information.
Different investigators have used different methodologies to evaluate the structure-function relationship in glaucoma. Garway-Heath et al.2 found that the visual sensitivity expressed in linear scale defined the structure-function relationship better than visual sensitivities expressed as a decibel (dB) scale. Bowd et al.3 showed that a linear fit between structure and function with visual sensitivity expressed as dB scale was comparable to a logarithmic fit in describing the structure-function relationship. Hood and Kardon1 showed that a simple linear model considering function in terms of visual sensitivity loss (total deviation plot on standard automated perimetry) also can well describe the structure-function relationship. An advantage of this model is that it estimated the visual loss and structural measurement by differentiating between RNFL thickness and supportive tissue (described in detail later).
The purpose of our study was to determine the sectoral and global structure-function relationship between SDOCT-derived RNFL, ONH and macular measurements, and visual sensitivity loss on standard automated perimetry (SAP), and also to evaluate this relationship using the model proposed by Hood and Kardon.1
METHODS
This was an observational study of participants included in the Diagnostic Innovations in Glaucoma Study (DIGS) and African Descent and Glaucoma Evaluation Study (ADAGES), which are prospective longitudinal studies designed to evaluate optic nerve structure and visual function in glaucoma conducted at the Hamilton Glaucoma Center, University of California, San Diego. Participants in both studies include normal subjects, patients with glaucoma and glaucoma suspects, who are longitudinally evaluated clinically and with several functional and imaging tests. Informed consent was obtained from all participants and the University of California San Diego Human Subjects Committee approved all methodology. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects.
Inclusion criteria were a best corrected visual acuity of 20/40 or better, spherical refraction within ±5.0 D and cylinder correction within ±3.0 D and open angles on gonioscopy. Eyes with coexisting retinal disease, uveitis, or non-glaucomatous optic neuropathy were excluded. All the participants underwent a comprehensive ophthalmic examination including review of medical history, visual acuity testing, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometry, gonioscopy, dilated fundoscopic examination with a 78-D lens, stereoscopic optic disc photography, and standard automated perimetry (SAP) with 24-2 Swedish Interactive Threshold Algorithm (SITA; Carl Zeiss Meditec Inc. Dublin, CA).
We included three groups of participants; normal subjects, glaucoma suspects and glaucoma patients. Inclusion was based on eyes and when both eyes of participants satisfied the inclusion criteria, both were included. When an eye had more than one examination over the follow-up time, each examination was included separately. Appropriate statistical methods were used to deal with the correlation of measurements from multiple examinations from the same eye and from both eyes of the same individual (see below). Eyes were classified as glaucomatous if they had repeatable (at least two consecutive) abnormal SAP test results on the 24-2 program of the Humphrey Visual Field Analyzer (Carl Zeiss Meditec, Dublin, CA). An abnormal SAP result was defined as having a pattern standard deviation (PSD) outside the 95% confidence limits and/or a glaucoma hemifield test (GHT) result outside normal limits, regardless of the appearance of the optic disc. Glaucoma suspects were defined as eyes with abnormal-appearing optic discs (presence of neuroretinal rim thinning or localized or diffuse RNFL defects indicative of glaucoma, i.e., glaucomatous optic neuropathy) by masked stereophotograph assessment without repeatable abnormal SAP results. Glaucoma suspects also included eyes with IOP > 22 mm Hg, but with healthy-appearing optic discs and without repeatable abnormal SAP results. Normal subjects were recruited from the general population through advertisement, as well as from the staff and employees of the University of California, San Diego. Normal control eyes had IOP of 21 mm Hg or less with no history of increased IOP and a normal SAP result. A normal SAP result was defined as a mean deviation (MD) and PSD within the 95% confidence limits, and a GHT result within normal limits.
Instrumentation
RTVue
SDOCT examination was performed with the RTVue (software version 4.0.5.39). The principles and protocol used have been explained earlier.17 The protocols used for imaging with RTVue in this study were ONH (optic nerve head) and GCC (ganglion cell complex). All patients had both protocols performed on the same day.
Optic nerve head measurements
The ONH protocol was used to obtain ONH measurements. It consists of 12 radial scans 3.4 mm in length (452 A scans each) and 6 concentric ring scans ranging from 2.5 to 4.0 mm in diameter (587 to 775 A scans each) all centered on the optic disc. ONH parameters considered in this study for the structure-function relationship analysis were the temporal (316° -45°), superior (46° –135°), nasal (136° –225°), inferior (226° –315°) and total neuroretinal rim area.
Peripapillary retinal nerve fiber layer measurements
The ONH protocol also generates a polar RNFL thickness map which is the RNFL thickness measured along a circle 3.45 mm in diameter centered on the optic disc. It gives the average RNFL thickness in the temporal (316° -45°), superior (46° –135°), nasal (136° –225°), inferior (226° –315°) quadrant as well as the overall average along the entire measurement circle. In addition, each quadrant is divided into 4 sectors and the software provides the RNFL thicknesses in each of these 16 sectors. For the study, we divided the superior and inferior quadrants into superotemporal (46° –90°), superonasal (91° –135°), inferotemporal (271° –315°) and inferonasal (226° –270°) quadrants.
