Abstract
Background
Previous studies have reported maternal exposure to airborne polycyclic aromatic hydrocarbons (PAH), as well as DNA adducts reflecting total PAH exposure, to be associated with reduced fetal growth. The role of diet, the main source of PAH exposure among non-smokers, remains uncertain.
Objective
To assess associations between birth weight, length and small size for gestational age (SGA) with maternal intakes of the genotoxic PAH benzo(a)pyrene [B(a)P] during pregnancy, exploring potential effect modification by dietary intakes of vitamin C, vitamin E, alpha- and beta-carotene, as well as glutathione S-transferase P1 (GSTP1) polymorphisms, hypothesized to influence PAH metabolism.
Methods
657 women in the INMA (Environment and Childhood) Project from Sabadell (Barcelona) were recruited during the first trimester of pregnancy. Dietary B(a)P and nutrient intakes were estimated from food consumption data. Genotyping was conducted for the Ile105Val variant of GSTP1. Multivariable models were used to assess associations between size at birth and dietary B(a)P, evaluating potential interactions with candidate nutrients and GSTP1 variants.
Results
There were significant interactions between elevated intakes of vitamin C (above the mean of 189.41mg/day) and dietary B(a)P during the first trimester of pregnancy in models for birth weight and length (P<0.05), but no interactions were found with other nutrients. B(a)P intakes were associated with significant reductions in birth weight and length (coefficient±SE for a 1-SD increase in B(a)P: −101.63±34.62 g and −0.38±0.16 cm, respectively) among women with low, but not high, vitamin C intakes. Elevated dietary B(a)P was also associated with increased risk of SGA births among women with low dietary vitamin C. Among these women, associations were strongest in those carrying the GSTP1 Val allele, associated with lower contaminant detoxification activity.
Conclusion
Results suggest that dietary B(a)P exposure may impair fetal growth, particularly in genetically susceptible populations, and that increasing maternal intakes of vitamin C may help to reduce any adverse effects.
Keywords: polycyclic aromatic hydrocarbons, benzo(a)pyrene, diet, pregnancy, vitamin C, glutathione S-transferase P1, fetal growth
1. INTRODUCTION
Recent epidemiological studies suggest prenatal exposure to polycyclic aromatic hydrocarbons (PAH) may be associated with adverse reproductive or child health outcomes, including low birth weight and length, preterm birth, reduced head circumference at birth, and lower scores on childhood tests of neurodevelopment (Choi et al. 2006;Choi et al. 2008;Perera et al. 2005;Perera et al. 2006). Most studies on reproductive effects of PAHs to date have assessed exposure either using personal monitoring equipment to assess atmospheric exposure, or have used levels of bulky DNA adducts as a marker of total PAH exposure, including both airborne and dietary sources. It has been demonstrated that PAHs cross the placenta barrier during pregnancy, and may therefore compromise fetal development (Autrup and Vestergaard 1996;Perera et al. 1999;Sanyal et al. 2007).
Diet is the main source of PAH exposure among individuals not exposed to high levels of tobacco smoke or with occupational exposures (Agency for Toxic Substances and Disease Registry (ATSDR) 1995;Butler et al. 1993;Hatagima 2002;Phillips 1999;Scherer et al. 2000;Suzuki and Yoshinaga 2007;Vyskocil et al. 2000). Benzo(a)pyrene [B(a)P] has been identified as human mutagen, carcinogen, and endocrine disruptor, and has been widely used as a marker of exposure to total carcinogenic PAHs (Agency for Toxic Substances and Disease Registry (ATSDR) 1995). However, it remains uncertain whether the dietary route of exposure may relate to birth outcomes.
Most studies to date on maternal PAH exposure and reproductive outcomes have not assessed the role of factors hypothesized to influence metabolism of these compounds. Antioxidant nutrients such as vitamin C, vitamin E, alpha-carotene or beta-carotene have been reported to reduce DNA damage related to PAH exposure, perhaps by inducing the activity of detoxifying enzymes such as GSTs, and could therefore help to protect against adverse health outcomes related to exposure to such contaminants (Bhuvaneswari et al. 2002;Kelvin et al. 2009;Mooney et al. 2005;Palli et al. 2000;van Lieshout et al. 1996). Additionally, the enzyme glutathione S-transferase P1 (GSTP1) is involved in detoxification of xenobiotic compounds including PAHs (Hatagima 2002;McCarty et al. 2009;Pavanello and Clonfero 2000;Watson et al. 1998). Polymorphisms in the GSTP1 gene are related to changes in enzymatic activity and hence detoxification capacity, and may therefore determine individual susceptibility to adverse health outcomes related to PAH exposure (Hatagima 2002;Pavanello and Clonfero 2000;Perera et al. 1999;Watson et al. 1998). Although antioxidant nutrients have been shown to modify the formation of DNA adducts in cord blood (Kelvin et al. 2009), to date, no previous studies have examined whether antioxidant intakes or GSTP1 polymorphisms modify associations between dietary PAH exposure and reproductive health outcomes.
In a previous study, we assessed levels of maternal dietary intakes of B(a)P, one of the most studied PAH compounds, and reported an association with lower birth weight (Duarte-Salles et al. 2010). This study aimed to further assess associations between intakes of B(a)P during pregnancy and multiple indicators of fetal growth impairment in a population-based birth cohort, focusing on whether maternal antioxidant intakes and the GSTP1 (Ile105Val) polymorphism in either mother or child may modulate this association.
2. METHODS
2.1. Study population
Data come from a population-based birth cohort established in Sabadell (Catalonia, Spain) as part of the INMA – INfancia y Medio Ambiente [Environment and Childhood] Project. Details of the study design have been described previously (Guxens et al. 2011;Aguilera et al. 2010). Briefly, between July 2004 and July 2006, 657 women were recruited during their first trimester of pregnancy. From the initial sample, birth weight was available for 614 (93%) and birth length for 604 (92%) newborns; main covariates including smoking, education level and dietary data were available for 586 (86%) and 574 (87%) of these children for each outcome, respectively. The analysis sample did not differ from those with missing data in terms of characteristics including child sex, birth weight, gestational age at birth, and maternal age (data not shown). Since smoking contributes substantially to B(a)P exposure (Agency for Toxic Substances and Disease Registry (ATSDR) 1995; Duarte-Salles et al. 2010), additional analyses were done excluding women who declared any smoking during pregnancy. This study was approved by the Clinical Research Ethical Committee of the Municipal Institute of Health Care (CEIC-IMAS), and informed consent was signed by all participants.
2.2. Dietary assessment and B(a)P intakes
Dietary intakes since the start of pregnancy were assessed at recruitment using a previously validated 101-item semi-quantitative food frequency questionnaire (Vioque J 2006). Intake frequencies ranged from “never or < once a month” to “≥ 6 times/day”. Daily intakes of foods and nutrients were estimated using standard portion sizes. Intakes of energy and nutrients, including intake of vitamin C, vitamin E, alpha-carotene and beta-carotene, were estimated using a database compiled based on Spanish nutrient tables (Farran A et al. 2003).
Estimation of B(a)P intakes has been described previously (Duarte-Salles et al. 2010). A compilation of the available data on food concentrations of B(a)P was undertaken to construct a food composition table for items in the food frequency questionnaire. Exclusion criteria for the selection of eligible values applicable to this study includes: (i) values published before 1990, (ii) values from highly polluted settings (e.g.: foods locally produced in Kuwait) (Husain et al. 1997), (iii) extreme outliers, and (iv) values for heterogeneous food groups instead of food items. When a published value was below the limit of detection or not detectable, the value assigned was the half of the detection limit. Average concentrations for B(a)P in each food were calculated using all available data for that food item. When no data was available for a food item that was asked at the FFQ, concentrations of B(a)P were imputed from food items with similar characteristics (e.g.: values for skimmed milk were imputed from semi-skimmed milk). Finally, daily B(a)P intake was estimated by multiplying food item concentrations of B(a)P by intakes of each food, which were then summed to estimate total intakes expressed as micrograms (µg) per day.
