Abstract
In this study we have investigated the potential of mycobacterial proteins as candidate subunit vaccines for bovine tuberculosis. In addition, we have explored the use of TLR-ligands as potential adjuvants in cattle. In vitro screening assays with whole blood from M. bovis-infected and BCG-vaccinated cattle demonstrated that fusion protein constructs were most commonly recognised, and the ID83 fusion protein was selected for further immunisation studies. Furthermore, glucopyranosyl lipid A (GLA) and resiquimod (R848), agonists for TLR4 and TLR7/8 respectively, stimulated cytokine production (IL-12, TNF-α, MIP-1β and IL-10) in bovine dendritic cell cultures, and these were formulated as novel oil-in-water emulsions (GLA-SE and R848-SE) for immunisation studies. Immunisation with ID83 in a water-in-oil emulsion adjuvant (ISA70) induced both cell mediated and humoral immune responses, as characterised by antigen-specific IFN-γ production, cell proliferation, IgG1 and IgG2 antibody production. In comparison, ID83 immunisation with the novel adjuvants induced weaker (ID83/R848-SE) or no (ID83/GLA-SE) antigen-specific IFN-γ production and cell proliferation. However, both did induce ID83-specific antibody production, which was restricted to IgG1 antibody isotype. Overall, these results provide encouraging preliminary data for the further development of ID83 in vaccine strategies for bovine TB.
INTRODUCTION
Bovine tuberculosis (TB) caused by the bacterial pathogen Mycobacterium bovis (M. bovis) poses a major economical and animal health problem to the farming community. Despite the current ‘test and slaughter’ control policy, the incidence of bovine TB in Great Britain remains high [1], and so vaccination of cattle against M. bovis infection is being considered as one of the long term control options for reducing the risk and incidence of bovine TB. The most promising vaccination strategies against bovine TB are based on a heterologous prime-boost approach, which involves priming the immune system with BCG followed by boosting with a subunit vaccine [2–4]. Thus, the identification of immunogenic proteins suitable for use as the ‘boosting’ subunit vaccine remains a high priority in TB research.
Given that purified protein or peptide antigens are poorly immunogenic when administered on their own [5], it is crucial that protein subunit vaccines are administered in a suitable adjuvant system. Most modern adjuvants consist of two components: (i) immunostimulants that act directly on the immune system to augment responses to vaccine antigens; and (ii) vehicles that ensure vaccine antigens are presented to the immune system in an optimal manner. One class of immunostimulants that have shown potential as adjuvants are the ligands of the Toll-like receptors (TLRs). TLRs are pattern recognition receptors expressed on numerous cells of the immune system, which bind several conserved molecules expressed by a wide variety of infectious agents resulting in the production of pro-inflammatory cytokines/chemokines and type I IFNs that mediate the host’s ability to eliminate the pathogen [6–9]. The performance of several TLR ligands as adjuvants have been (or are currently being) evaluated in numerous human clinical trials. These include ligands for TLR2 (e.g. Pam3Cys and palmitic acid), TLR3 (e.g. poly I:C derivatives), TLR4 (e.g. MPLA), TLR5 (e.g. flagellin), TLR7/8 (e.g. imiquimod), and TLR9 (e.g. CpG oligonucleotides) (reviewed in [10]). The second crucial component of an adjuvant system is the vehicle for delivery of the antigen/adjuvant. Immunisation of mice with a commercial influenza vaccine (Fluzone) plus a synthetic TLR4 ligand (lipid A) as an aqueous formulation was not as effective in generating cellular immune responses when compared to Fluzone plus lipid A formulated as an oil-in-water emulsion [11], highlighting the need for careful preparation of the adjuvant to induce the most desirable results.
In this paper, we have screened a library of proteins for their immunogenicity in M. bovis-infected and M. bovis bacillus Calmette-Guérin (BCG)-vaccinated cattle, with the aim of identifying suitable candidates for sub-unit vaccines for bovine TB. In addition, we have assessed the performance of glucopyranosyl lipid A (GLA) and resiquimod (R848) (TLR4 and TLR7/8 agonists respectively) as adjuvants when formulated in an oil-in-water emulsion.
MATERIALS AND METHODS
In vitro antigen screening
(i) Cattle
All animals were housed at the Animal Health and Veterinary Laboratories Agency at the time of blood sampling, and procedures were conducted within the limits of a United Kingdom Home office license under the Animal (Scientific Procedures) Act 1986, which were approved by the local ethical review committee. Heparinised blood samples were obtained from 22 naturally M. bovis-infected, single intradermal cervical comparative tuberculin (SICCT)-positive reactors from herds known to have bovine tuberculosis. A detailed post mortem examination of these animals revealed visible TB lesions in all but three animals, confirming the presence of active disease. Heparinised blood samples were also obtained from a total of 9 animals vaccinated with BCG Danish (Statens Serum Institutet, Copenhagen, Denmark) as previously described [12].