Macular measurements
The ganglion cell complex (GCC) protocol consists of one horizontal line scan 7 mm in length (467 A scans) followed by 15 vertical line scans 7 mm in length (each 400 A scans) and at 0.5 mm intervals centered 1 mm temporal to the fovea. GCC protocol is designed to measure the inner retinal thickness which includes the nerve fiber layer, ganglion cell layer and the inner plexiform layer, collectively called the GCC. The parameters generated by the GCC analysis and considered for analysis in this study were the average inner retinal thickness, superior inner retinal thickness (0°–180°) and inferior inner retinal thickness (181° –360°).
In addition to the inner retinal thickness parameters, GCC protocol also measures the full retinal thickness at the macula. The full retinal thickness parameters obtained with GCC protocol were the full retina thickness average, superior and inferior retinal thickness average.
Standard Automated Perimetry
Visual field data was divided into sectors based on the map proposed by Garway-Heath et al (figure 1).2 This map relates sectors of ONH with their corresponding visual field sectors. For example, the inferotemporal optic disc sector relates to the superonasal visual field sector and the superotemporal optic disc sector relates to the inferonasal visual field sector. The same relationship as with ONH sectors was used for the RNFL sectors. The square in the center of the visual field in figure 1 shows the area that relates to the macular region. SAP-measured visual sensitivity loss was calculated by first converting the dB scale values at each test location on the total deviation numerical plot to a linear scale (reciprocal of Lambert scale) using the following formula.
Figure 1.
The relationship between visual field sectors and optic disc regions/retinal nerve fiber layer quadrants based on the map by Garway-Heath et al.2 The square centered on the fovea shows the area used to assess the relationship between central visual field sectors and macular regions.
Then values from all test points within the visual field sectors corresponding to the anatomic sectors described above with each scanning protocol were averaged. The average visual sensitivity loss per sector was used as such in the linear scale and also converted back to the dB scale for the analysis.
Only good quality RTVue images, as defined by a signal strength index (SSI) of ≥30 were used for analysis. Only images and visual field testing pairs acquired within 6 months of each other were included for the analysis.
STATISTICAL ANALYSIS
Descriptive statistics included mean and standard deviation for normally distributed variables and median, first quartile, and third quartile values for non-normally distributed variables. Analysis of variance or Kruskal-Wallis tests were used to evaluate visual field parameter and clinical differences between normal, suspect and glaucoma subjects. As measurements from both eyes from a subject, and from different examinations of a subject are correlated, a nested model was used for comparison between the groups. Eye and visit were nested within the subject and subject as a variable was nested within the group.
Structure–function associations were investigated by using linear (y= ax + b) regression between rim area, RNFL thickness and macular thickness, and visual sensitivity loss expressed both in linear and dB scale. The results are reported as R2. Locally weighted scatterplot smoothing (lowess) curves were also used to fit the relationship graphically. Lowess is a modelling method which combines the linear least square regression with the non linear regression.18 It does this by fitting simple models to localized subsets of the data to build up a function that describes the deterministic part of the variation in the data, point by point. Lowess curve has the advantage in describing the structure-function relationship because it does not need the specification of a function (for example, linear, quadratic, etc) to fit a model to all of the data in a given sample.
Details of the model proposed by Hood and Kardon are explained elsewhere.1 This model makes some basic assumptions to evaluate the structure-function relationship. It proposes that the RNFL thickness, R, measured with OCT is made up of two components, thickness due to retinal ganglion cell axons, called signal or so and the residual thickness due to glial cells and blood vessels called base level or b so that the measured RNFL thickness is given by the equation,
It also proposes that visual sensitivity decreases, as the signal so decreases, but the residual b does not change. So the above equation is written as
where D is the loss of visual sensitivity on dB scale, represented on the total deviation numeric map. Base level or b is taken as the RNFL thickness corresponding to a decrease in the visual sensitivity of more than 10 dB (compared to the age matched normal) on total deviation numeric plot.
Converting the visual sensitivity loss to linear scale, the above equation can be represented as
where T is the visual sensitivity loss in a linear scale.
Statistical analyses were performed using commercial software (Stata ver. 10.0; StataCorp, College Station, TX). A p value of ≤ 0.05 was considered statically significant.
RESULTS
Functional and structural measurements among the healthy, suspect and glaucoma cohorts are shown in Table 1. Age and visual field parameters were significantly different between the glaucoma and normal group, and between the glaucoma and the suspect group, but were not significantly different between the normal and the suspect group. Temporal and nasal ONH rim area were significantly different between the normal and the suspect, and normal and the glaucoma groups, but were not significantly different between the suspect and the glaucoma groups. All the other structural parameters were significantly different between the normal, suspect and the glaucoma groups.
Table 1.