2.3. Genotyping
Maternal blood samples were collected at recruitment and cord blood at delivery. DNA was extracted from 646 maternal blood and 519 cord blood samples (511 mother-child pairs) using the Chemagic MAgnetic Sparateor technology (Chemagen) at the Spanish National Genotyping Centre (CEGEN). DNA was quantified using dsDNA fluorescent detection (PicoGreen, Molecular Probes) and normalized to 75–100 ng/µl. GSTP1 Ile105Val polymorphism (rs1695, A/G) was genotyped with KASPar technology (KBioscience) at the CEGEN following manufacturer’s instructions. Genotyping quality was validated with the inclusion of three HapMap trios with known genotypes. Moreover, one of these HapMap samples was used as an inter-plate replicate. Additionally, 190 samples (17%) were also genotyped using a Pyrosequencing assay (Biotage) as described elsewhere (Morales et al. 2009). Ninety-nine per cent of the samples analyzed simultaneously by KASPar and Pyrosequencing showed consistent genotypes. Four mother-child pairs (0.7% of the total cohort) were excluded from the analysis due to discordant genotypes between the methods (N=2) or due to Mendelian errors (N=2). Finally, genotypes were obtained for 635 out 646 maternal (98%) and 484 out 519 (93%) child blood samples respectively, of which 565 (89%) and 464 (89%) had diet information and were included in the analysis. The analysis sample with information on maternal GSTP1 did not differ from those with missing data, however, those with child GSTP1 genotype data had slightly higher birth weight, length and gestational age compared with those with child GSTP1 missing data (data not shown). All assays were performed blinded to fetal growth indicators and dietary B(a)P intakes. GSTP1 genotypes were in Hardy-Weinberg equilibrium in the cohort (P > 0.05). Because of the low frequency of Val/Val homozigotes, we combined the GSTP1 Ile/Val and Val/Val genotypes as in dominant genetic models for subsequent analyses. The frequency of the 105Val allele was 0.33 in the mother and 0.34 in the child.
2.4. Birth outcomes and other variables
Birth weight and length were recorded by specially trained midwives at delivery. Gestational age was calculated from the date of the last menstrual period reported at recruitment and confirmed using ultrasound measures in the first trimester. Ultrasound estimates were used when estimates based on last menstrual period and ultrasound differed by ≥ 7 days (n=91; 16%). Infants with birth weight below the 10th percentile of a Spanish reference population were classified as small size for gestational age (SGA) (Carrascosa et al. 2004).
Information on maternal age, parental occupation (Grupo de Trabajo de la SEE - Álvarez-Dardet C et al. 1995), the highest achieved level of parental education (≥university, secondary, ≤primary), parental region of origin (European, other) and ethnicity (Caucasian, other), parity, pre-pregnancy weight, maternal height, and paternal weight and height were collected by questionnaire in the first trimester of pregnancy. Tobacco use (women who reported smoking throughout pregnancy) and passive smoke exposure (women residing with an active smoker or exposed to tobacco smoke at place of work) during pregnancy were collected in the third trimester. Women were categorized as non-smokers (women not exposed to tobacco smoke), passive smokers (non-smokers exposed to passive smoking), and active smokers. Maternal cotinine levels were measured in maternal urine collected in the third trimester of pregnancy, using the Cotinine Micro-Plate EIA Kit (Ora Sure Technologies, Inc.).
B(a)P is absorbed onto fine particles and studies have found moderate to high correlations between atmospheric concentrations of fine particles, B(a)P and nitrogen dioxide (NO2) (Hazenkamp-von Arx et al. 2004; Tham et al. 2008; Wilhelm et al. 2011) since most of these pollutants, including B(a)P and NO2, come from the traffic (Lodovici et al. 2003; Varea et al. 2011; Wilhelm et al. 2011). As exposure to NO2 during pregnancy has been related to reproductive outcomes (Ballester et al. 2010), in this study we assessed possible confounding using residential outdoor NO2 as a proxy indicator. NO2 was estimated by land-use regression modelling as described previously (Aguilera et al. 2008).
2.5. Statistical analysis
Descriptive analyses were stratified by maternal levels of vitamin C intake. Within strata of vitamin C (< or ≥ the mean of 189.41 mg/day), measures of size at birth (birth weight, birth length, and SGA) and maternal characteristics were described across population-wide tertiles of B(a)P intake (lowest two vs. highest tertile). Statistical significance was evaluated using χ2, Student’s t-test, or Wilcoxon-Mann-Whitney tests for categorical, continuous, and non-normally distributed continuous variables, respectively.
Multivariate linear regression models were used to examine the relationship between dietary B(a)P intakes during pregnancy and birth weight and length, and logistic regression was used to assess associations with SGA birth. Modification of associations with B(a)P by maternal intakes of candidate nutrients during pregnancy were tested by including interaction terms in the regression models; interactions with vitamin C were significant using B(a)P either continuously or in tertiles (P<0.10). No significant interactions were found between dietary B(a)P and vitamin E, alpha-carotene or beta-carotene intakes (interaction P>0.05). Models were therefore stratified by elevated vitamin C intakes. Generalized additive models (GAMs) were used to evaluate the linearity of the relationship between dietary B(a)P and birth outcomes among women with high vs. low intakes of vitamin C. Relationships between B(a)P and birth weight or length were linear in both strata (P for gain in linearity >0.10 in GAM models), and therefore results are reported as coefficients for a 1 SD increase in B(a)P with standard errors (SE). However, results were similar using tertiles of B(a)P intake (not shown). For SGA, associations with dietary B(a)P were nonlinear (P for gain in linearity <0.10); results are thus reported as ORs for the highest vs. lowest two tertiles of B(a)P intakes, with 95% CIs. Models were further stratified by GSTP1 Ile105Val genotypes in order to evaluate whether the associations with dietary B(a)P exposure differed by maternal and child GSTP1 polymorphism. Due to the small number of SGA infants, it was not possible to further stratify analyses for this outcome by GSTP1 variants.
Covariates in final models were sex of the child, gestational age, nulliparity, active or passive smoking during pregnancy, maternal region of origin (European and non-European), education level (≥university, secondary and ≤primary), height, pre-pregnancy weight, and energy intakes (kilocalories/day). Other covariates (maternal age, parental occupation, parental ethnicity, father’s country of origin, father’s weight and height, marital status, maternal cotinine levels during pregnancy, and atmospheric NO2) were tested as potential confounders but were excluded from final models for parsimony, as they did not meaningfully affect our estimates (change-in-estimate <10%). Adjusting for maternal serum levels of polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE) also had no meaningful effect. Similarly, we confirmed that results were comparable after adjusting for maternal intakes of food groups such as fruits (including juices) and vegetables, intakes of antioxidant vitamin-containing supplements, shellfish and processed meat (main sources of B(a)P dietary intakes), as well as intakes other micronutrients hypothesized to protect against the formation of adducts, namely vitamin E, alpha-carotene and beta-carotene (Kelvin et al. 2009) (results not shown). Associations were also similar after excluding women who actively smoked during pregnancy (n=97), preterm births (<37 weeks gestation, n=13), low birth weight children (n=25) or non-European and non-Caucasian children (n=76) (latter three not shown). Delta-betas were used to exclude the most influential observations in the multivariate analyses; no meaningful changes were observed. Data were analyzed using STATA 10.1 (Stata Corporation, College Station, Texas).