(ii) Stimulation of whole blood cultures
A total of 54 recombinant proteins from the Infectious Disease Research Institute (IDRI) protein repository were evaluated for immunogenicity in cattle at a final concentration of 10μg/ml. Included in this set were six novel fusion proteins (ID71, ID83, ID87, ID91, ID93 and ID97) consisting of at least two different proteins. Bovine tuberculin purified protein derivative (PPD-B, AHVLA, Weybridge, UK) and Staphylococcal enterotoxin B (SEB, Sigma-Aldrich, UK) were used as positive controls at a final concentration of 10μg/ml and 1μg/ml respectively, while RPMI 1640 medium alone (Gibco Life Technologies, Paisley, UK) was included as negative control. Whole-blood aliquots (250μl) were added in duplicate to antigen in 96-well plates and incubated at 37°C in the presence of 5% CO2 for 24 hours, after which plasma supernatants were harvested and stored at −80°C until required.
(iii) IFN-γ enzyme-linked immunosorbent assay (ELISA)
Quantification of IFN-γ in the plasma supernatants was determined using the Bovigam ELISA kit (Prionics AG, Switzerland). A result was considered positive if the optical density at 450 nm (OD450) with antigen minus the OD450 without antigen (ΔOD450) was >0.1 in both of the duplicate wells.
In vitro adjuvant screening
(i) Stimulation of bovine monocyte-derived dendritic cells (MDDC)
Bovine MDDC were generated as previously described [13]. Briefly, PBMC were isolated from cattle whole blood using Histopaque 1077 (Sigma Aldrich), following which bovine CD14+ monocytes were isolated using MACS anti-CD14 MicroBeads (Miltenyi Biotec, Bisley, Surrey, UK). CD14+ cells were cultured at 37°C in the presence of 5% CO2 for 3 days in complete medium (RPMI 1640 containing 25mM HEPES, 10% FCS, 1% NEAA, 5 x10−5M β-mercaptoethanol, 100U/ml penicillin and 100μg/ml streptomycin [Gibco Life Technologies]) in the presence of 1000U/ml equine GM-CSF (supplied by Falko Steinbach, Department of Virology, AHVLA) and 4ng/ml bovine IL-4 (AbD-Serotec, Kidlington, Oxon, UK ). Bovine MDDC were stimulated with aqueous suspensions of (a) GLA, the synthetic TLR4 agonist was bulk manufactured for IDRI by Avanti Polar Lipids, Inc. (Alabaster, AL), (b) R848 (Invivogen, San Diego, CA), or (c) a mix of GLA and R848 (5:1 ratio) for 24 hours, following which supernatants were harvested and stored at −80°C until required.
(ii) Cytokine multiplex assay
Simultaneous detection of bovine MIP-1β, TNF-α, IL-1β, IL-6, IL-10 and IL-12 was performed using the MSD multiplex platform (Meso Scale Discovery, Gaithersburg, MD, USA) as previously described [14, 15].
ID83 immunisation study
(i) Immunisation strategy
The TLR agonists GLA and R848 were formulated at IDRI (Seattle, WA) in an oil-in-water stable emulsion (SE) (i.e. GLA-SE and R848-SE) [16], while the mineral oil-based ISA70 [a ready-to-use oily vaccine adjuvant based on high-grade injectable mineral oil (Seppic, France)] was prepared as a water-in-oil adjuvant as previously described [17]. GLA-SE and R848-SE were used at a dose of 5μg and 1μg per injection respectively, while the ID83 fusion protein was used at a dose of 10μg per injection. 20 calves (ca. 6 months of age) from bovine TB-free herds were allocated to one of five study groups (four animals per group) that were injected via the intramuscular route with 100μl of either: (a) PBS; (b) ID83 in ISA70 adjuvant; (c) ID83 in GLA-SE adjuvant; (d) ID83 in R848-SE adjuvant; or (e) ID83 in GLA-SE + R848-SE adjuvant. Animals were injected at week 0 and at week 4, while whole blood and serum samples were collected at weeks 0, 2, 4, 6 and 8 for immunological assays.
(ii) Stimulation of whole blood cultures and IFN-γ ELISA
Whole blood (250μl/well) was stimulated in duplicate wells with the ID83 fusion protein or the individual protein components (Rv1813, Rv2608 and Rv3620c) at final concentrations of 5μg/ml. Pokeweed mitogen (PWM; 5μg/ml, Sigma Aldrich) and RPMI 1640 medium alone were included as positive and negative controls respectively. Plasma supernatants were harvested after 24 hours and the level of IFN-γ quantified using the Bovigam ELISA kit (Prionics) and recombinant bovine IFN-γ as a standard (Thermo Scientific, UK). Results are expressed as ΔIFN-γ concentration (IFN-γ concentration of antigen stimulated cultures minus IFN-γ concentration of negative control stimulated cultures).