Structural and functional characteristics of the participants.
| Normal (84 examinations from 80 eyes of 47 participants) | Glaucoma suspect (235 examinations from 199 eyes of 130 participants) | Glaucoma (260 examinations from 213 eyes of 146 participants) | p value | |
|---|---|---|---|---|
| Age (years) | 63 (54.5, 67) | 66 (55, 72) | 72 (62, 77) | <0.001 |
| Race (African American) | 5 (11%) | 22 (17%) | 43 (29%) | 0.001 |
| Visual field parameters | ||||
| Mean deviation (dB) | 0.15 (−0.55, 0.76) | −0.17 (−1.04, 0.53) | −2.76 (−5.61, −1.43) | <0.001 |
| Pattern standard deviation (dB) | 1.49 (1.34, 1.67) | 1.55 (1.35, 1.74) | 3.07 (2.15, 6.67) | <0.001 |
| Visual field index (%) | 100 (99, 100) | 99 (99, 100) | 95 (87, 98) | <0.001 |
| Optic nerve head parameter (mm2) | ||||
| Total rim area | 1.20 (1.03, 1.52) | 0.93 (0.73, 1.23) | 0.76 (0.48, 1.16) | <0.001 |
| Temporal rim area | 0.17 (0.10, 0.27) | 0.09 (0.04, 0.17) | 0.07 (0.02, 0.17) | <0.001 |
| Superior rim area | 0.34 (0.28, 0.41) | 0.29 (0.22, 0.37) | 0.23 (0.14, 0.35) | <0.001 |
| Nasal rim area | 0.35 (0.3, 0.41) | 0.31 (0.22, 0.38) | 0.27 (0.15, 0.38) | <0.001 |
| Inferior rim area | 0.35 (0.3, 0.43) | 0.24 (0.17, 0.34) | 0.17 (0.09, 0.30) | <0.001 |
| RNFL parameter (μm) | ||||
| Temporal RNFL | 78.47 (66.22, 86.55) | 70 (64.02, 78.37) | 64.45 (56.03, 73.65) | <0.001 |
| Superotemporal | 132.63 (119.53, 146.46) | 121.12 (106.54, 134.34) | 100.96 (88.34, 117.56) | <0.001 |
| Superonasal | 114.58 (100.11, 128.97) | 107.02 (94.26, 120.02) | 92.07 (82.97, 108.67) | <0.001 |
| Nasal | 77.78 (68.62, 87.56) | 71.95 (66.21, 81) | 64.44 (58.21, 75.69) | <0.001 |
| Inferonasal | 125.01 (111.83, 137.06) | 113.63 (101.87, 127.84) | 99.66 (89.23, 116.43) | <0.001 |
| Inferotemporal | 137.79 (126.98, 155.21) | 130.09 (117.27, 143.58) | 100.62 (85.74, 124.68) | <0.001 |
| Average | 102.75 (95.12, 111.02) | 96.09 (88.1, 102.65) | 81.45 (74.40, 93.56) | <0.001 |
| Inner retina parameter (μm) | ||||
| Average | 94.84 (89, 99.87) | 89.82 (84.34, 94.49) | 82.74 (75.52, 89.85) | <0.001 |
| Superior inner | 95.28 (87.88, 100.82) | 89.66 (83.44, 95.24) | 83.93 (77.34, 91.67) | <0.001 |
| Inferior inner | 94.69 (89.99, 100.13) | 90.01 (84.06, 94.41) | 81.23 (72.81, 89.67) | <0.001 |
| Full retina thickness (μm) | ||||
| Average | 267.84 (252.86, 279.21) | 259.55 (251.75, 268.45) | 252.95 (242.34, 263.84) | <0.001 |
| Superior full | 268.67 (252.96, 282.13) | 261.02 (251.94, 270.07) | 255.94 (245.18, 266.96) | <0.001 |
| Inferior full | 266.37 (252.2, 276.16) | 257.11 (250.79, 267.42) | 250.51 (239.10, 261.64) | <0.001 |
Table 2 shows the structure-function associations between ONH rim area (expressed in linear scale) and visual sensitivity loss expressed in dB and linear scales in the corresponding visual field sectors. Table 3 shows the structure-function associations between RNFL thickness (expressed in linear scale) and visual sensitivity loss expressed in dB and linear scales in the corresponding visual field sectors. Table 4 shows the structure-function associations between inner macular and full macular thickness (expressed in linear scale) and visual sensitivity loss expressed in dB and linear scales in the corresponding visual field sectors. The strongest associations were found between RNFL thickness at the arcuate regions and visual field total deviation in their corresponding sectors, and the inner retinal thickness at the macula and visual field total deviation in the corresponding sector. Associations between visual field total deviation and optic nerve head measurements were weakest. Associations were similar with visual sensitivity loss considered both on decibel and linear scale.
Table 2.