3. RESULTS
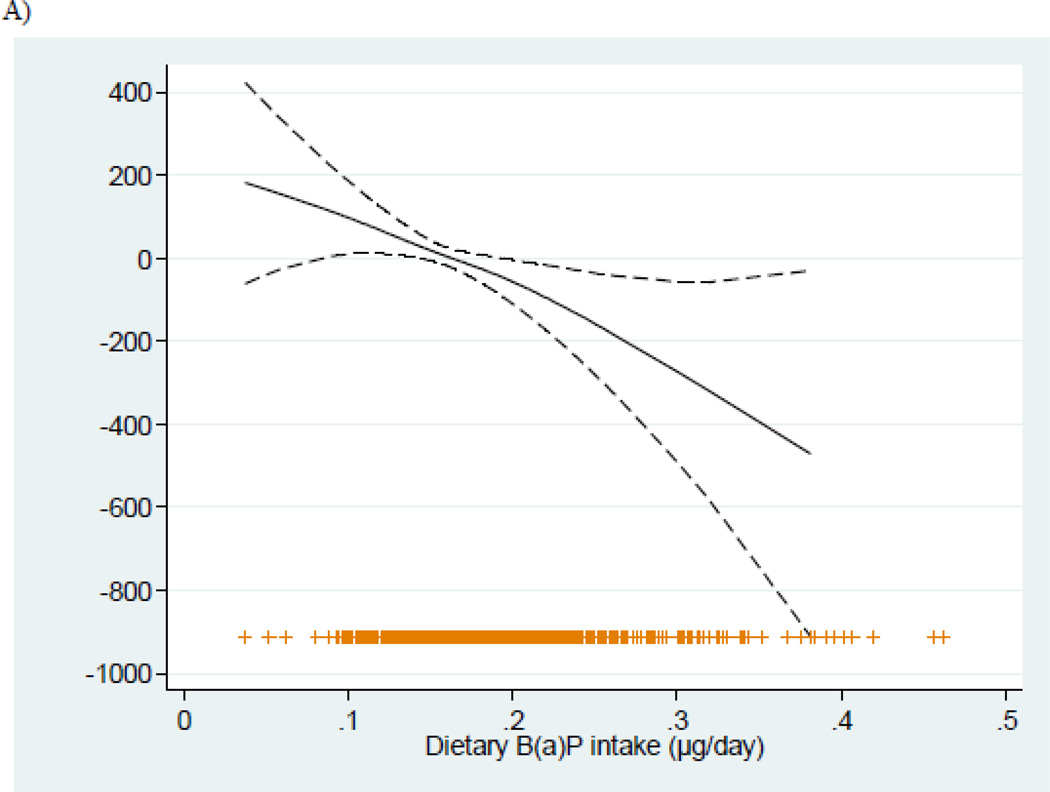
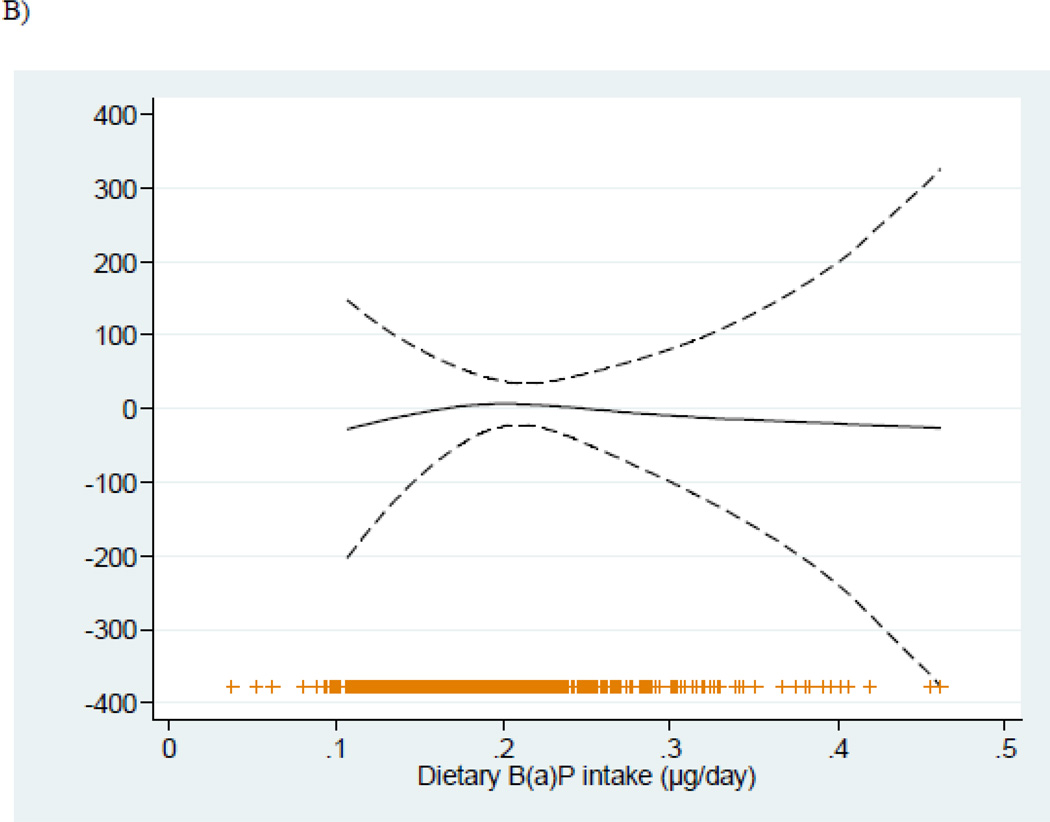
Overall, the mean±SD intake of dietary B(a)P was 0.19±0.6 µg/day. Sample characteristics are presented stratified by levels of vitamin C intake, as associations between dietary B(a)P and size at birth differed in infants with high (≥ the mean of 189.41 mg/day) vs. low maternal intakes of this nutrient (interaction P<0.10 for multiple outcomes) (Table 1). Statistically significant differences in birth weight, birth length and SGA by level of dietary B(a)P were observed only among women with lower vitamin C intakes. In this group of women, the mean birth weight and birth length were significantly lower and the frequency of SGA births was higher in those in the highest tertile vs. lower tertiles of B(a)P intake (3073 vs. 3274 g, 49.50 vs. 48.57 cm and 6.95% vs. 17.39% respectively, P <0.05). Among women with high vitamin C intakes, there was no difference in size at birth across tertiles of dietary B(a)P.
Table 1.