(iii) PBMC proliferation assay
PBMC were isolated from whole blood using Histopaque 1077 (Sigma Aldrich), washed twice with HBSS and re-suspended in cell culture medium. PBMC (2x105 cells per well) were stimulated for 5 days in triplicate wells of a 96-well plate with the ID83 fusion protein or the individual protein components (Rv1813, Rv2608 and Rv3620c) at final concentrations of 5μg/ml. PWM (5μg/ml) and RPMI 1640 medium alone were included as positive and negative controls respectively. For the last 16 hours of culture, wells were pulsed with 1 μCi of [Methyl-3H]-thymidine before being harvested using a Harvester 96 Mach III (TomTec Inc, Hamden, CT, USA). Lymphocyte proliferation was assessed by the incorporation of 3H-thymidine as measured using a MicroBeta2 2450 (Perkin Elmer, Waltham, MA, USA), triplicate wells averaged and results are expressed as Δcpm (cpm of antigen stimulated cultures minus cpm of negative control stimulated cultures).
(vi) Serology assays
(a) Bovine total IgG ELISA: Maxisorp 96-well plates (Nunc™, Thermo Fisher Scientific, Denmark) were coated overnight with 0.5 μg/well of ID83 fusion protein, washed with PBS containing 0.1% Tween 20 (wash buffer) and blocked for 2 hours by the addition of PBS containing 2% casein and 0.05% Tween 20 (casein blocking buffer). Wells were washed, serum samples (diluted 1:100 in casein blocking buffer) added and plates incubated for 2 hours, after which the plates were washed and a sheep anti-bovine IgG HRP-conjugated antibody (AbD-Serotec; diluted 1:5000 in casein blocking buffer) added for one hour. After a final wash, 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Sigma Aldrich) was added for 10 minutes. The colour reaction was stopped by the addition of 0.5 M H2SO4, and optical densities (ODs) were measured at 450nm. ID83-specific ODs were calculated by subtracting the OD readings from control wells not coated with ID83. (b) Bovine isotype IgG ELISA: ID83-specific bovine IgG1 and IgG2 levels were evaluated as described above, but with the following modifications. Firstly, the blocking buffer was switched to PBS containing 3% bovine serum albumin (BSA), which was shown to perform better than the casein blocking buffer and negated the need for non-ID83 coated control wells (data not shown). Secondly, sheep anti-bovine IgG isotype specific HRP-conjugated antibodies (either anti-bovine IgG1 or anti-bovine IgG2) were used in the detection step (AbD-Serotec; both diluted 1:5000 in BSA blocking buffer).
Statistical analysis
Statistical analysis was performed using GraphPad Instat 3 software (GraphPad Software Inc, USA), while area under the curve (AUC) calculations for individual animal immune responses over the course of the study were performed using GraphPad Prism 5 software (GraphPad Software Inc).
RESULTS
Selection of antigen candidate
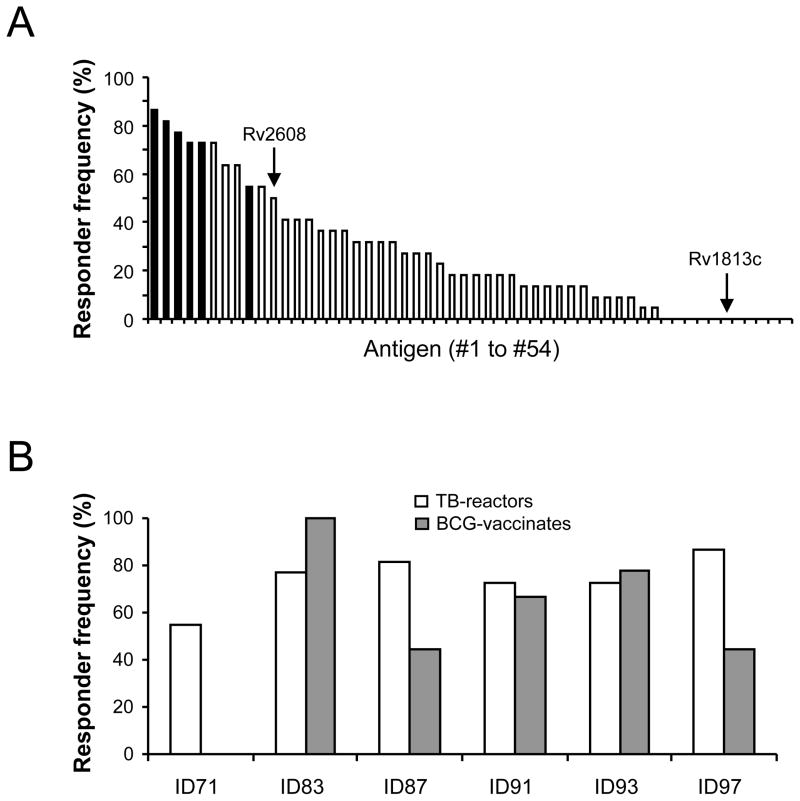
To identify antigens that show potential as subunit vaccine candidates for bovine TB, a total of 54 proteins from the IDRI protein repository were screened for their ability to stimulate an IFN-γ response in whole blood from TB-reactor cattle. All animals responded to the SEB and PPD-B positive controls (data not shown). Of the 54 IDRI proteins, 11 (20%) failed to induce an IFN-γ response in any animal studied, while the remaining 43 (80%) were recognised to various degrees with responder frequencies ranging from 5 to 86% (Figure 1A). Of note, the most frequently recognised antigens in TB-reactor animals were the fusion proteins (filled bars), where all six induced responses in at least half of the animals studied. The immunogenicity of the fusion proteins were further explored in non-infected BCG vaccinated cattle (figure 1B), where all but one fusion protein induced IFN-γ production in these animals, with the most frequent responses being detected against the ID83 fusion protein. Thus, due to its high levels of immunogenicity both in TB-reactor and in BCG-vaccinated animals, ID83 was selected for further evaluation in an immunisation study.