Structure-function associations with optic nerve head rim area.
| Sector | Loss of visual sensitivity in corresponding sector (linear scale) | Loss of visual sensitivity in corresponding sector (decibel scale) | ||
|---|---|---|---|---|
|
| ||||
| R2 | P | R2 | P | |
|
| ||||
| Temporal Rim area | 0.02 | 0.006 | 0.02 | <0.001 |
| Superior Rim area | 0.06 | <0.001 | 0.07 | <0.001 |
| Nasal Rim area | 0.01 | 0.02 | 0.02 | 0.02 |
| Inferior Rim area | 0.08 | <0.001 | 0.08 | <0.001 |
| Total Rim area | 0.05 | 0.001 | 0.07 | <0.001 |
Table 3.
Structure-function associations with retinal nerve fiber layer thickness measurements (R2 and p values).
| Sector | Loss of visual sensitivity in corresponding sector (linear scale) | Loss of visual sensitivity in corresponding sector (decibel scale) | ||
|---|---|---|---|---|
|
| ||||
| R2 | P | R2 | P | |
|
| ||||
| Temporal | 0.10 | <0.001 | 0.12 | <0.001 |
| Supero-temporal | 0.22 | <0.001 | 0.19 | <0.001 |
| Supero-nasal | 0.09 | <0.001 | 0.09 | <0.001 |
| Nasal | 0.06 | <0.001 | 0.08 | <0.001 |
| Infero-nasal | 0.08 | <0.001 | 0.08 | <0.001 |
| Infero-temporal | 0.26 | <0.001 | 0.22 | <0.001 |
| Average RNFL | 0.17 | <0.001 | 0.19 | <0.001 |
Table 4.
Structure-function associations with inner retinal and full macular thickness measurements (R2 and p values).
| Sector | Loss of visual sensitivity in corresponding sector (linear scale) | Loss of visual sensitivity in corresponding sector (decibel scale) | ||
|---|---|---|---|---|
|
| ||||
| R2 | P | R2 | P | |
|
| ||||
| Inner retinal | ||||
| Superior | 0.19 | <0.001 | 0.22 | <0.001 |
| Inferior | 0.30 | <0.001 | 0.27 | <0.001 |
| Average | 0.25 | <0.001 | 0.28 | <0.001 |
| Full macula | ||||
| Superior | 0.09 | <0.001 | 0.10 | <0.001 |
| Inferior | 0.14 | <0.001 | 0.13 | <0.001 |
| Average | 0.12 | <0.001 | 0.13 | <0.001 |
The strength of the association varied by diagnostic group. The association between structure and function in normal subjects in our study was not statistically significant for any structural parameter, with R2 values between 0.00 (for all ONH and most of macular inner retinal thickness measurements) and 0.02 (for average RNFL thickness measurement). The R2 in glaucoma suspects ranged from 0.00 (p=0.99, for nasal rim area) to 0.03 (p=0.04, for macular full thickness superior average) on the dB scale and 0.00 (p=0.91, for nasal rim area) to 0.03 (p=0.03, for macular full thickness superior average) on the linear scale. The R2 in glaucoma patients ranged from 0.01 (p=0.29, for nasal rim area) to 0.29 (p<0.001, for macular inner retinal average) on the dB scale and 0.00(p=0.49, for nasal rim area) to 0.32 (p<0.001, for macular inner retinal inferior average) on the linear scale.
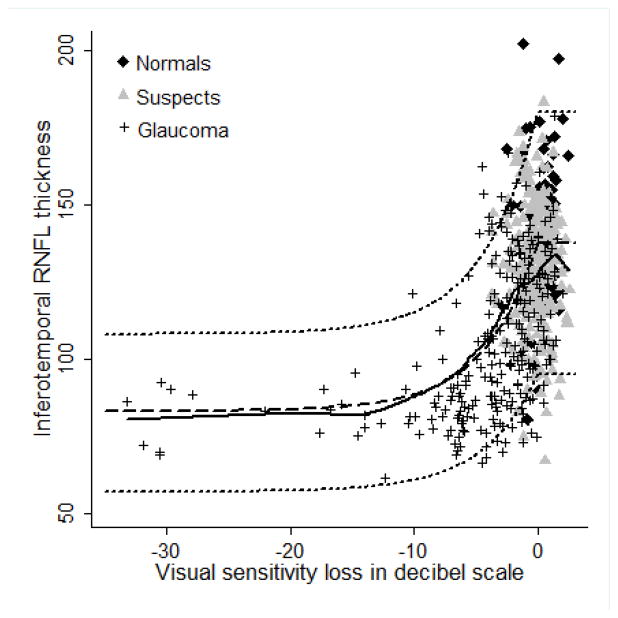