Maternal dietary B(a)P intakes, fetal growth indicators and key confounders across B(a)P and Vitamin C intakes during 1st trimester of pregnancy in the INMA-Sabadell cohort
| < Vitamin C mean intake |
≥ Vitamin C mean intake |
|||||||
|---|---|---|---|---|---|---|---|---|
| All (n=338) |
B(a)P- Tertile 1 and 2 (n=292) |
B(a)P- Tertile 3 (n=46) |
p Value* |
All (n=248) |
B(a)P- Tertile 1 and 2 (n=99) |
B(a)P- Tertile 3 (n=149) |
p Value* |
|
|
Dietary B(a)P intake (µg/day), mean±SD |
0.16 ± 0.04 |
0.15 ± 0.03 |
0.23 ± 0.03 |
< 0.001 |
0.22 ± 0.06 |
0.17 ± 0.02 |
0.25 ± 0.05 |
< 0.001 |
|
Newborn characteristics |
||||||||
| Birth weight (g), mean±SD |
3247 ± 417 |
3274 ± 399 |
3073 ± 488 |
0.001 | 3263 ± 421 |
3275 ± 430 |
3255 ± 416 |
0.356 |
| Birth length (cm), mean±SD (n=574) |
49.38 ± 1.92 |
49.50 ± 1.87 |
48.57 ± 2.07 |
0.001 | 49.44 ± 1.85 |
49.46 ± 1.93 |
49.42 ± 1.81 |
0.668 |
| SGA, % | 8.22 | 6.95 | 17.39 | 0.016 | 7.26 | 7.07 | 7.38 | 0.926 |
| Sex (male), % | 48.82 | 49.32 | 45.65 | 0.644 | 51.21 | 51.52 | 51.02 | 0.937 |
|
Maternal GSTP1 Ile105Val genotypes, %a |
||||||||
| Ile/Ile | 45.26 | 47.52 | 31.11 | 47.06 | 49.47 | 45.45 | ||
| Ile/Val | 44.04 | 42.91 | 51.11 | 41.18 | 42.11 | 40.56 | ||
| Val/Val | 10.70 | 9.57 | 17.78 | 0.069 | 11.76 | 8.42 | 13.99 | 0.421 |
|
Child GSTP1 Ile105Val genotypes, %b |
||||||||
| Ile/Ile | 47.83 | 52.70 | 14.29 | 37.23 | 46.25 | 30.56 | ||
| Ile/Val | 40.21 | 35.68 | 71.42 | 51.07 | 46.25 | 54.63 | ||
| Val/Val | 11.96 | 11.62 | 14.29 | < 0.001 |
11.70 | 7.50 | 14.81 | 0.056 |
|
Maternal characteristics |
||||||||
| Age (years), mean±SD |
31.29 ± 4.25 |
31.18 ± 4.29 |
31.96 ± 4.01 |
0.383 | 31.68 ± 4.49 |
31.98 ± 3.72 |
31.49 ± 4.94 |
0.374 |
| Pre-pregnancy weight (kg), mean±SD |
62.42 ± 11.56 |
62.19 ± 11.02 |
63.84 ± 14.58 |
0.933 | 62.95 ± 13.89 |
64.62 ± 15.14 |
61.85 ± 12.93 |
0.130 |
| Height (cm), mean±SD |
162.66 ± 5.95 |
162.63 ± 5.86 |
162.87 ± 6.54 |
0.399 | 162.19 ± 6.26 |
162.28 ± 6.22 |
162.13 ± 6.30 |
0.427 |
| Gestational age (weeks), mean±SD |
39.58 ± 1.38 |
39.66 ± 1.36 |
39.09 ± 1.41 |
0.011 | 39.52 ± 1.34 |
39.47 ± 1.38 |
39.55 ± 1.31 |
0.590 |
| Nulliparous, % | 55.62 | 59.25 | 32.61 | 0.001 | 58.47 | 59.60 | 57.72 | 0.769 |
| Exposure to tobacco smoke, % |
||||||||
| Passive smokers | 35.50 | 33.90 | 45.65 | 37.90 | 30.30 | 42.95 | ||
| Active smokers | 17.16 | 15.75 | 26.09 | 0.017 | 15.73 | 9.09 | 20.13 | 0.001 |
| Vitamin C intake (mg/day), mean±SD |
125.44 ± 37.82 |
122.98 ± 37.77 |
141.06 ± 34.61 |
0.002 | 276.59 ± 86.65 |
243.86 ± 53.90 |
298.34 ± 97.03 |
< 0.001 |
| Energy (kcal/day), mean±SD |
2587 ± 575 |
2085 ± 405 |
2627 ± 454 |
< 0.001 |
2159 ± 451 |
2202 ± 362 |
2843 ± 548 |
< 0.001 |
| Maternal education (primary or less), % |
26.63 | 23.29 | 47.83 | 0.002 | 31.05 | 28.28 | 32.89 | 0.590 |
|
Atmospheric nitrogen dioxide (NO2) (µg/m3), mean±SDc |
32.31 ± 8.82 |
32.25 ± 8.87 |
32.64 ± 8.63 |
0.767 | 31.56 ± 8.69 |
29.46 ± 6.19 |
32.86 ± 9.72 |
0.051 |
SD, standard deviation; SGA, small size for gestational age.
Available for n=565 (96% of the analysis sample).
Available for n=464 (79% of the analysis sample).
Available for n=542. Estimation of outdoor exposure at home addresses during pregnancy (Aguilera et al. 2010).
p-value from χ2, Student’s t-test, or Wilcoxon-Mann-Whitney tests.
Dietary B(a)P intakes were associated significantly with lower birth weight in the total sample after multivariate adjustment (coefficient±SE for 1-standard deviation increase of B(a)P intake: −44.60±21.94g), but no significant associations were found for birth length and SGA (coefficient±SE: −0.13±0.09, and OR: 2.06, 95%CI: 0.90 – 4.70, respectively) (results not shown).
As shown (Table 2, Figure 1), increasing dietary B(a)P intakes were associated with significant reductions in birth weight and length among women with low, but not high, vitamin C intakes. Multivariable-adjusted coefficients±SE for birth weight and length associated with each 1-standard deviation increase of B(a)P intake were −101.63±34.62g and −0.38±0.16cm respectively in the group with low vitamin C intakes, and 2.05±29.56g and 0.10±0.13cm in the high intake group (P-value for interactions were 0.026 and 0.088 respectively). Similar results were found after excluding women who actively smoked during pregnancy (n=97) (Table 2). Among non-smokers with low vitamin C intakes, multivariable-adjusted coefficients±SE for birth weight and length were −88.26±41.36g and −0.37±0.18cm respectively, and −3.71±32.46g and 0.04±0.13cm in the high vitamin C intake group.
Table 2.
Associations between fetal growth indicators and dietary B(a)P intakes during the 1st trimester of pregnancy by Vitamin C intakea
| Birth weight |
Birth length |
||||||
|---|---|---|---|---|---|---|---|
| Dietary B(a)P intake (µg/day) | N | β, 1 SD increase | SE | p-Value | β, 1 SD increase | SE | p-Value |
| < Mean Vitamin C intake | |||||||
| All women* | 338 | ||||||
| Crude | −109.31 | 32.12 | 0.001 | −0.48 | 0.15 | 0.002 | |
| Adjusted | −101.63 | 34.62 | 0.004 | −0.38 | 0.16 | 0.017 | |
| Non-smokers during pregnancy | 280 | ||||||
| Crude | −71.96 | 38.17 | 0.060 | −0.36 | 0.17 | 0.044 | |
| Adjusted | −88.26 | 41.36 | 0.034 | −0.37 | 0.18 | 0.048 | |
| ≥ Mean Vitamin C intake | |||||||
| All women | 248 | ||||||
| Crude | 10.62 | 27.27 | 0.697 | 0.02 | 0.12 | 0.844 | |
| Adjusted | 2.05 | 29.56 | 0.945 | 0.10 | 0.13 | 0.439 | |
| Non-smokers during pregnancy | 209 | ||||||
| Crude | 19.12 | 30.63 | 0.533 | −0.01 | 0.13 | 0.962 | |
| Adjusted | −3.71 | 32.46 | 0.909 | 0.04 | 0.13 | 0.784 | |
SE, standard error.