Figure 1. Selection of the ID83 fusion protein for vaccination studies.
(A) Responder frequency of 22 TB reactor animals to 54 recombinant proteins from the IDRI protein repository. Filled bars denote responses to novel IDRI fusion proteins. (B) Responder frequency of 22 TB reactor and between 5 and 9 (depending on the protein) BCG vaccinated animals to the 6 IDRI fusion proteins. In both graphs, the responder frequency is based on IFN-γ production as the readout.
In vitro evaluation of adjuvant immunogenicity
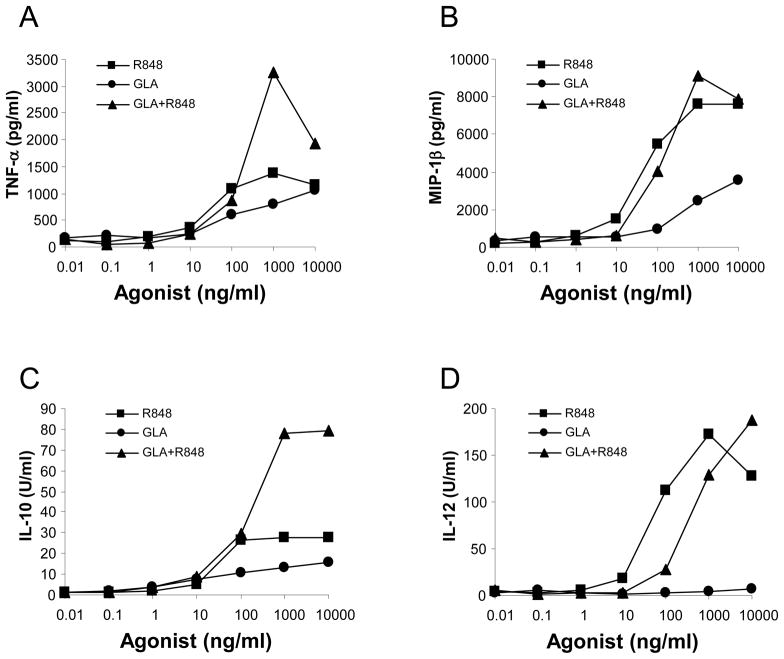
One mechanism by which adjuvants may exert their effect is through the activation of innate immunity by inducing dendritic cells to secrete cytokines [10]. To investigate whether the adjuvant components GLA or R848 activated bovine innate immunity, aqueous preparations of these TLR ligands were used to stimulate bovine MDDC and cytokine production (MIP-1β, TNF-α, IL-1β, IL-6, IL-10 and IL-12) enumerated. Whilst no increases in IL-1β or IL-6 levels were observed (data not shown), bovine MDDC secreted MIP-1β, TNF-α, IL-10 in responses to stimulation with GLA or R848 (figure 2). Although IL-12 production was also observed in response to stimulation with R848, little production was seen to GLA alone (figure 2D). Overall, MDDC secreted higher levels of all four cytokines following stimulation with R848 compared to GLA. Although marginal for TNF-α and IL-10 (figure 2A and 2C respectively), this was most striking for MIP-1β and IL-12 (figure 2B and 2D respectively). Of note, a potential synergistic effect in the production of TNF-α and IL-10 was observed when MDDC were treated simultaneously with R848 and GLA.
Figure 2. Cytokine production by bovine MDDC following stimulation with GLA and R848.
Bovine MDDC were cultured for 24 hours in the presence of increasing concentrations of GLA, R848 or a mixture of GLA and R848. Quantification of (A) TNF-α, (B) MIP-1β, (C) IL-10 and (D) IL-12 was performed using a cytokine multiplex assay. Data shown is representative of two experiments.