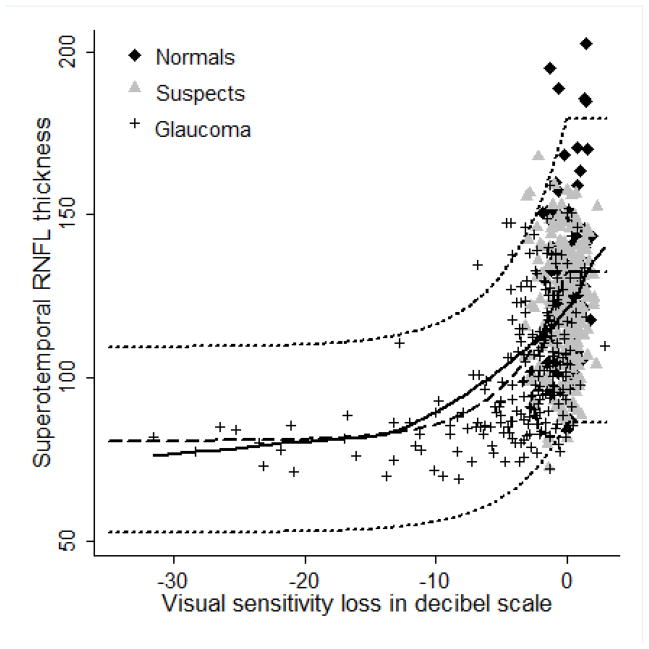
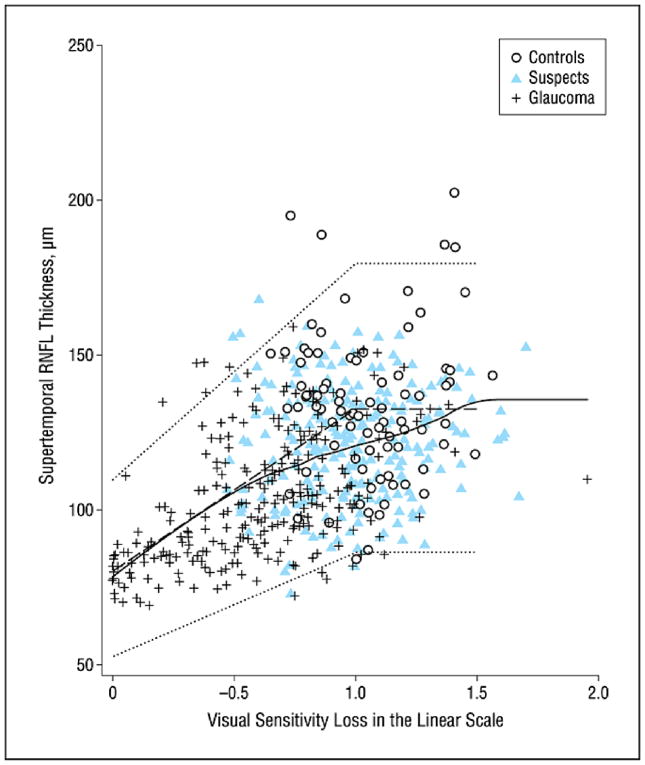
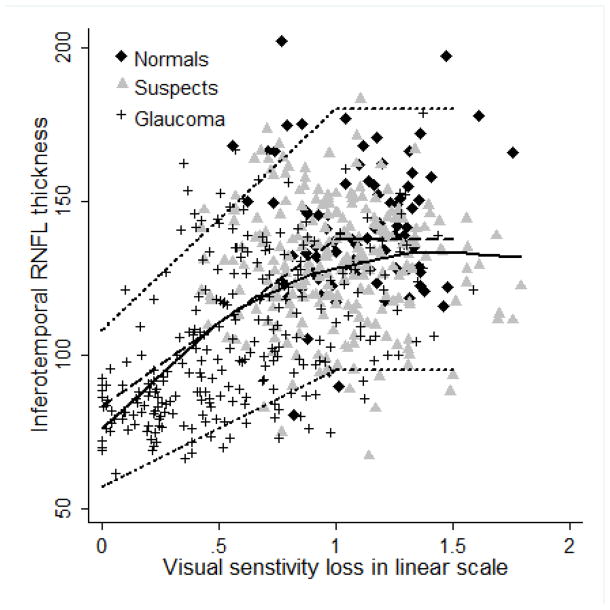
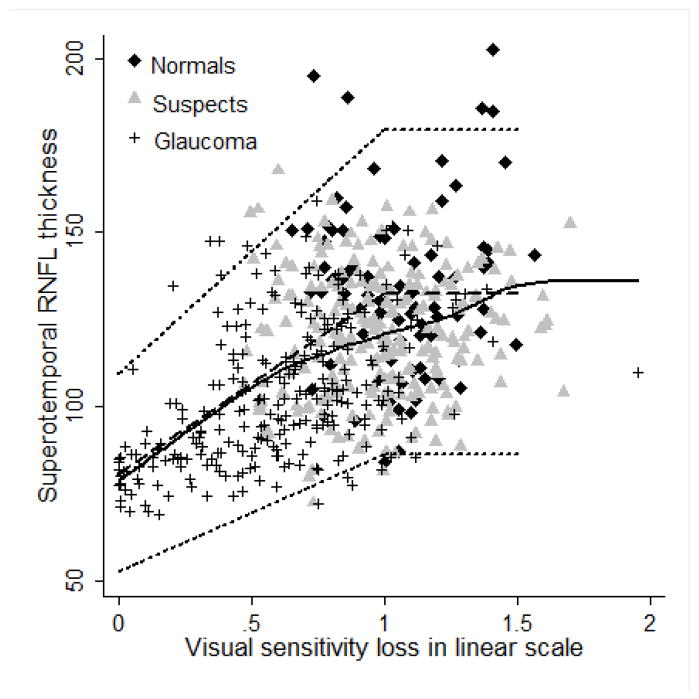
Figures 2–5 show the model proposed by Hood and Kardon fit to the inferotemporal and superotemporal RNFL sectors in our data. The base level b was calculated as the RNFL thickness corresponding to a mean loss of sensitivity of 10 dB or lower in the inferotemporal (19 eyes) and superotemporal RNFL (19 eyes) sectors. For the inferotemporal RNFL sector, mean value of b was 83.26 μm and for the superotemporal RNFL sector, it was 80.74 μm. The three theoretical curves (dotted lines) in the figures, representing the mean and the 95% prediction interval were plotted according to the method proposed by Hood and Kardon.1 The b in the inferotemporal quadrant was 60% of the mean inferotemporal RNFL thickness in normal subjects (137.79 μm) and b in the superotemporal quadrant was 61% of the mean superotemporal RNFL thickness in normal subjects (132.63 μm). The central dashed lines in the figures were derived by joining the mean RNFL thickness in the inferotemporal and superotemporal sectors of normal subjects with 60% and 61% of this thickness respectively. The short dashed lines above and below the dashed line were derived by connecting the value 2 standard deviations (SD) above and below the mean RNFL thickness in the inferotemporal and superotemporal sectors of normal subjects with 60% and 61% of these values (2 SD above and below mean) respectively. Figures 2 and 3 show the model with the visual sensitivity loss in dB units and figures 4 and 5 show the visual sensitivity loss in linear scale. To check the goodness of fit, we calculated the number of points falling outside the short dashed lines. For the inferotemporal RNFL sector-superonasal visual field association (dB scale), 24 glaucoma, 13 suspect and 5 normal eyes (total of 7.3%) fell outside the short dashed lines. For the superotemporal RNFL sector-inferonasal visual field association (dB scale), 10 glaucoma, 3 suspect and 5 normal eyes (3.1%) fell outside.
Figure 2.
Linear model showing the relationship between inferotemporal retinal nerve fiber layer (RNFL) thickness and superonasal sector visual sensitivity loss. Solid black line represents the lowess curve fitting our data. Dashed line and the two short dashed lines above and below the dashed line represent the 50th, 95th and 5th percentile of the model.
Figure 5.
Linear model showing the relationship between superotemporal retinal nerve fiber layer (RNFL) thickness and inferonasal sector visual sensitivity loss. Solid black line represents the lowess curve fitting our data. Dashed line and the two short dashed lines above and below the dashed line represent the 50th, 95th and 5th percentile of the model.