Multivariable models adjusted for sex of the child, gestational age, nulliparity, exposure to tobacco smoke during pregnancy, maternal region of origin, education level of the mother, maternal height and prepregnancy weight and energy intakes. β coefficients are for a 1-standard deviation increase of dietary B(a)P.
p for interaction between dietary B(a)P intake and elevated Vitamin C intake among all women: Birth weight = 0.026, and Birth length = 0.088.
Figure 1.
Relationship (and 95% confidence intervals) between dietary B(a)P intakes during pregnancy and birth weight adjusted for sex of the child, gestational age, nulliparity, smoking during pregnancy, maternal region of origin, education level of the mother, maternal height, pre-pregnancy weight, and energy intakes, stratified by the mean of vitamin C (189.41 mg/day). Adjusted general additive models for birth weight in A) < Vitamin C mean intake, and B) > Vitamin C mean intake.
After further stratifying by GSTP1 Ile105Val polymorphism in either mother or child, associations between higher intakes of dietary B(a)P and reduced birth weight and length were strong and significant only among those with the 105Val allele. As before, these associations were observed only among women with low vitamin C intakes (Table 3); P-value for interaction between dietary B(a)P and elevated Vitamin C intake among women with low detoxification capacity were 0.035 for birth weight and 0.038 for birth length. After stratifying based on both maternal and fetal genotype simultaneously, associations between dietary B(a)P and birth size among these women were strongest when the 105Val allele was present in both mother and child (n=105; multivariate-adjusted association −158.64±57.01g and −0.54±0.26cm for birth weight and length respectively), vs. in only one member of the pair (n=78; −12.87±77.39g and −0.15±0.37cm, respectively). When neither member had the Val variant, multivariable-adjusted associations were null.
Table 3.
Associations between fetal growth indicators and dietary B(a)P intakes during the 1st trimester of pregnancy by Vitamin C intake and GSTP1 Ile105Val polymorphism in mother and childa
| Birth weight |
Birth length |
||||||
|---|---|---|---|---|---|---|---|
| Dietary B(a)P intake (µg/day) | N | β, 1 SD increase | SE | p-Value | β, 1 SD increase | SE | p-Value |
| < Mean Vitamin C intake* | |||||||
| By maternal GSTP1 Ile105Val genotypesb * | 326 | ||||||
| Crude | |||||||
| Ile/Ile | 148 | −36.67 | 50.36 | 0.468 | −0.15 | 0.27 | 0.585 |
| Ile/Val or Val/Val | 179 | −149.81 | 42.99 | 0.001 | −0.68 | 0.18 | <0.001 |
| Adjusted | |||||||
| Ile/Ile | 148 | −18.94 | 54.41 | 0.728 | −0.09 | 0.29 | 0.764 |
| Ile/Val or Val/Val | 179 | −145.06 | 48.60 | 0.003 | −0.50 | 0.20 | 0.014 |
| By child GSTP1 Ile105Val genotypes b ** | 276 | ||||||
| Crude | |||||||
| Ile/Ile | 132 | −66.26 | 61.21 | 0.281 | −0.19 | 0.27 | 0.487 |
| Ile/Val or Val/Val | 144 | −135.78 | 44.50 | 0.003 | −0.60 | 0.22 | 0.007 |
| Adjusted | |||||||
| Ile/Ile | 132 | −44.72 | 67.06 | 0.506 | −0.13 | 0.29 | 0.649 |
| Ile/Val or Val/Val | 144 | −130.79 | 46.20 | 0.008 | −0.48 | 0.23 | 0.037 |
| ≥ Mean Vitamin C intake | |||||||
| By maternal GSTP1 Ile105Val genotypes | 238 | ||||||
| Crude | |||||||
| Ile/Ile | 112 | 13.79 | 42.47 | 0.746 | 0.01 | 0.19 | 0.950 |
| Ile/Val or Val/Val | 126 | −1.86 | 36.99 | 0.960 | −0.03 | 0.16 | 0.866 |
| Adjusted | |||||||
| Ile/Ile | 112 | −34.91 | 44.76 | 0.437 | −0.04 | 0.19 | 0.853 |
| Ile/Val or Val/Val | 126 | 11.92 | 42.37 | 0.779 | 0.11 | 0.18 | 0.531 |
| By child GSTP1 Ile105Val genotypes | 188 | ||||||
| Crude | |||||||
| Ile/Ile | 70 | 2.34 | 59.71 | 0.969 | 0.21 | 0.27 | 0.438 |
| Ile/Val or Val/Val | 118 | −6.36 | 37.88 | 0.867 | −0.03 | 0.18 | 0.858 |
| Adjusted | |||||||
| Ile/Ile | 70 | −26.63 | 62.73 | 0.673 | 0.29 | 0.29 | 0.332 |
| Ile/Val or Val/Val | 118 | −51.25 | 45.64 | 0.264 | −0.01 | 0.21 | 0.942 |
SE, standard error.
Multivariable models adjusted for sex of the child, gestational age, nulliparity, exposure to tobacco smoke during pregnancy, maternal region of origin, education level of the mother, maternal height and prepregnancy weight and energy intakes. β coefficients are for a 1-standard deviation increase of dietary B(a)P.
Associations between dietary B(a)P and fetal growth indicators were unchanged in the subsample for which maternal/child GSTP1 polymorphisms was available.
p for interaction between dietary B(a)P intake and elevated Vitamin C intake among women with low detoxification capacity (GSTP1 = Ile/Val or Val/Val; n=305): Birth weight = 0.035, Birth length = 0.038.
p for interaction between dietary B(a)P intake and elevated Vitamin C intake among children with low detoxification capacity (GSTP1 = Ile/Val or Val/Val; n=262): Birth weight = 0.170, Birth length = 0.287.
In multivariate logistic regression models (Table 4), elevated intakes of B(a)P were associated with an increased risk of SGA (OR: 3.51; 95% CI: 1.16 – 10.59) among women below the mean intake of vitamin C. However, no association was found between dietary B(a)P and SGA among women with high vitamin C intake (OR: 0.81; 95% CI: 0.23 – 2.75).
Table 4.
Associations between SGA births and dietary B(a)P intakes during the 1st trimester of pregnancy by level of maternal Vitamin C intakea
| SGA |
|||||
|---|---|---|---|---|---|
| Dietary B(a)P intake (µg/day) | N | SGA - N (%) | OR | 95% CI | p-Value |
| < Vitamin C mean intake | |||||
| Crude | |||||
| B(a)P-Tertile 1 and 2 | 292 | 20(6.85) | 1.00 | ||
| B(a)P-Tertile 3 * | 46 | 8(17.39) | 2.86 | 1.18 – 6.95 | 0.020 |
| Adjusted b | |||||
| B(a)P-Tertile 1 and 2 | 1.00 | ||||
| B(a)P-Tertile 3 * | 3.51 | 1.16 – 10.59 | 0.026 | ||
| ≥ Vitamin C mean intake | |||||
| Crude | |||||
| B(a)P-Tertile 1 and 2 | 99 | 7 (7.07) | 1.00 | ||
| B(a)P-Tertile 3 | 149 | 11(7.38) | 1.05 | 0.39 – 2.80 | 0.926 |
| Adjusted b | |||||
| B(a)P-Tertile 1 and 2 | 1.00 | ||||
| B(a)P-Tertile 3 | 0.81 | 0.23 – 2.75 | 0.731 | ||
SGA, small size for gestational age.
Multivariable models adjusted for sex of the child, gestational age, nulliparity, exposure to tobacco smoke during pregnancy, maternal region of origin, education level of the mother, maternal height and prepregnancy weight and energy intakes.