ID83 immunisation study
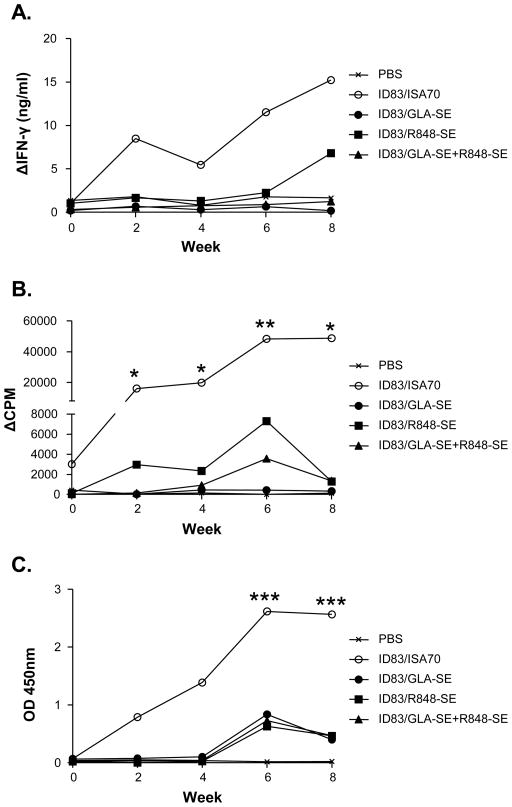
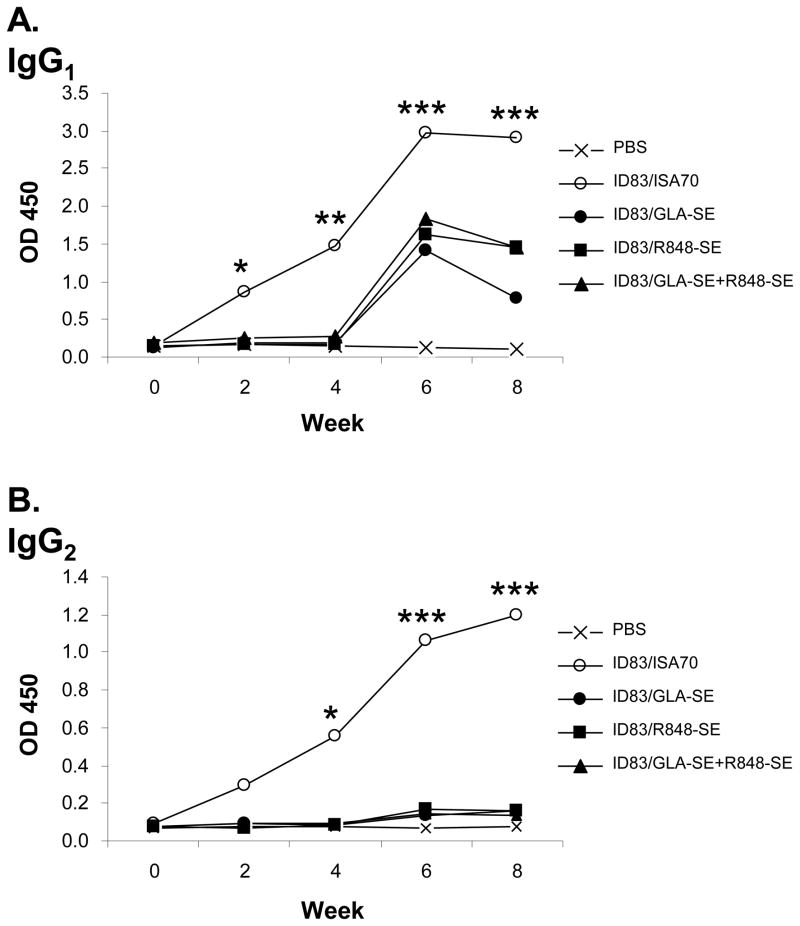
Having demonstrated (i) the immunogenicity of the ID83 fusion protein and (ii) the potential of GLA and R848, when used alone or in combination, to induce cytokine production in bovine innate immune cells, we next evaluated the efficacy of ID83 when delivered in different adjuvant systems (as detailed in material and methods). The ISA70 formulation was included as a control in this study as its potential as an adjuvant for protein vaccination in cattle has been previously demonstrated [17]. As shown in figure 3A, ID83-specific IFN-γ responses were first detected in the ID83/ISA70 group two weeks after the initial injection, which were further boosted following the second injection at week 4. Although no IFN-γ responses were detectable in any of the other groups following the first immunisation, ID83-specific IFN-γ responses were observed in the ID83/R848-SE group at week 8 (i.e. 4 weeks post second immunisation), albeit at lower levels compared to the ID83/ISA70 group. Similar to the IFN-γ response, increased ID83-specific PBMC proliferation was observed two weeks after the initial immunisation in the ID83/ISA70 group, which again appeared to be boosted after the second immunisation at week 4 (figure 3B). Although at lower levels when compare to the ID83/ISA70 group, PBMC proliferative responses were also observed in the ID83/R848-SE group two weeks after the first immunisation, which were transiently boosted following the second immunisation. Weaker transient responses were also observed in the ID83/GLA-SE+R848-SE group after the second immunisation, while no ID83-specific proliferative responses were seen in the ID83/GLA-SE or PBS control groups. Lastly, ID83-specific bovine IgG was detected in the serum of ID83/ISA70 immunised animals two weeks after the first injection, which peaked at week 6 and remained high for the duration of the experiment (figure 3C). Although not detected after the first injection, serum ID83-specific IgG was detected in all other ID83-immunised animals after the second injection, albeit at lower levels compared to the ID83/ISA70 group. Vaccine induced ID83-specific humoral responses were further evaluated by individually quantifying IgG1 and IgG2 antibody isotypes. With the exception of the PBS control group, all other groups demonstrated ID83-specific IgG1 isotype responses (figure 4A) with kinetics similar to that seen for total IgG (figure 3C). In contrast, only immunisation with ID83/ISA70 induced ID83-specific IgG2 isotype responses (figure 4B).