Figure 3.
Linear model showing the relationship between superotemporal retinal nerve fiber layer (RNFL) thickness and inferonasal sector visual sensitivity loss. Solid black line represents the lowess curve fitting our data. Dashed line and the two short dashed lines above and below the dashed line represent the 50th, 95th and 5th percentile of the model.
Figure 4.
Linear model showing the relationship between inferotemporal retinal nerve fiber layer (RNFL) thickness and superonasal sector visual sensitivity loss. Solid black line represents the lowess curve fitting our data. Dashed line and the two short dashed lines above and below the dashed line represent the 50th, 95th and 5th percentile of the model.
DISCUSSION
We evaluated the structure-function relationship in glaucoma using the SDOCT-derived ONH, RNFL and the macular thickness parameters, and found that the strongest associations were for the RNFL measurements at the arcuate areas and the inner retinal thickness measurements at the macula. To the best of our knowledge this is the first study to evaluate the structure-function relationship to the RNFL, macula and ONH measurements of SDOCT.
The RNFL sectors with the strongest association with visual sensitivity loss in our study were the inferotemporal, superotemporal and average measurements. This is similar to the results reported by Horn et al.,19 who evaluated the structure-function relationship using SDOCT derived RNFL thickness measurements. This is also similar to the results reported by previous studies using Stratus OCT.3–6 The reported R2 in different studies have been variable, ranging from 0.33 in the study by Bowd et al.3 to 0.56 in the study by Miglior et al.5 Differences in the R2 between studies are likely to occur because of differences in the populations and severity of glaucoma of included patients. It has been shown that the strength of association between structure and function is weak in normal subjects, suspect and early glaucoma because the range of visual sensitivity loss is too narrow in these populations.20 The glaucoma cohort in our study had milder visual field loss (median MD of −2.52dB) compared to the study by Miglior et al. (median MD of −7.8dB).5
The structure-function associations for the inner retinal thickness parameters were similar to those found with the inferotemporal and superotemporal RNFL sectors. This could be related to the improvement in software analysis of SDOCT compared to time-domain OCT macular thickness data which now concentrates on the inner retinal layers instead of all the retinal layers at the macula. Such improvement has been made possible by the higher resolution of SDOCT compared to time-domain OCT, enabling better identification of the different retinal layers.