N for adjusted and crude models was the same.
p for interaction = 0.075.
4. DISCUSSION
In this study, higher maternal dietary B(a)P intakes during the first trimester of pregnancy were related to significant reduction both in birth weight and length, and an increase in risk of SGA among women with low dietary vitamin C intakes. There were significant interactions between dietary B(a)P and vitamin C intakes for birth weight and birth length. Although the number of SGA births was small, elevated B(a)P intakes were also associated with a significant increase in the risk of SGA birth, again among women with low vitamin C intakes. Moreover, associations with birth weight and length were stronger in the presence of the GSTP1 105Val allele in mothers or children, associated with lower rates of detoxification of contaminants such as B(a)P, and persist among non-smokers. Our findings suggest, for the first time, that higher maternal intakes of vitamin C during pregnancy as well as genetic polymorphisms related to detoxification of these contaminants, may help to attenuate any adverse effects of maternal dietary B(a)P on fetal growth. Further research is needed to confirm that vitamin C may help to reduce such adverse effects among women more genetically susceptible to these contaminants.
These findings suggest that prenatal exposure to B(a)P specifically from dietary sources may be related to impaired fetal growth. Earlier studies have reported significant negative associations between maternal PAH exposure, estimated based on levels of bulky DNA adducts or personal measures of atmospheric PAH exposure, with birth weight, birth length and SGA in populations from the United States (Choi et al. 2006;Choi et al. 2008;Perera et al. 2003;Perera et al. 2004), Poland (Choi et al. 2006;Perera et al. 1998) and the Czech Republic (Dejmek et al. 2000). Bulky DNA adducts are a biomarker of overall PAH exposure, including PAHs from the diet, as well as from tobacco smoke and contaminated air (Pavanello et al. 2006). Although diet is recognized as the main source of PAH exposure for non-occupationally exposed individuals and non-smokers (Agency for Toxic Substances and Disease Registry (ATSDR) 1995;Phillips 1999;Scherer et al. 2000;Suzuki and Yoshinaga 2007), most studies exploring the role of PAHs in fetal growth have not specifically examined the role of dietary intakes of these compounds, nor the role of interactions with dietary factors or genetic polymorphisms involved in the metabolism and detoxification of PAHs.
Dietary exposure to PAHs is common, as these compounds are widespread throughout the diet, due to food exposure to contaminated air, soil and water as well as processing and cooking methods (e.g. drying, smoking, grilling, roasting or frying) that increase concentration of PAHs, including B(a)P, in numerous foods (Agency for Toxic Substances and Disease Registry (ATSDR) 1995). In a previous analysis, we reported that dietary B(a)P was inversely associated with birth weight (Duarte-Salles et al. 2010). To our knowledge, besides our analyses, there are only two prior epidemiologic studies that have examined the role of prenatal exposure to B(a)P specifically from diet on fetal growth (Perera et al. 2004;Jedrychowski et al. 2011). One study estimated dietary PAHs based on a limited number of food items (smoked, grilled or barbequed food intakes) and reported very weak inverse associations with indicators of size at birth (Perera et al. 2004). The other study found a significant negative effect of maternal intake of barbecued meat during the third trimester of pregnancy on birth weight, independent of atmospheric PAH exposure measured using personal air monitors (Jedrychowski et al. 2011). The present study is the first that examines the role of prenatal dietary B(a)P exposure estimated taking into account all dietary sources, as we have previously identified other food groups, such as shellfish, cereals or fats and oils, as important sources and determinants of B(a)P and total PAH intakes (Duarte-Salles et al. 2010;Ibanez et al. 2005). Evidence of the importance of taking into account all sources of B(a)P exposure to adequately estimate exposure has been previously provided by Sinha et al, who found a stronger effect of dietary B(a)P in risk of colorectal adenoma when using B(a)P estimated from the whole diet than when using B(a)P estimated only from meat intakes (Sinha et al. 2005).
In this population, negative associations between dietary B(a)P and size at birth were observed only among women with lower intakes of vitamin C. Antioxidant nutrients, including vitamin C, have been shown to reduce the formation of bulky DNA adducts related to PAH exposure (Kelvin et al. 2009;Mooney et al. 2005;Palli et al. 2000). It is possible that vitamin C could be a marker for intakes of an antioxidant-rich diet or other aspects of dietary quality which may help to counter potential genotoxic effects of B(a)P and other contaminants. To better understand the role of vitamin C, we also assessed interactions of dietary B(a)P with fruit and vegetable intakes, which have been also related to reduced levels of DNA adducts (Palli D 2000). These interactions were not statistically significant (P>0.10), though stratified results were consistent with those observed for vitamin C, as there were somewhat stronger negative associations with B(a)P in women with low fruit and vegetable intakes.
Other micronutrients with antioxidant properties have also been hypothesized to protect against the formation of DNA adducts. Kelvin et al. (Kelvin et al. 2009) found that relationships between prenatal exposure to atmospheric PAH and adduct levels in cord blood were strongest among infants with low serum concentrations of alpha-tocopherol and carotenoids. However, biomarkers of vitamin C were not examined in that study. In our study we did not observe significant interactions with vitamin E, alpha-carotene or beta-carotene intakes in associations between dietary B(a)P and fetal growth indicators. Associations varied little across levels of these nutrients. For example, we found that multivariable-adjusted coefficients±SE for birth weight associated with 1-standard deviation increase of B(a)P intake were −48.54±39.07g in the group of women with low vitamin E intake (≤ the mean of 11.26mg/day), and −23.02±25.97g in the high intake group. The lack of modulation by these nutrients may be partly explained by differences in dietary sources in the Spanish vs. US food cultures. Given the high intakes of olive oil in Spain, added oils—which may also contain B(a)P—are the predominant source of dietary vitamin E (Garcia-Closas et al. 2006), in contrast to the wider variety of food sources of this nutrient in the US, including ready-to-eat cereals, breads and fruits and vegetables (Maras et al. 2004).
Although laboratory experiments support the existence of a negative effect of B(a)P exposure on fetal growth (Agency for Toxic Substances and Disease Registry (ATSDR) 1995), mechanisms through which PAHs may influence fetal growth are uncertain. However, it has been shown that these compounds are capable of crossing the placental barrier (Autrup and Vestergaard 1996;Perera et al. 1999;Sanyal et al. 2007). A number of mechanisms linking PAHs to fetal growth have been postulated. For example, PAHs bind to receptors regulating the induction of P450 enzymes, which may decrease the uptake of oxygen and nutrients; similar consequences may also be related to binding of these chemicals to receptors related to insulin and growth factor metabolism (Guyda 1991). In in vitro studies, B(a)P exposure has been shown to affect early trophoblast proliferation due to the interaction with growth factor receptors (Dejmek et al. 2000;Zhang et al. 1995).
Previous studies have shown the GSTP1 105Val allele to decrease conjugation and detoxification of PAH compounds (Butkiewicz et al. 2000;Whyatt et al. 2000). Our findings suggest that genetic variation in GSTP1 may increase susceptibility to adverse effects of dietary B(a)P on size at birth. Earlier studies exploring incidence of asthma in relation to exposure to tobacco smoke and air pollution, both sources of PAH exposure, have also found the GSTP1 105Val allele to be influential in the development of respiratory symptoms in children (Lee et al. 2004;Lee et al. 2007;Romieu et al. 2006).