Figure 3. Immunisation with ID83 induces cellular and humoral immune responses.
Cattle (four per group) were immunised at week 0 and week 4 as detailed in the material and methods. Blood samples were taken at 2 weekly intervals for assessment of (A) ID83-specific whole blood IFN-γ production, (B) ID83-specific PBMC proliferation, and (C) ID83-specific IgG serum antibody levels. Median responses for each group are plotted. * p<0.05, ** p<0.01, *** p<0.001, nonparametric ANOVA with Dunn’s Multiple Comparison Test (versus the PBS control group at each time point).
Figure 4. Immunisation with ID83/ISA70 induces production of ID83-speficic IgG2 serum antibodies.
Cattle (four per group) were immunised at week 0 and week 4 as detailed in the material and methods, and blood samples taken at 2 weekly intervals for assessment of (A) ID83-specific IgG1, or (B) ID83-specific IgG2 serum antibody levels. Median responses for each group are plotted. * p<0.05, ** p<0.01, *** p<0.001, nonparametric ANOVA with Dunn’s Multiple Comparison Test (versus the PBS control group at each time point).
DISCUSSION
Our initial screening experiments revealed that ID83 induced IFN-γ responses in TB-reactor animals, suggesting that proteins present in this fusion construct are targets of the immune system during natural infection with M. bovis. Indeed, the immunogenicity of this fusion protein was further confirmed in the immunisation study, wherein injection with ID83 in ISA70 adjuvant induced both cellular (IFN-γ production and PBMC proliferation) and humoral (serum IgG) immune responses. To date, promising vaccine strategies for bovine TB utilize a prime-boost approach, whereby the efficacy of a ‘priming’ BCG vaccination is significantly improved following ‘boosting’ with DNA [2], protein [4], or viral subunit vaccines [3, 18]. As shown in figure 1B, ID83-specific responses were observed in BCG vaccinated animals, suggesting that BCG vaccination primes, amongst other things, responses to the proteins contained in the fusion construct. Furthermore, mice immunised with ID83 formulated in an oil-in-water emulsion containing a TLR4 agonist showed significant reduction in bacterial burden following aerosol challenge with M. tuberculosis, demonstrating a protective role for immune responses targeted to these proteins [19]. Taken together, these data provide good rationale for the further development of ID83 as a cattle subunit vaccine, particularly in the setting of prime-boost strategies in combination with BCG.
The ID83 fusion protein consists of the following three proteins: Rv2608 (a member of the PE/PPE protein family); Rv1813c (a member of the DosR regulon protein family) and Rv3620c (a member of the ESAT-6 like protein family). Rv2608 and Rv1813c were tested as individual proteins in our initial screening experiments (figure 1, arrowed). Rv2608 induced IFN-γ production in half of the TB reactor animals studied, however Rv1813c failed to induce a response in any animal. Interestingly, the lack of immune response to Rv1813c is consistent with previous data showing limited recognition of other DosR regulon gene products in TB reactor cattle [20]. Although Rv3620c is encoded by a gene which is deleted from the M. bovis genome, we have previously shown that this protein is also immunogenic in TB reactor cattle[21], presumably due to its high degree of homology with other members of the ESAT-6 like protein family that are expressed in M. bovis [22]. As shown in figure 3, immunisation with ID83/ISA70 primed both proliferative and IFN-γ responses to this fusion protein. To gain insight as to whether these responses were directed against a single dominant protein or to multiple proteins contained within ID83, we also analysed cell mediated immune responses to the individual proteins Rv2608, Rv3620c and Rv1813c. Rv3620c specific IFN-γ production was observed in immunised animals, irrespective of the adjuvant regime used, while Rv2608 specific responses were only apparent in the ISA70 adjuvant group (Supplemental figure S1). Little, if any, Rv1813c specific IFN-γ production was observed. Thus, these results were consistent with the data from naturally infected TB-reactor animals, demonstrating the immunogenicity of Rv3620c and Rv2608. Furthermore, the lack of an IFN- γ response to the Rv1813c protein in TB-reactor animals may be due in part to the poor immunogenicity (with regards to induction of IFN- γ responses) of this protein in cattle, rather than issues in the level of expression of this potential latency-specific antigen during active disease. In contrast to the IFN-γ data, PBMC proliferation experiments revealed a broader repertoire of protein responses in ID83 immunised animals (Supplemental figure 2). Although there was a trend in stronger responses to Rv2608, PBMC proliferation was also observed with Rv3620c and Rv1813c, suggesting that these proteins may contain immunogenic regions that induce T cell proliferation independent of IFN-γ secretion or may act on other cell subsets present in PBMC (e.g. B-cells and NK cells).