Association between ONH sectors and VF loss was weak. There are no reports on the structure-function relationship using the SDOCT derived ONH parameters. Previous studies have used HRT derived ONH sectors to determine this relationship.2–3, 7 Garway-Heath et al.2 reported a R2 of 0.38 between the temporal neuroretinal rim area and central visual field mean visual sensitivity in dB scale on quadratic regression and a R2 of 0.30 on linear regression using reciprocal of Lambert scale. Bowd et al.3 reported a R2 of 0.16 between inferotemporal ONH rim area and superonasal visual field sector sensitivity on linear regression. One of the possible reasons for a weak structure-function association may be a greater variability in the rim area measurement compared to the RNFL measurement as measured by SDOCT.21–22 In addition, this may also be because of the weaker performance of the RTVue software for topographic assessment of the ONH compared to its macular and RNFL thickness evaluation algorithms.
We used the visual sensitivity loss as determined on the total deviation numeric plot as the functional measure, as described by Hood and Kardon,1 and not the visual threshold as has been used in most of the other studies. Total deviation numeric plot adjusts the visual sensitivity loss according to the age of the subject. In this way the age related variability in the functional measurement is minimized. For the structural measurements, though there are no age corrected values, the change with age has been reported to be small.23–25 We used lowess curves to estimate the structure-function relationship. The advantage of the lowess curve is that it does not need the specification of a function to model the relationship in a given sample. The shape of the lowess curves shows the lag between structural and functional components in glaucoma. In early stages of glaucoma, the decline in RNFL thickness is rapid and there is a lag in the visual sensitivity loss. But as the glaucoma damage becomes severe, RNFL thickness reaches a base level beyond which only the visual sensitivity declines.
We also evaluated the structure-function relationship using the model proposed by Hood and Kardon1 and demonstrated that their linear model fits our structure-function data well. Lowess curve fitting our data was very similar to the predicted curve according to the Hood and Kardon’s model (central dashed line in the figures). The number of points lying outside the proposed 95% prediction lines (2 short dashed lines in the figures) was fewer in the superotemporal sector compared to the inferotemporal sector. Hood and Kardon demonstrated that the RNFL thickness in the arcuate regions reached a floor level (b) at a mean sensitivity loss of 10 dB, beyond which there was no significant decline in the RNFL thickness. They estimated that the floor level was close to 33% of the normal RNFL thickness in the arcuate sectors measured with the Stratus OCT. We found that the floor level with RTVue was significantly higher (close to 80 μm). It was 60% of the normal RNFL thickness in the arcuate sectors. A possible limitation in the estimation of ‘b’ from our data is that the number of eyes with a mean visual sensitivity loss of >10dB in both the inferotemporal and superotemporal RNFL sectors was only 19. As can be seen in Figures 2 and 3, there were substantial number of glaucomatous eyes with an average visual sensitivity loss per sector of 5 to 10 dB, with a RNFL thickness of below 80 μm. When we considered ‘b’ as 33% of the normal RNFL thickness at the arcuate areas, as found by Hood and Kardon with Stratus OCT, and plotted the graphs as in figures 2 and 3, close to 25% of glaucomatous eyes were above the 95th percentile line. Determination of the base level b with SDOCT needs more work with a good number of eyes with severe visual sensitivity loss. It is important to note that recently a non-linear model to evaluate structure-function relationship in glaucoma has been proposed by Harwerth et al.26 We however did not evaluate the model proposed by Harwerth et al separately as both these models have been shown to have similar accuracies for grouped data.26
Overall the relationship between structure and function in our study was weak to moderate. Different factors have been proposed to explain the imperfect relationship between structure and function. Important among them are the eyes which show a lag in either the structural or the functional test during the course of the disease. Similarly there may be eyes wherein the structure to function correspondence map proposed by Garway-Heath et al. might fail leading to a weak association between structure and function.
In conclusion, we found that the strongest associations between structure and function using SDOCT were found for the RNFL measurements at the arcuate areas and the inner retinal thickness measurements at the macula. The linear model proposed by Hood and Kardon is useful to study the structure-function relationship in glaucoma.
Acknowledgments
Supported in part by CAPES Ministry of Education, Brazil grant BEX1327/09-7 (MTL), NEI EY08208 (PAS), NEI EY11008 (LMZ), Participant retention incentive grants in the form of glaucoma medication at no cost (Alcon Laboratories Inc., Allergan, Pfizer Inc., SANTEN Inc.).