In addition to a comprehensive assessment of dietary B(a)P, other important strengths of this study include the exploration of synergies with dietary antioxidants, and the availability of maternal and child data on a genetic polymorphism. Although we were able to asses for confounding from a wide array of socioeconomic and lifestyle factors, it is possible that uncontrolled confounding still remains since there are other factors that we were not able to consider. One limitation of this study is the lack of data on atmospheric PAH exposure. However, we were able to account for other key sources of PAH exposure, namely exposure to both active and passive tobacco smoke. We were also able to use exposure to atmospheric NO2, a marker of air pollution, as a proxy measure of atmospheric PAH. Although direct estimates of airborne PAH were not available, studies have shown moderate to strong correlations between PAH in air and other markers of air pollution such as NO2 (Tham et al. 2008; Wilhelm et al. 2011). Moreover, in the absence of a strong correlation between dietary and atmospheric PAHs, which we have no reason to expect in this population, potential confounding by this variable is likely to be modest. Another limitation is the modest sample size, particularly after stratifying by vitamin C intake and genotype. This was a limitation particularly for the analysis of SGA births, as it was not possible to further stratify models for this outcome by the GSTP1 polymorphism. Moreover, our results were robust after substantially (20%) increasing or decreasing the levels of exposure in a randomly selected 20% subset as a sensitivity analyses. We know of no reason for the association between GSTP1 alleles and maternal dietary B(a)P observed in our population among subjects with low maternal vitamin C intakes, and believe this occurred by chance. Though further study is required to confirm these findings, the consistency of our results after various sensitivity analyses, including excluding immigrants, suggest the findings are robust, and do not suggest that maternal vitamin C or B(a)P intake are markers of population strata correlated with ancestry.
4.1. Conclusion
This study provides evidence that prenatal exposure to B(a)P from dietary sources may reduce birth weight and length and increase risk of SGA birth in women with low vitamin C intakes during pregnancy. Presence of the GSTP1 Val-105 allele seems to increase susceptibility to effects of maternal B(a)P intakes on fetal growth indicators. Future analyses are needed to confirm this finding in populations with different dietary habits.
Highlights.
Fetal growth indicators and maternal intakes of benzo(a)pyrene [B(a)P] in pregnancy.
Subjects from the INMA (Environment and Childhood) Project in Sabadell.
Significant interactions between elevated vitamin C intakes and dietary B(a)P.
B(a)P related with lower birth weight and length in women with low vitamin C intakes.
Associations were strongest in subjects carrying the GSTP1 Val allele.
ACKNOWLEDGEMENTS
The authors are grateful to Silvia Fochs, Anna Sànchez Maribel López, Nuria Pey, and Muriel Ferrer for their assistance in contacting the families and administering the questionnaires. This study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176, CB06/02/0041), Spanish Ministry of Health (FIS-PI041436, FIS-PI081151), Generalitat de Catalunya-CIRIT 1999SGR 00241, and Fundación Roger Torné. The authors have no conflicts of interest to declare.
Abbreviations
- PAH
polycyclic aromatic hydrocarbons
- B(a)P
benzo(a)pyrene
- GSTP1
glutathione S-transferase P1
- SGA
small size for gestational age
- INMA study
Environment and Childhood study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs).:Department of Health and Human Services, Public Health Service. Atlanta, GA: U.S; 1995. [PubMed] [Google Scholar]
- Aguilera I, Garcia-Esteban R, Iniguez C, Nieuwenhuijsen MJ, Rodriguez A, Paez M, et al. Prenatal Exposure to Traffic-Related Air Pollution and Ultrasound Measures of Fetal Growth in the INMA-Sabadell Cohort. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera I, Sunyer J, Fernandez-Patier R, Hoek G, Aguirre-Alfaro A, Meliefste K, et al. Estimation of outdoor NO(x), NO(2), and BTEX exposure in a cohort of pregnant women using land use regression modeling. Environ Sci Technol. 2008;42:815–821. doi: 10.1021/es0715492. [DOI] [PubMed] [Google Scholar]
- Autrup H, Vestergaard AB. Transplacental transfer of environmental genotoxins--polycyclic aromatic hydrocarbon-albumin in nonsmoking women. Environ Health Perspect. 1996;104(Suppl 3):625–627. doi: 10.1289/ehp.96104s3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester F, Estarlich M, Iniguez C, Llop S, Ramon R, Esplugues A, et al. Air pollution exposure during pregnancy and reduced birth size: a prospective birth cohort study in Valencia, Spain. Environ Health. 2010;9:6. doi: 10.1186/1476-069X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari V, Velmurugan B, Nagini S. Induction of glutathione-dependent hepatic biotransformation enzymes by lycopene in the hamster cheek pouch carcinogenesis model. J Biochem Mol Biol Biophys. 2002;6:257–260. doi: 10.1080/10258140290030843. [DOI] [PubMed] [Google Scholar]
- Butkiewicz D, Grzybowska E, Phillips DH, Hemminki K, Chorazy M. Polymorphisms of the GSTP1 and GSTM1 genes and PAH-DNA adducts in human mononuclear white blood cells. Environ Mol Mutagen. 2000;35:99–105. [PubMed] [Google Scholar]
- Butler JP, Post GB, Lioy PJ, Waldman JM, Greenberg A. Assessment of carcinogenic risk from personal exposure to benzo(a)pyrene in the Total Human Environmental Exposure Study (THEES) Air Waste. 1993;43:970–977. doi: 10.1080/1073161x.1993.10467179. [DOI] [PubMed] [Google Scholar]
- Carrascosa A, Yeste D, Copil A, Almar J, Salcedo S, Gussinye M. [Anthropometric growth patterns of preterm and full-term newborns (24–42 weeks' gestational age) at the Hospital Materno-Infantil Vall d'Hebron (Barcelona)(1997–2002] An Pediatr (Barc ) 2004;60:406–416. doi: 10.1016/s1695-4033(04)78299-5. [DOI] [PubMed] [Google Scholar]
- Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect. 2008;116:658–665. doi: 10.1289/ehp.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejmek J, Solansky I, Benes I, Lenicek J, Sram RJ. The impact of polycyclic aromatic hydrocarbons and fine particles on pregnancy outcome. Environ Health Perspect. 2000;108:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Salles T, Mendez MA, Pessoa V, Guxens M, Aguilera I, Kogevinas M, et al. Smoking during pregnancy is associated with higher dietary intake of polycyclic aromatic hydrocarbons and poor diet quality. Public Health Nutr. 2010:1–10. doi: 10.1017/S1368980010001278. [DOI] [PubMed] [Google Scholar]
- Farran A, Zamora R, Cervera P. Tablas de composición de alimentos CESNID. Barcelona: Edicions Universitat de Barcelona/McGraw-Hill; 2003. [Google Scholar]
- Garcia-Closas R, Berenguer A, Gonzalez CA. Changes in food supply in Mediterranean countries from 1961 to 2001. Public Health Nutr. 2006;9:53–60. doi: 10.1079/phn2005757. [DOI] [PubMed] [Google Scholar]
- Grupo de Trabajo de la SEE - Álvarez-Dardet C, Alonso J, Domingo A, Regidor E. La medición de la clase social en ciencias de la salud. Barcelona: SG Editores; 1995. [Google Scholar]