GLA and R848 exert their effect through interaction with TLRs present on the surface of innate immune cells. In vitro experiments demonstrated that GLA stimulated the secretion of IL-12 and the pro-inflammatory cytokines IL-6 and TNF-α in mice and human dendritic cells [23]. R848 activates cells through interaction with either TLR7 or TLR8 [24], and has also been shown to induce the production of IL-12 and several pro-inflammatory cytokines from both human [25, 26] and mice [27] innate immune cells. As shown herein, these immunostimulatory properties of GLA and R848 can also be extended to cattle. Both reagents induced production of pro-inflammatory cytokines (TNF-α and MIP-1β) and the immunoregulatory cytokine IL-10, while R848 also induced production of the immunoregulatory cytokine IL-12 in bovine dendritic cells. However, differences were observed in the potency of the two TLR ligands, with R848 inducing greater levels of all four cytokines. Of particular interest was the relative production of IL-10 and IL-12, where area under the curve analysis demonstrated R848 induced an IL-12-polarised response, GLA induced an IL-10-polarised response, while stimulation with both TLR ligands induced a more balanced response (Supplemental table S1). Given the key roles of IL-12 and IL-10 in promoting Th1-like cell mediated immunity and regulating T-cell responses respectively, we hypothesised that immunisation in the presence of R848-SE, when compared to GLA-SE, would result in qualitative and/or quantitative differences in vaccine-induced immune responses. Our results demonstrated that vaccine-induced cell-mediated immune responses were evident in cattle immunised with ID83 in adjuvants containing R848-SE, but were absent when GLA-SE was used as the sole adjuvant (figure 3A and 3B). Thus, analysing the stimulatory effects of adjuvants on bovine dendritic cells in vitro, with particular emphasis on the relative levels of induced IL-12 and IL-10 production, may provide a useful tool in pre-screening novel adjuvants for potential induction of cell-mediated immune responses when used as adjuvants in vaccination studies.
Immunisation in either R848 or GLA adjuvants induced humoral immune responses, which were restricted to the production of ID83-specific IgG1 isotype antibodies. In contrast, immunisation with ID83 in ISA70 adjuvant induced both IgG1 and IgG2 isotype responses. Previous studies in mice have suggested a link between the induction of Th1-like immune responses and the generation of IgG2 isotype antibody, where addition of either the Th1-like cytokine IFN-γ [28] or Th1-polarised T-cell clones [29] induced antigen-specific B-cells to secrete IgG2a. Furthermore, in vivo administration of recombinant IFN-γ stimulated production of antigen-specific IgG2a [30]. Thus, we speculate that the greater induction of an antigen-specific IFN-γ response observed when ID83 is delivered in ISA70 adjuvant (compared to R848 or GLA) drives the subsequent generation of ID83-specific IgG2 antibodies, which are not observed when the immunisations are delivered in adjuvants containing R848 or GLA.
In summary, we have identified ID83 as a target of the immune system in both M. bovis-infected and BCG-vaccinated cattle. Furthermore, immunisation with this fusion protein induced both cell-mediated and humoral immune responses when used in combination with the ISA70 water-in-oil adjuvant. Taken together, these data provide encouraging preliminary data for the further development of ID83 in vaccine strategies for bovine TB. Indeed, the fusion protein ID93, which contains all of the individual proteins of ID83, will soon enter into phase I clinical studies for human TB. Although GLA-SE and R848-SE failed to prime strong ID83-specific cell mediated immune responses when used as adjuvants, they did show stimulatory activity on bovine innate immune cells, as demonstrated by the induction of pro-inflammatory cytokines in bovine dendritic cell cultures. Therefore, we believe that the results presented herein warrant further vaccination studies, particularly addressing antigen and adjuvant dose optimisation, alongside evaluating the effect of GLA and R848 when delivered in the ISA70 water-in-oil system. Given that ID83 was a target of the immune system in BCG-vaccinated cattle, future studies should also evaluate the immunogenicity of adjuvanted ID83 when used as a ’boost’ in BCG ‘primed’ vaccinated animals. These experiments would ultimately allow for the rational design of a vaccination/challenge study to assess the efficacy of immunisation with ID83 against bovine TB.