Footnotes
Financial Disclosure: HL Rao: none; MT Leite: none; LM Zangwill: Heidelberg Engineering (F), Carl Zeiss (F), Optovue (F); RN Weinreb: Optovue (C, F), Topcon (C, F,R), Heidelberg Engineering (F), Carl Zeiss (C, F); PA Sample: Carl Zeiss (F), Heidelberg Engineering (F), Welch Allyn (F); FA Medeiros: Carl Zeiss (F, R), Heidelberg Engineering (R).
References
- 1.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007 Nov;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2213–2220. [PubMed] [Google Scholar]
- 3.Bowd C, Zangwill LM, Medeiros FA, et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2006 Jul;47(7):2889–2895. doi: 10.1167/iovs.05-1489. [DOI] [PubMed] [Google Scholar]
- 4.El Beltagi TA, Bowd C, Boden C, et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology. 2003 Nov;110(11):2185–2191. doi: 10.1016/S0161-6420(03)00860-1. [DOI] [PubMed] [Google Scholar]
- 5.Miglior S, Riva I, Guareschi M, et al. Retinal sensitivity and retinal nerve fiber layer thickness measured by optical coherence tomography in glaucoma. Am J Ophthalmol. 2007 Nov;144(5):733–740. doi: 10.1016/j.ajo.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Ferreras A, Pablo LE, Garway-Heath DF, Fogagnolo P, Garcia-Feijoo J. Mapping standard automated perimetry to the peripapillary retinal nerve fiber layer in glaucoma. Invest Ophthalmol Vis Sci. 2008 Jul;49(7):3018–3025. doi: 10.1167/iovs.08-1775. [DOI] [PubMed] [Google Scholar]
- 7.Iester M, Mikelberg FS, Courtright P, Drance SM. Correlation between the visual field indices and Heidelberg retina tomograph parameters. J Glaucoma. 1997 Apr;6(2):78–82. [PubMed] [Google Scholar]
- 8.Nassif N, Cense B, Park B, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004 Feb 9;12(3):367–376. doi: 10.1364/opex.12.000367. [DOI] [PubMed] [Google Scholar]
- 9.Wojtkowski M, Srinivasan V, Ko T, Fujimoto J, Kowalczyk A, Duker J. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Express. 2004 May 31;12(11):2404–2422. doi: 10.1364/opex.12.002404. [DOI] [PubMed] [Google Scholar]
- 10.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4290–4296. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung CK, Cheung CY, Weinreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008 Nov;49(11):4893–4897. doi: 10.1167/iovs.07-1326. [DOI] [PubMed] [Google Scholar]
- 12.Menke MN, Knecht P, Sturm V, Dabov S, Funk J. Reproducibility of nerve fiber layer thickness measurements using 3D fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5386–5391. doi: 10.1167/iovs.07-1435. [DOI] [PubMed] [Google Scholar]
- 13.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009 Jun;93(6):775–781. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung CK, Cheung CY, Weinreb RN, et al. Retinal Nerve Fiber Layer Imaging with Spectral-Domain Optical Coherence Tomography A Variability and Diagnostic Performance Study. Ophthalmology. 2009 May 21; doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Sung KR, Kim DY, Park SB, Kook MS. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology. 2009 Jul;116(7):1264–1270. 1270e1261. doi: 10.1016/j.ophtha.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology. 2009 Jul;116(7):1271–1277. doi: 10.1016/j.ophtha.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao HL, Zangwill LM, Weinreb RN, Sample PA, Alencar LM, Medeiros FA. Comparison of Different Spectral Domain Optical Coherence Tomography Scanning Areas for Glaucoma Diagnosis. Ophthalmology. 2010 May 19; doi: 10.1016/j.ophtha.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland WS, Devlin SJ. Locally-Weighted Regression: An Approach to Regression Analysis by Local Fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 19.Horn FK, Mardin CY, Laemmer R, et al. Correlation between local glaucomatous visual field defects and loss of nerve fiber layer thickness measured with polarimetry and spectral domain OCT. Invest Ophthalmol Vis Sci. 2009 May;50(5):1971–1977. doi: 10.1167/iovs.08-2405. [DOI] [PubMed] [Google Scholar]
- 20.Ajtony C, Balla Z, Somoskeoy S, Kovacs B. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by optical coherence tomography. Invest Ophthalmol Vis Sci. 2007 Jan;48(1):258–263. doi: 10.1167/iovs.06-0410. [DOI] [PubMed] [Google Scholar]
- 21.Caprioli J, Miller JM. Optic disc rim area is related to disc size in normal subjects. Arch Ophthalmol. 1987 Dec;105(12):1683–1685. doi: 10.1001/archopht.1987.01060120081030. [DOI] [PubMed] [Google Scholar]
- 22.Budde WM, Jonas JB, Martus P, Grundler AE. Influence of optic disc size on neuroretinal rim shape in healthy eyes. J Glaucoma. 2000 Oct;9(5):357–362. doi: 10.1097/00061198-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000 Jan;118(1):22–26. doi: 10.1001/archopht.118.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007 Jun;114(6):1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007 May;114(5):921–926. doi: 10.1016/j.ophtha.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010 Jul;29(4):249–71. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]