- Guxens M, Ballester F, Espada M, Fernandez MF, Grimalt JO, Ibarluzea J, et al. Cohort Profile: The INMA--INfancia y Medio Ambiente--(Environment and Childhood) Project. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- Guyda HJ. Metabolic effects of growth factors and polycyclic aromatic hydrocarbons on cultured human placental cells of early and late gestation. J Clin Endocrinol Metab. 1991;72:718–723. doi: 10.1210/jcem-72-3-718. [DOI] [PubMed] [Google Scholar]
- Hazenkamp-von Arx ME, Götschi Fellmann T, Oglesby L, Ackermann-Liebrich U, Gíslason T, Heinrich J, et al. PM2.5 assessment in 21 European study centers of ECRHS II: Method and first winter results. J Air Waste Manag Assoc. 2003;53(5):617–628. doi: 10.1080/10473289.2003.10466189. [DOI] [PubMed] [Google Scholar]
- Hatagima A. Genetic polymorphisms and metabolism of endocrine disruptors in cancer susceptibility. Cad Saude Publica. 2002;18:357–377. doi: 10.1590/s0102-311x2002000200002. [DOI] [PubMed] [Google Scholar]
- Ibanez R, Agudo A, Berenguer A, Jakszyn P, Tormo MJ, Sanchez MJ, et al. Dietary intake of polycyclic aromatic hydrocarbons in a Spanish population. J Food Prot. 2005;68:2190–2195. doi: 10.4315/0362-028x-68.10.2190. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Tang D, Stigter L, Mroz E, Flak E, et al. Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: Birth cohort study in Poland. Nutrition. 2011 Nov 11; doi: 10.1016/j.nut.2011.07.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvin EA, Edwards S, Jedrychowski W, Schleicher RL, Camann D, Tang D, et al. Modulation of the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev. 2009;18:2262–2268. doi: 10.1158/1055-9965.EPI-09-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Lin YC, Lee YC, Wang JY, Hsiue TR, Guo YL. Glutathione S-transferase P1 gene polymorphism and air pollution as interactive risk factors for childhood asthma. Clin Exp Allergy. 2004;34:1707–1713. doi: 10.1111/j.1365-2222.2004.02099.x. [DOI] [PubMed] [Google Scholar]
- Lee YL, Lee YC, Guo YL. Associations of glutathione S-transferase P1, M1, and environmental tobacco smoke with wheezing illness in school children. Allergy. 2007;62:641–647. doi: 10.1111/j.1398-9995.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Venturini M, Marini E, Grechi Dm Dolara P. Polycyclic aromatic hydrocarbons air levels in Florence, Italy, and their correlation with other air pollutants. Chemosphere. 2003;50(3):377–382. doi: 10.1016/s0045-6535(02)00404-6. [DOI] [PubMed] [Google Scholar]
- Maras JE, Bermudez OI, Qiao N, Bakun PJ, Boody-Alter EL, Tucker KL. Intake of alpha-tocopherol is limited among US adults. J Am Diet Assoc. 2004;104:567–575. doi: 10.1016/j.jada.2004.01.004. [DOI] [PubMed] [Google Scholar]
- McCarty KM, Santella RM, Steck SE, Cleveland RJ, Ahn J, Ambrosone CB, et al. PAH-DNA adducts, cigarette smoking, GST polymorphisms, and breast cancer risk. Environ Health Perspect. 2009;117:552–558. doi: 10.1289/ehp.0800119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney LA, Madsen AM, Tang D, Orjuela MA, Tsai WY, Garduno ER, et al. Antioxidant vitamin supplementation reduces benzo(a)pyrene-DNA adducts and potential cancer risk in female smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:237–242. [PubMed] [Google Scholar]
- Morales E, Julvez J, Torrent M, de CR, Guxens M, Bustamante M, et al. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am J Epidemiol. 2009;169:1327–1336. doi: 10.1093/aje/kwp067. [DOI] [PubMed] [Google Scholar]
- Palli D, Vineis P, Russo A, Berrino F, Krogh V, Masala G, et al. Diet, metabolic polymorphisms and dna adducts: the EPIC-Italy cross-sectional study. Int J Cancer. 2000;87:444–451. doi: 10.1002/1097-0215(20000801)87:3<444::aid-ijc21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pavanello S, Clonfero E. Biological indicators of genotoxic risk and metabolic polymorphisms. Mutat Res. 2000;463:285–308. doi: 10.1016/s1383-5742(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Pavanello S, Pulliero A, Saia BO, Clonfero E. Determinants of anti-benzo[a]pyrene diol epoxide-DNA adduct formation in lymphomonocytes of the general population. Mutat Res. 2006;611:54–63. doi: 10.1016/j.mrgentox.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM, et al. Recent developments in molecular epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(Suppl 3):451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, et al. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Health Perspect. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Romieu I, Ramirez-Aguilar M, Sienra-Monge JJ, Moreno-Macias H, Rio-Navarro BE, David G, et al. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur Respir J. 2006;28:953–959. doi: 10.1183/09031936.06.00114905. [DOI] [PubMed] [Google Scholar]
- Sanyal MK, Mercan D, Belanger K, Santella RM. DNA adducts in human placenta exposed to ambient environment and passive cigarette smoke during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:289–294. doi: 10.1002/bdra.20346. [DOI] [PubMed] [Google Scholar]
- Scherer G, Frank S, Riedel K, Meger-Kossien I, Renner T. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiol Biomarkers Prev. 2000;9:373–380. [PubMed] [Google Scholar]
- Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2030–2034. doi: 10.1158/1055-9965.EPI-04-0854. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occup Environ Health. 2007;81:115–121. doi: 10.1007/s00420-007-0188-x. [DOI] [PubMed] [Google Scholar]
- Tham Y, Takeda K, Sakugawa H. Exploring the Correlation of Particulate PAHs, Sulfur Dioxide, Nitrogen Dioxide and Ozone, A Preliminary Study. Water Air and Soil Pollution. 2008;194:5–12. [Google Scholar]
- van Lieshout EM, Peters WH, Jansen JB. Effect of oltipraz, alpha-tocopherol, beta-carotene and phenethylisothiocyanate on rat oesophageal, gastric, colonic and hepatic glutathione, glutathione S-transferase and peroxidase. Carcinogenesis. 1996;17:1439–1445. doi: 10.1093/carcin/17.7.1439. [DOI] [PubMed] [Google Scholar]
- Varea M, Galindo N, Gil-Moltó J, Pastor C, Crespo J. Particle-bound polycyclic aromatic hydrocarbons in an urban, industrial and rural area in the western Mediterranean. J Environ Monit. 2011;13(9):2471–2476. doi: 10.1039/c1em10163c. [DOI] [PubMed] [Google Scholar]
- Vioque J. Validez de la evaluación de la ingesta dietética. In: Serra Magem L, Aranceta J, editors. Nutrición y Salud Pública. Métodos, bases científicas y aplicaciones. 2nd Edition. Barcelona: Masson. In.; 2006. [Google Scholar]
- Vyskocil A, Fiala Z, Chenier VV, Krajak L, Ettlerova E, Bukac J, et al. Assessment of multipathway exposure of small children to PAH. Environ Toxicol Pharmacol. 2000;8:111–118. doi: 10.1016/s1382-6689(00)00032-6. [DOI] [PubMed] [Google Scholar]
- Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Perera FP, Jedrychowski W, Santella RM, Garte S, Bell DA. Association between polycyclic aromatic hydrocarbon-DNA adduct levels in maternal and newborn white blood cells and glutathione S-transferase P1 and CYP1A1 polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9:207–212. [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health. 2011;10:89. doi: 10.1186/1476-069X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Connor EE, Chegini N, Shiverick KT. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation, and secretion of human chorionic gonadotropin in human placental cell lines. Biochem Pharmacol. 1995;50:1171–1180. doi: 10.1016/0006-2952(95)00253-v. [DOI] [PubMed] [Google Scholar]