Supplementary Material
Highlights.
Novel fusion protein constructs are frequently recognised in M. bovis infected cattle
TLR4 and TLR7/8 agonists induce cytokine production in bovine dendritic cells
ID83 immunisation in ISA70 adjuvant induces cell mediated and humoral immune responses
ID83 immunisation in TLR-agonist adjuvants induces mainly humoral immune responses
Acknowledgments
This study was funded by the Department for Environment, Food and Rural affairs (DEFRA), UK. Prior work for the development of TB antigens included in this manuscript was supported by the National Institutes of Health grants AI-044373 and AI-078054, and contracts AI-25479 and HHSN272200800045C. We are grateful to the staff of the Animal Service Unit for their dedication to the welfare of the animals housed at AHVLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DEFRA. The UK Chief Veterinary Officer’s 2009 report on animal health and welfare. 2010 http://wwwdefragovuk/corporate/about/who/cvo/documents/2009reportpdf.
- 2.Skinner MA, Wedlock DN, de Lisle GW, Cooke MM, Tascon RE, Ferraz JC, et al. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect Immun. 2005;73:4441–4. doi: 10.1128/IAI.73.7.4441-4444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vordermeier HM, Villarreal-Ramos B, Cockle PJ, McAulay M, Rhodes SG, Thacker T, et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect Immun. 2009;77:3364–73. doi: 10.1128/IAI.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedlock DN, Denis M, Skinner MA, Koach J, de Lisle GW, Vordermeier HM, et al. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infect Immun. 2005;73:3540–6. doi: 10.1128/IAI.73.6.3540-3546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Hagan DT, Rappuoli R. Novel approaches to vaccine delivery. Pharmaceutical research. 2004;21:1519–30. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual review of immunology. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 10.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29:3341–55. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–63. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 12.Vordermeier HM, Cockle PC, Whelan A, Rhodes S, Palmer N, Bakker D, et al. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin Diagn Lab Immunol. 1999;6:675–82. doi: 10.1128/cdli.6.5.675-682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirson C, Jones GJ, Steinbach S, Besra GS, Vordermeier HM. Differential effects of Mycobacterium bovis - derived polar and apolar lipid fractions on bovine innate immune cells. Vet Res. 2012;43:54. doi: 10.1186/1297-9716-43-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coad M, Clifford D, Rhodes SG, Hewinson RG, Vordermeier HM, Whelan AO. Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses. Vet Res. 2010;41:14. doi: 10.1051/vetres/2009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones GJ, Pirson C, Hewinson RG, Vordermeier HM. Simultaneous measurement of antigen-stimulated interleukin-1 beta and gamma interferon production enhances test sensitivity for the detection of Mycobacterium bovis infection in cattle. Clin Vaccine Immunol. 2010;17:1946–51. doi: 10.1128/CVI.00377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick TS. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids and surfaces B, Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Vordermeier HM, Dean GS, Rosenkrands I, Agger EM, Andersen P, Kaveh DA, et al. Adjuvants induce distinct immunological phenotypes in a bovine tuberculosis vaccine model. Clin Vaccine Immunol. 2009;16:1443–8. doi: 10.1128/CVI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing Mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect Immun. 2006;74:1416–8. doi: 10.1128/IAI.74.2.1416-1418.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin SL, Bertholet S, Kahn M, Zharkikh I, Ireton GC, Vedvick TS, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones GJ, Pirson C, Gideon HP, Wilkinson KA, Sherman DR, Wilkinson RJ, et al. Immune responses to the enduring hypoxic response antigen Rv0188 are preferentially detected in Mycobacterium bovis infected cattle with low pathology. PLoS One. 2011;6:e21371. doi: 10.1371/journal.pone.0021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones GJ, Gordon SV, Hewinson RG, Vordermeier HM. Screening of predicted secreted antigens from Mycobacterium bovis reveals the immunodominance of the ESAT-6 protein family. Infect Immun. 2010;78:1326–32. doi: 10.1128/IAI.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 23.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nature immunology. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 25.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cellular immunology. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 26.Ida JA, Shrestha N, Desai S, Pahwa S, Hanekom WA, Haslett PA. A whole blood assay to assess peripheral blood dendritic cell function in response to Toll-like receptor stimulation. Journal of immunological methods. 2006;310:86–99. doi: 10.1016/j.jim.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small antiviral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature immunology. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 28.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 29.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.